Abstract
Background
Infantile hemangioma (IH) is the commonest vascular tumor affecting children that appears in the first 2 weeks of life and follows a proliferative phase that continues during the first year of life. After then, it undergoes involution, which lasts for several months or years depending on the size, site, gender, and development of complications. The purpose of this prospective study was to evaluate the correlation of age and duration of propranolol therapy to the outcomes of infantile hemangioma.
Methods
A prospective study included 28 patients with IH in which the propranolol therapy was initiated in a dose of 3 mg/kg/day divided into two to three doses. The surface area of IH was calculated monthly using AutoCAD software. Treatment with propranolol was discontinued when there was no more decrease in the surface area for two consecutive visits. Eleven males and 17 females completed the study. The age at initiation of therapy ranged from 2 to 16 months while at the end of therapy it was 9 to 23 months.
Results
The mean difference percent of surface area at 6 months was (51.1 ± 16.3), while at the end of the treatment course was (75.0±16.8) which was statistically significant (P < 0.0001). In addition, a significant inverse correlation was found between the age at the beginning of treatment and the difference percent of surface area. A similar inverse correlation was observed between the age at the beginning of treatment and the duration of treatment.
Conclusion
In addition to the safety and efficacy of propranolol therapy for IH, a higher response rate can be gained with early treatment and a prolonged course of therapy. In addition, propranolol therapy should be continued until there is no more response for two consecutive months regardless of the age of therapy initiation and the duration of treatment.
Similar content being viewed by others
Background
Infantile hemangioma (IH) represents the commonest benign tumor during infancy, and it is the most common type of vascular tumors affecting children [1]. It can be seen in 4–10% of children and having female sex preponderance [2].
The characteristic proliferative phase of IH takes place in the first year of life, with maximal growth occurring during the first 6 months after birth, during which it increases in size, depth, and color, with increasing possibility of bleeding and ulceration. The involution phase starts thereafter and may continue up to 5 or even 10 years [3].
Many strategies had been adopted for the treatment of IH, among them are B-blockers which emerged to be the first choice. In 2008, “Leaute-Labreze” was the first to describe the effectiveness of propranolol in the regression of IH when the drug was prescribed for a baby with hypertrophic cardiomyopathy [4]. From this date on, many researchers investigated the use of B-blockers in this context. In March 2014, propranolol got US Food and Drug Administration approval for its use in treating infants aged more than 5 weeks with IH in a dose of 3 mg/kg/day for 6 months duration. The same report recorded 11.4% rebound growth of IH that necessitated retreatment [5, 6].
Although the exact mechanism of action is still not clear, propranolol exerts its effect possibly through vasoconstriction consequent on decrease of the release of nitrous oxide and inhibition of angiogenesis by downregulating the expression of endothelial growth factor (EGV) and to a lesser extent the basic fibroblast growth factors (bFGF); in addition, there would be an apoptosis of capillary endothelial cells [7,8,9].
The present study aims to evaluate the effect of age and duration of propranolol therapy on outcomes of patients with IH intending to get the best results with the least side effects and least incidence of rebound growth
Method
A prospective study was carried on a pediatric age group complaining from infantile hemangioma. The children visited a pediatric surgery clinic from July 2015 until October 2017. The study was approved by the Medical College Ethical and Scientific Committee.
During the study period, 54 patients with IH underwent a thorough history and clinical examination that proved the diagnosis. Ultrasound and Doppler studies were adjuvant in certain cases. The inclusion criteria were fascial, genital, and complicated IH. In addition to cosmetic and disfigurement caused by the hemangioma. The exclusion criteria were “PHACE” syndrome, bradycardia with or without hypotension, wheezy chest, and history of hypoglycemia. Accordingly, 28 patients with IH fulfill the criteria. After that, the parents or caregivers of eligible patients were educated about the condition and the treatment protocol with obtaining their written informed consent.
Treatment protocol
Propranolol was prescribed to all cases in a dose of 3 mg/kg/day in two or three divided doses. Parents were instructed to use the drug daily with a monthly visit to assess the response, adjust the dose, and to record any side effects or complications of treatment. The parents were advised to increase feeding especially for infants below 6 months of age to avoid hypoglycemia and to phone call or visit the clinic when they experience any changes in their child before the monthly visit. Before starting the treatment and in every monthly visit, the lesions were measured in dimensions according to their geometric shape and photographed. The surface areas were calculated using AutoCAD 2017 software which is commonly used in the processing of medical images [10, 11] and compared with the last reading. Treatment with oral propranolol was stopped gradually over a period of 2 weeks when no decrease in the surface area of the lesion for two consecutive visits was observed. After the stoppage of treatment, the patients were followed up for 4 to 6 months to exclude the rebound growth of the IH.
