Abstract
Background
Bladder nephrogenic adenoma (BNA) is a rare benign proliferative pathology of its mucosa. The etiopathogenesis of the disease remains uncertain, and the treatment is still challenging.
Case presentation
In this article, we present a clinical case of bladder nephrogenic adenoma in a 6-year-old boy. Clinical symptoms of the disease appeared 3 years after surgical treatment of obstructive megaureter. Histopathological and immunohistochemical examination of glandular polyp biopsies allowed excluding the tumor process and making a diagnosis.
Conclusion
Nephrogenic adenomas demonstrate a variety of morphologic patterns that may occasionally be confused with malignant processes. We described the endoscopic characteristics of BNA and characterized its histological and immunohistochemical features in a boy after ureteroneocystostomy.
Similar content being viewed by others
Background
Bladder nephrogenic adenoma (BNA) is a rare disease described only in scanty papers. In male, this disease occurs twice often as in female. The age range of the pathology is quite wide but only one-tenth of cases occur in children. BNA is a benign proliferative mucosa pathology at which the tubular, and papillary lesions have a villous and partially glandular structure. They are more often found in the bladder neck and urethra. The etiology and pathogenesis of BNA remain still unclear [1,2,3,4].
Case presentation
A 3-year-old patient was admitted to the hospital with complaints of fever and urination of small volumes. The findings of urinalysis were leukocyturia, proteinuria, and bacteriuria. After the full-patient examination based on the obtained data, the right-sided obstructive megaureter was diagnosed. The patient underwent Cohen’s ureteroneocystostomy on the right side with resection of the distal narrowed part of the ureter and tailoring of the enlarged area along the length according to Hendren’s technique. The patient was examined 1 year after surgery. According to the uroradiological examination, no violation for contrast self-excretion from the urinary tract and other urodynamic disorders in the postoperative period was observed. The parameters of dynamic renal scintigraphy showed that the function of the operated kidney approached the contralateral.
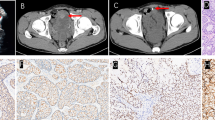
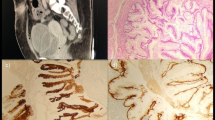
Three years after ureterocystoneostomy, the child developed recurrent hematuria painful frequent urination of small volumes, and urgent urinary incontinence. On ultrasonography: the urinary bladder had a clear but irregular contour; the bladder walls were compacted and thickened; on an internal contour, the multiple bulk protrusions were visualized. On cystoscopic examination, the mucosal layer of the bladder was pale pink and two orifices were found. In the vesical trigone region with the spread to the right side wall of the bladder, the different glandular polyps (foci of mucosal lesions in the form of «cauliflower» in the areas from 0.5 × 0.5 cm to 2.5 × 3 cm with vascular germination) were revealed. The lesions had a papillary shape. Papillae were short and without branching (Fig. 1). We endoscopically excised all lesions, which were bleeding and looking like polyps and sent obtained biopsies for histopathological examination. The histological specimens with hematoxylin-eosin and histochemical methods were stained.
According to the histopathological examination, bladder nephrogenic adenoma was diagnosed. The areas of different histological structures with the presence of those that were similar to glands, cysts, and papillae as well as various variants of their combinations in the specimens were found (Figs. 2 and 3). The BNA was externally covered with cubic epithelium and had vacuolated cytoplasm and round (sometimes vesicular) nuclei with small nucleoli («hobnail cells»). Lymphocyte, macrophage, plasma cell, and neutrophilic leukocyte infiltration in its stroma was weakly expressed (Figs. 2 and 3).
