Abstract
Background
Extralobar pulmonary sequestration–a congenital lung malformation characterized by nonfunctional lung tissue with its own visceral pleura and without a connection to the normal tracheobronchial tree–is often surgically resected given the potential for infectious complications. We report a case of a child with extralobar pulmonary sequestration in a rare and challenging intradiaphragmatic location, which made preoperative identification and planning difficult.
Case presentation
A 2-year-old boy presented for follow-up of a large left extralobar pulmonary sequestration initially diagnosed on an antenatal ultrasound. Follow-up imaging, including prenatal MRI and postnatal CT angiography, was inconclusive on the precise location of the extralobar pulmonary sequestration in relation to the diaphragm but did reveal a subdiaphragmatic arterial supply from a branch off the celiac trunk. Planned resection with diagnostic thoracoscopy revealed the mass to seemingly be below the diaphragm; however, subsequent abdominal laparoscopy identified it to be within the diaphragm. Once the supplying vessel off the celiac trunk was controlled and divided, the mass was circumferentially excised from the edges of the diaphragmatic muscle. The remaining diaphragmatic defect was then closed, and the patient did well postoperatively.
Conclusions
This case demonstrates the difficulty of making a definitive diagnosis of intradiaphragmatic extralobar pulmonary sequestration without operative intervention. CT angiography and identification of the sequestration’s arterial supply may not be conclusive in determining the precise location if in close proximity to the diaphragm.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Pulmonary sequestration (PS), also called bronchopulmonary sequestration, is defined as accessory foregut formation of nonfunctional lung tissue that does not contribute to the normal tracheobronchial tree and receives anomalous arterial blood supply from the systemic circulation [1, 2]. Two types of PS are known: the abnormality shares visceral pleura with a normal adjacent lung lobe (intralobar pulmonary sequestrations, IPS) or, less commonly, the abnormality contains its own visceral pleura outside of the normal lungs (extralobar pulmonary sequestrations, EPS). We present the case of an intradiaphragmatic EPS (IDEPS), an exceedingly rare subtype of EPS, located in the left hemidiaphragm of a 2-year-old boy.
Case presentation
A 2-year-old boy presented to Pediatric Surgery for follow-up of a large left chest mass initially found on a routine antenatal anatomy ultrasound. Follow-up ultrasound revealed a bilobed left-sided mass with a systemic arterial feeder to the lower portion, raising the question of a sequestration. It was unclear if the lesion was located in the upper abdomen or lower chest. Prenatal MRI done to rule out diaphragmatic hernia showed a well-encapsulated posterior left lower lobe pulmonary malformation with a systemic feeding vessel but was again inconclusive whether the mass was intra-thoracic or intra-abdominal.
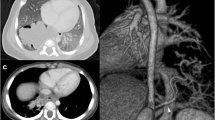
The patient was born at term via an uncomplicated vaginal delivery. Chest x-ray at birth showed a left lower lobe opacity consistent with possible bronchopulmonary sequestration. He was observed in the NICU where his vitals remained within normal limits prior to discharge home on day 2 of life. He was followed postnatally by Pediatric Surgery without the development of respiratory distress or pulmonary infection. At 20 months of age, the patient underwent CT angiography (CTA) for pre-procedure planning. The CTA of his chest showed a 3.6 × 1.6 × 3.5 cm irregular soft tissue mass within the left lower hemithorax abutting the diaphragm anteromedially with arterial blood supply from an accessory branch off the celiac trunk (Fig. 1); and the radiologist thought it appeared to be extralobar and supradiaphragmatic. Upon our review of the CTA, it remained unclear whether the EPS was above or below the diaphragm, yet the irregular contour of the left hemidiaphragm led us to agree that it was more likely located intra-thoracically and abutting the diaphragm. Thoracoscopic evaluation of the left chest with possible laparoscopy for resection of the EPS was planned.
