Abstract
Background
The surgical repair of H-type tracheo-oesophageal fistulas situated below the level of the second thoracic vertebra requires an open thoracotomy or a thoracoscopy. We describe a novel technique that allows for the use of a cervical incision to repair a fistula situated in the thorax, thus diminishing surgical risk.
Case presentation
In this report, we describe a 3-day-old term baby with an H-type tracheo-oesophageal fistula where flexible bronchoscopy and gastroscopy were used to cannulate the fistula with a soft ureteric catheter. This allowed for it to be tractioned into the cervical region where it was surgically dissected and isolated. There was no need for re-intervention in the first 3 months after surgery.
Conclusion
Flexible bronchoscopy-aided cannulation of H-type fistulas can assist in intraoperative identification of the fistulous tract as well as help traction it into a surgically more accessible area like the cervical region.
Similar content being viewed by others
Background
One of the most frequent complications encountered following neonatal H-type fistula repair is vocal cord paresis secondary to injury to the recurrent laryngeal nerve [1]. From an anatomical point of view, it is not uncommon to have additional fistulous tracts or tracheal and laryngeal anomalies associated with tracheo-oesophageal fistulas. Fistulas situated in the chest are usually approached via the thorax, either by using an open thoracotomy or a thoracoscopy, which both carry a significant risk in the neonatal population [1]. Therefore, a preoperative flexible bronchoscopic assessment of the vocal cord function and the anatomy of the trachea is of use and has become a standard practice in our institution. In addition, we describe how the flexible bronchoscope can be used as an adjunct to facilitate repairs via a cervical incision of fistulas situated in the thorax, thus improving the surgical risk profile.
Case presentation
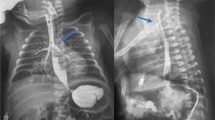
We would like to report the case of a term neonate who presented at 3 days of age with blue spells and choking on milk feeds. An oesophagogram confirmed the suspected diagnosis of an H-type tracheo-oesphageal fistula with intact distal oesophagus. The fistula was located at the level of the third thoracic vertebra. A decision to proceed with surgical repair was made.
After induction of anaesthesia, a size 1.5 laryngeal mask was sited through which the baby was spontaneously breathing and, when required, assisted by the attending anaesthetist. A 2.8-mm Olympus flexible bronchoscope was passed via the laryngeal mask into the subglottic space and subsequently into the trachea. The fistula to the oesophagus and its distance from the carina were identified. There were no additional tracheo-bronchial anomalies. A 0.028-in floppy tip guidewire was passed through the working channel of the bronchoscope into the fistula and the bronchoscope withdrawn whilst stabilising the guidewire with an aim to leave it in situ. The bronchoscope was then repassed adjacent to the guidewire and a 4-French ureteric catheter (open-end flexi tip ureteral catheter, Cook Medical) was then passed over the guidewire to sit in the fistula under direct vision. The guidewire was then removed, the ureteric catheter fixated at the mouth and a size 3-microcuff endotracheal tube passed orally with the tip distal to the trachea-oesophageal fistula to secure the airway. An 8-mm gastroscope was advanced into the oesophagus and the distal ureteric catheter grasped and brought out to the mouth and secured to itself, in effect creating a loop through the fistula secured at the mouth (Fig. 1).
A Fluoroscopy image delineating the trajectory of this H-type fistula. B Bronchoscopy image of the tracheal opening of the fistula with the carina visible in the distance. C Bronchoscopy image of the guidewire being place through the working channel into the fistula. D Bronchoscopy image the ureteric catheter being advanced over guidewire. E Oesophageal endoscopy demonstrating nasogastric tube (white) and ureteric catheter (blue) from the oesophageal end of the fistula. F Ureteric catheter being grabbed with the forceps via the working channel of the gastroscope. G Distal end of ureteric catheter being taken out of the mouth while the anaesthetist is securing the proximal end of the catheter and the endotracheal tube
The patient was then positioned and draped for the surgical procedure. Through a right cervical incision, the sternocleidomastoid muscle and the carotid sheath were retracted laterally and the trachea identified. The recurrent laryngeal nerve was identified and preserved as the tracheo-oesophageal groove was followed into the upper mediastinum. Traction on the catheter looped through the fistula aided identification of the fistula and brought it superiorly towards the cervical incision, aiding dissection and isolation of the fistula. Stay sutures were then applied to the trachea and oesophagus superior and inferior to the fistula, the catheter withdrawn and the fistula divided. A size 8-French nasogastric tube was passed. The tracheal and oesophageal defects were then closed with simple interrupted sutures.