The immunohistochemical research method was used for identification of the pathological lesions histogenesis at the cellular level and its specific antigenic properties. According to its results, normal urothelial cells revealed a positive immunohistochemical reaction with antibodies to GATA3 (Cell Marque, clone L50-823), p40 (Biocare, clone BC28), and cytokeratin 7 (DAKO, clone OV-TL 12/30). In glandular-like epithelial cells the immunohistochemical response was positive with antibodies to cytokeratin 7 (DAKO, clone OV-TL 12/30), heterogeneous positive reaction to CD57 (DAKO, clone TB-01) and negative with antibodies to Wilms’ tumor antigen (DAKO, clone 6F-H2), alpha-methylacyl-CoA racemase (AMACR, p504s) (DAKO, clone 13H4), cathepsin K (Cell Marque, clone 3F9), CDX-2 (DAKO, clone DAK-CDX2), p40 (Biocare, clone BC28), and GATA3 (Cell Marque, clone L50-823). With antibodies to vimentin (DAKO, clone V9) received a negative reaction in epithelial cells and a positive one in the stroma (Fig. 4).
Six months after endoscopic resection of tumor-like formations, cystoscopy revealed no bladder mucosa lesions.
Discussion
There are various factors that lead to the development of BNA: infectious, traumatic, and toxic agents [5]. According to the literature, this illness has been stated to be associated with surgery for congenital malformations of the urinary system in children and prostate pathology in adults [4, 6, 7]. For clinical course, the dysuric symptoms with urgent urinary incontinence and sometimes hematuria are typical [1, 4, 7, 8]. Signs of bladder mucosa inflammation usually are detected cystoscopically—red velvet areas with exophytic structures which can be papillary, polypoid, fungating, or sessile. Mostly they are cystic and sometimes pseudoinfiltrative formations with small thin papillary structures on the mucosa surface. In 20% of cases, the lesions are multiple and large [2, 4, 5].
BNAs include structures that look like cysts, papillae, and tubules. These histological structures consist of small hollow tubules that are microscopically similar to mesonephric. The formations are usually lined with a single layer of mitotically inactive, bland cuboidal, and hobnail cells or low columnar epithelium. Nephrogenic adenoma borders with the urothelium which has structure of vascular cystic tubules lined with hobnail cells [2, 9].
Small clusters and single-scattered cells that have vacuolated cytoplasm and central nuclei are cytologically detected. The cell nuclei have evenly distributed chromatin with small nucleoli. Up to 10% of cells have a pale, almost transparent cytoplasm and small nuclei without nucleoli in them. Sometimes small pseudopapillary clusters of cells with irregular nuclear membranes and prominent nucleoli are also detected [2, 4, 5]. In the surrounding tissues, inflammatory infiltrate consisting of plasma cells, neutrophils, lymphocytes, and stroma edema is detected [3]. In general, BNA has a benign course, even with significant cytological atypia [1].
At the immunohistochemical examination, BNA by positive staining of PAX2, PAX8, and CA-125 is characterized. Moreover, some authors consider that positive staining of PAX2 confirms the nephrogenic adenoma origin from renal tubules [10]. At the same time, positive staining is also obtained with cytokeratin 7, racemase, α-methylacyl-CoA racemase (AMACR), P504S, CAM5.2, CK20, CD10, EMA, pancytokeratin (AE1/AE3), and keratin (for fibromyxoid variant) [4, 9]. Negative staining with PSA, CK20, CD10, CK903, and p63 is also observed. Despite this, with these antibodies, the staining may be focally positive too [9, 10].
Conclusion
Thus, nephrogenic adenomas demonstrate a variety of morphologic patterns that may occasionally be confused with malignant processes. In our article, we described the endoscopic characteristics of BNA and characterized its histological and immunohistochemical features in a boy after ureteroneocystostomy.
The pathogenesis of BNA in our patient was due to the migration of renal tubules glandular epithelium cells with their bladder mucosa dissemination and invasion after vesicoureteral obstruction removal, as well as bladder mucosa metaplasia due to traumatic injury during surgery.
The presented clinical and diagnostic data allows completing and enlarging the currently available evidence about bladder nephrogenic adenoma in children.