Upon entry into the chest, a mass was seen in the posterior recess of the left hemidiaphragm and appeared to be deep to a layer of tissue continuous with the diaphragm. The diaphragm overlying the mass was noted to be thinner than usual with the mass appearing as a distinct bulge beneath the diaphragm (Fig. 2). Due to these findings, it was concluded that the mass was located intra-abdominally and laparoscopic entry into the abdomen was gained. Careful dissection was used to divide the uppermost short gastric vessels, mobilize the attachment between the left lateral segment of the liver and the diaphragm, and move the spleen away from the diaphragm. A bulge within the left hemidiaphragm was identified, indicating the mass was actually intradiaphragmatic. A prominent vessel coming from the area of the celiac trunk supplied the mass and it was controlled and divided (Fig. 3). The peritoneum overlying the mass was divided and the mass (3.5 × 2.9 × 1.3 cm, 4.8 g) was circumferentially excised from the edges of the diaphragmatic muscle and removed in its entirety (Fig. 4). The diaphragmatic defect in the left hemidiaphragm was repaired primarily (Fig. 5).
The patient recovered uneventfully postoperatively and was discharged home on postoperative day 1. The patient followed up in our clinic on postoperative day 18 and was recovering well without issue. Histopathological examination revealed an EPS containing a congenital pulmonary airway malformation type II.
Discussion
Pulmonary sequestration is a congenital lung malformation lacking respiratory function and supplied by systemic circulation, most commonly from the descending aorta and celiac trunk. The cause of these malformations remains unknown but likely involves abnormal budding of the developing lungs [3]. Identification of pulmonary sequestrations can usually be done by diagnostic imaging showing a soft tissue mass with a non-pulmonary feeding vessel. Postnatally, pulmonary sequestrations may be asymptomatic or present with dyspnea, cyanosis, chronic cough, wheezing, and recurrent pneumonia. Overall, sequestrations are generally considered for resection given the potential for infections [2] and confusion with malignant lesions [4]. Though extralobar sequestrations are much less prone to infectious complications because of their distinct pleura and systemic venous drainage [5], it is still recommended to remove asymptomatic EPS lesions to avoid infection and possible subsequent pneumonitis. Minimally invasive surgical techniques improve cosmetic outcomes and decrease recovery time for these patients.
Extralobar cases account for 25% of pulmonary sequestrations and rarely present below the diaphragm, with up to 15% being subdiaphragmatic [4, 5]. Even more rare are intradiaphragmatic extralobar pulmonary sequestrations (IDEPS). These are proposed to arise from an accessory bud forming at the level of the diaphragm with subsequent embryological fusion of the diaphragm enclosing the EPS within its musculature [4].
Contrast-enhanced CT is the gold standard for investigating the anatomy and arterial supply of pulmonary sequestrations prior to surgical resection [6]. However, as in the present case, it may be unclear from preoperative imaging, including CTA, where the EPS is anatomically located in relation to the diaphragm. Preoperative identification of true intradiaphragmatic sequestration is especially challenging with subsequent difficulty in the selection of the best surgical approach [7].
Kargl et al. sought to categorize the arterial blood supply for 21 cases of PS and CPAM as supradiaphragmatic, subdiaphragmatic, or both. Subdiaphragmatic blood supply was categorized into those arising off the abdominal aorta and celiac trunk, with all except one of these cases representing extralobar pulmonary sequestrations [8]. The majority of EPS lesions are therefore supplied by subdiaphragmatic vessels, regardless of the EPS itself being above or below the diaphragm. Therefore, the location of extralobar pulmonary sequestrations should not be entirely assumed based on its arterial supply.
Due to the rarity of IDEPS, there is limited literature regarding their diagnostic workup and management, and controversy continues to exist on whether a thoracic or abdominal surgical approach should be taken. In their case series, Nijagal et al. agreed that preoperative imaging studies may provide discordant results on the locations of EPS [9]. They recommended that preoperative planning include preparation for a combined thoracoscopic and laparoscopic approach with subsequent removal of the mass using the approach that provides superior visualization [9]. Oreglio et al. noted that, although arterial supply originated from the celiac trunk in their cases, approaching via the thoracic cavity was still preferred for its increased visualization and feasibility in reaching the IDEPS [10]. However, a potential risk of using a thoracic approach is retraction of the feeding vessel into the abdominal cavity resulting in intra-abdominal bleeding that may be missed [10]. There are advantages and disadvantages of both thoracoscopic and laparoscopic approaches and a combined thoracoscopic-laparoscopic approach may be preferred for its superior visualization.