The child was returned to the neonatal intensive care unit. The endotracheal tube was removed the following day and enteral feeds commenced. The child was fully orally fed and discharged on postoperative day 3. There were no signs of recurrence at the 3 months of follow-up consultation.
Follow-up in a multidisciplinary team setting consisting of general surgeons, ear, nose and throat surgeons and respiratory physicians is warranted [2].
Cannulation of the trachea-oesophageal fistula has been described in the literature either by using a rigid or a flexible bronchoscope. Different probes have been utilized, as described in Table 1.
In this case, the use of bronchoscopic visualisation and passage of a catheter through the fistula enabled the following:
-
Preoperative assessment of vocal cord function [6].
-
Exclusion of additional fistulous tracts or associated anomalies such as laryngeal cleft tracheomalacia, complete tracheal rings or vascular airway compression.
-
Confirmation of the level of the fistula and ascertaining the optimal surgical approach.
-
Safe identification and isolation of the fistula via a cervical incision.
The authors’ caution against using a rigid tip guidewire as it may lead to inadvertent perforation of the tracheo-bronchial tree and subsequent pneumothorax or pneumomediastinum.
Conclusions
We report no intraprocedural complications of this technique and believe it is successful in reducing surgical risk in the subset of neonates with H-type oesophageal fistulas.
Availability of data and materials
Available upon request.
References
Fallon SC, Langer JC, Peter SD, Tsao KJ, Kellagher CM, Lal DR. Congenital H-type tracheoesophageal fistula: a multicenter review of outcomes in a rare disease. J Pediatr Surg. 2017;52:1711–4.
Gendler Y, Seguier-Lipszyc E, Silbermintz A, Hain M, Stern Y, Kravarusic D, et al. Aerodigestive clinics as emerging pediatric care model: the first 100 patients in Israel. Isr Med Assoc J. 2021;23:569–75.
Amat F, Heraud M-C, Scheye T, Canavese M, Labbe A. Flexible bronchoscopic cannulation of an isolated H-type tracheoesophageal fistula in a newborn. J Pediatr Surg. 2012;47:E9–E10.
Cuestas G, Rodriguez V, Millan C, Munzon PB, Munzon GB. H-type tracheoesophageal fistula in the neonatal period: difficulties in diagnosis and different treatment approaches. A case series. Arch Argent Pediatr. 2020;118(1):47–60.
Sim J, Hong J. Double H-type tracheoesophageal fistulae: a case report. Adv Pediatr Surg. 2018;24(2):94–9.
Zani A, Jamal L, Cobellis G, Wolinska J, Fung S, Propst E, et al. Long-term outcomes following H-type tracheoesophageal fistula repair in infants. Pediatr Surg Int. 2017;33(2):187–90.
Acknowledgements
Nil
Funding
The procedure reflects every day clinical practice, so no additional funding was required for this case report.
Author information
Authors and Affiliations
Contributions
LP and AN drafted the manuscript and performed the bronchoscopic procedures. SN and IY performed gastroscopy and surgery. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This case report reflects every day clinical practice, and therefore, ethics approval was granted.
Consent for publication
A consent was obtained from the parents for anonymous publication of the case.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Procopiuc, L., Naqvi, S., Yardley, I. et al. Preoperative flexible bronchoscopy-aided cannulation of a neonatal H-type tracheo-oesophageal fistula assists intraoperative identification of the fistulous tract. Ann Pediatr Surg 18, 69 (2022). https://doi.org/10.1186/s43159-022-00207-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43159-022-00207-x