Availability of data and materials
All data regarding the present study is available by contacting the corresponding author.
Abbreviations
- BNA:
-
Bladder nephrogenic adenoma
- AMACR:
-
Alpha-methylacyl-CoA racemase
- CK:
-
Cytokeratin
- EMA:
-
Epithelial membrane antigen
- PSA:
-
Prostate-specific antigen
- H&E:
-
Hematoxylin and eosin
References
Yi Y, Wu A, Cameron AP. Nephrogenic adenoma of the bladder: a single institution experience assessing clinical factors. Int Braz J Urol. 2018;44(3):506–11. https://doi.org/10.1590/S1677-5538.IBJU.2017.0155.
Kunju LP. Nephrogenic adenoma: report of a case and review of morphologic mimics. Arch Pathol Lab Med. 2010;134(10):1455–9. https://doi.org/10.5858/2010-0226-CR.1.
Crook TJ, Mead Z, Vadgama B, Malone PS. A case series of nephrogenic adenoma of the urethra and bladder in children: review of this rare diagnosis, its natural history and management, with reference to the literature. J Pediatr Urol. 2006;2(4):323–8. https://doi.org/10.1016/j.jpurol.2006.02.004.
Gandhi JS Nephrogenic metaplasia. PathologyOutlines.com website. Available from: https://www.pathologyoutlines.com/topic/bladdernephroadenoma.html. Accessed 14 Dec 2021.
Venyo AK. Nephrogenic adenoma of the urinary bladder: a review of the literature. Int Sch Res Notices. 2015. https://doi.org/10.1155/2015/704982.
Zougkas K, Kalafatis M, Kalafatis P. Nephrogenic adenoma of the urinary bladder. Int Urol Nephrol. 2004;36(4):513–7. https://doi.org/10.1007/s11255-004-0848-7.
Chen CS, Cheng CL. Nephrogenic adenoma of the urinary bladder: clinical experience and review of the literature. J Chin Med Assoc. 2006;69(4):166–8. https://doi.org/10.1016/S1726-4901(09)70199-6.
Kuzaka B, Pudełko P, Powała A, Górnicka B, Radziszewski P. Nephrogenic adenoma of the urinary bladder: a report of three cases and a review of the literature. Ann Transplant. 2014;19:153–6. https://doi.org/10.12659/AOT.889441.
Safaei A, Farzaneh MR, Amin Sharifi AR. Immunohistochemistery study in a case of nephrogenic adenoma of bladder. Iran J Med Sci. 2012;37(2):137–40.
Tong GX, Melamed J, Mansukhani M, Memeo L, Hernandez O, Deng FM, et al. PAX2: a reliable marker for nephrogenic adenoma. Mod Pathol. 2006;19(3):356–63. https://doi.org/10.1038/modpathol.3800535.
Acknowledgements
None.
Funding
There is no funding to report.
Author information
Authors and Affiliations
Contributions
Concept and study design: Andrii Nakonechnyi. Methods and experimental work: Rostyslav Nakonechnyy. Results analysis and conclusions: Andrii Nakonechnyi. Manuscript preparation: Rostyslav Nakonechnyy. All authors revised the final version. Both authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out in compliance with the principles of the Declaration of Helsinki, the Council of Europe Convention on Human Rights and Biomedicine, ICH GCP, and relevant laws of Ukraine.
This study was approved by the Commission on Ethics of Research, Experimental Development and Research of Danylo Halytsky Lviv National Medical University with approval number 9 from 18.11.2019 year.
All patients entered the study after signing the informed consent and the case report is based on CARE guidelines.
Consent for publication
Written informed consent was obtained from the patient(s), and all data were analyzed and published anonymously.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rostyslav, N., Andrii, N. Bladder nephrogenic adenoma as a complication of surgical correction of megaureter. Ann Pediatr Surg 18, 77 (2022). https://doi.org/10.1186/s43159-022-00215-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43159-022-00215-x