Conclusion
In conclusion, this case presents the surgical resection of a pulmonary sequestration found in a rare and challenging location. CTA, though gold standard and essential for preoperative planning, may not be conclusive in identifying the precise location of extralobar pulmonary sequestrations when in close proximity to the diaphragm. Additionally, the arterial supply of EPS does not necessarily need to mandate a surgical approach. There is no consensus on the best surgical approach for IDEPS and a hybrid approach with both laparoscopy and thoracoscopy may provide both improved visualization and ability to identify the feeding vessel with little increased morbidity to the patient.
Availability of data and materials
Not applicable.
Abbreviations
- CPAM:
-
Congenital pulmonary airway malformation
- CT:
-
Computerized tomography
- CTA:
-
Computerized tomography angiography
- EPS:
-
Extrapulmonary sequestration
- IDEPS:
-
Intradiaphragmatic extralobar pulmonary sequestration
- IPS:
-
Intralobar pulmonary sequestration
- NICU:
-
Neonatal intensive care unit
- MRI:
-
Magnetic resonance imaging
- PS:
-
Pulmonary sequestration
References
Chakraborty RK, Modi P, Sharma, S. Pulmonary sequestration. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2022. https://pubmed.ncbi.nlm.nih.gov/30335347/. Accessed 20 Nov 2022.
Huang D, Habudin A, Yuan M, Yang G, Cheng K, Luo D, et al. The clinical management of extralobar pulmonary sequestration in children. Pediatr Pulmonol. 2021;56(7):2322–7.
Hackam DJ, Upperman J, Grikscheit T, Wang K, Ford HR. Pediatric Surgery. In: Brunicardi F, Andersen DK, Billiar TR, Dunn DL, Kao LS, Hunter JG, et al., editors. Schwartz's principles of surgery. 11th ed. McGraw Hill; 2019.
McAteer J, Stephenson J, Ricca R, Waldhausen JHT, Gow KW. Intradiaphragmatic pulmonary sequestration: advantages of the thoracoscopic approach. J Pediatr Surg. 2012;47(8):1607–10.
Savic B, Birtel FJ, Tholen W, Funke HD, Knoche R. Lung sequestration: report of seven cases and review of 540 published cases. Thorax. 1979;34(1):96–101.
Brown EG, Marr C, Farmer DL. Extralobar pulmonary sequestration: the importance of intraoperative vigilance. J Pediatr Surg Case Rep. 2013;1(4):74–6.
Federico, MJ, Baker CD, Deboer EM, Kupfer O, Martiniano SL, Stillwell P, et al. Respiratory Tract & Mediastinum. In: Hay Jr. WW, Levin MJ, Abzug MJ, Bunk M, editors. Current diagnosis & treatment: pediatrics. 25th ed. McGraw Hill; 2020.
Kargl S, Schlader F, Scala M, Kammel J. Vascular anatomy in congenital lung lesions–description and classification. Front Pediatr. 2022. https://doi.org/10.3389/fped.2022.900538.
Nijagal A, Jelin E, Feldstein VA, Courtier J, Urisman A, Jones KD, et al. The diagnosis and management of intradiaphragmatic extralobar pulmonary sequestrations: a report of 4 cases. J Pediatr Surg. 2012;47(8):1501–5.
Oreglio C, Tocchioni F, Ghionzoli M, Buccoliero A, Morabito A, Morini F. Intradiaphragmatic pulmonary sequestrations: a surgical challenge. Case Series Front Surg. 2023. https://doi.org/10.3389/fsurg.2023.1181007.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RW, LB, and AR performed the surgical procedure. AR, TT, and CL drafted the manuscript. LB and RW supervised and advised in the editing and preparation of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient’s guardian for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramos, AK.K.J., Teramae, T.R., Liang, C.G.Z. et al. The complexities in diagnosing intradiaphragmatic extrapulmonary sequestration: a case report. Ann Pediatr Surg 19, 35 (2023). https://doi.org/10.1186/s43159-023-00270-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43159-023-00270-y