Statistical analysis
Analysis of data was carried out using the available statistical package of SPSS-24 (Statistical Packages for Social Sciences version 24). Data were presented in simple measures of frequency, percentage, mean, standard deviation, and range (minimum–maximum values).
Paired t test and ANOVA were used for testing the significance of difference in percent of surface area. Pearson correlation was calculated for the correlation between two quantitative variables with its t-test for testing the significance of correlation. The correlation coefficient value (r) either positive (direct correlation) or negative (inverse correlation) with value < 0.3 represents no correlation, 0.3–< 0.5 represents weak correlation, 0.5–< 0.7 moderate strength, and > 0.7 strong correlation. In addition to correlation, the r2 was calculated (the coefficient of determination), i.e., when the value of r = 0.58, then r2 = 0.34, this means that 34% of the variation in the values of y may be accounted for by knowing values of x or vice versa. Statistical significance was considered whenever the P value was equal to or less than 0.05.
Results
Twenty eight patients out of 52 completed the study after they met the inclusion criteria. Figure 1 shows the flow diagram of the participants in the study from assessment for enrollment through intervention to the outcomes.
Boys represented 39.3% while girls constituted 60.7% in a male: female ratio of 1:1.5. The age of the patients at the beginning of the study ranged from 2–16 months (mean 5.7 ± 3.9); while at the end of the study, the age ranged from 9–23 months (mean15.9 ± 3.5). Treatment duration ranged from 6 to 15 months with a mean of (10.2 ± 2.6). The head was the commonest site of IH in 8 patients (28.5%). Seven patients (25%) had complications (bleeding ± ulceration) before starting the treatment, while two patients only showed rebound growth after cessation of treatment (Table 1).
No serious side effects developed during the treatment period that required discontinuation of therapy apart from sleep disturbance in one infant and development of wheezy chest in another that required a temporary stoppage of the treatment for 5 days.
A greater difference percent of surface area can be achieved when the treatment started early (Table 2), and there is a significant strong inverse correlation between age “at the beginning of treatment” and difference percent of surface area (R2 linear = 0.482 at 0.01 level, P = 0.0001) (Fig. 2).
The mean difference percent of surface area that had been achieved after 6 months of treatment was 51.1 ± 16.3 and at the end of therapy was 75.0 ± 16.8 with a significant P value = 0.0001 (Table 3 and Fig. 3)
Figure 4 shows that those patients who started propranolol therapy at early ages required a more prolong course of treatment to achieve regression of lesions with an inverse correlation between age “at the beginning of treatment” and the duration of treatment (R2 = − 0.234 at 0.01 level, P = 0.009).
Discussion
Many studies had established the efficacy of propranolol in treating complicated or problematic IH, but till now there is no consensus regarding the optimal duration of treatment with scarcity of research emphasizing the effect of age on the expected rate of regression. In this study, we used oral propranolol therapy as the first line of treatment for cases of IH starting at early ages as we can as possible and continued for variable periods with analysis of our data to decide the best age of starting treatment and the recommended duration of therapy
There is a general agreement that propranolol is effective in lightening up and reducing the size of IH when given early in the proliferative phase of development [12, 13]. According to Fuchsmann et al. [14] and Smithson et al. [15], these changes continued thereafter. Theories suggest that propranolol impedes the growth of hemangiomas by its antiangiogenic activity and induction of apoptosis when given early. Nevertheless, several case studies have further provided evidence of the dramatic response of massive, proliferating, life-threatening, and involuting lesions to propranolol therapy [16,17,18]. In the present paper, we noticed that the earlier initiation of propranolol therapy within the proliferative phase, the more reduction in the different percentage of surface area with a significant inverse correlation (R2 linear = 0.482 at 0.01 level, P = 0.0001) (Table 2, Figs. 2 and 5). A previous Turkish study showed that there is no statistically significant relation between the age of initiation of propranolol therapy and the response rate, but there was a significant increase in the response rate in association with the duration of therapy [19].
The mean difference percentage of the surface area of IH at 6 months of treatment was 51%, while it was 75% at the end of completed treatment with a statistically significant P value of 0.0001 (Table 3). A considerable number of patients continue to exhibit a significant reduction in the difference percentage of surface area when treatment extended for more than 6 months (as shown in Fig. 3) and those patients who did not show any difference in the percentage of surface area for two consecutive months of propranolol therapy were actually stopped exhibit regression of the lesion and considered for termination of treatment.
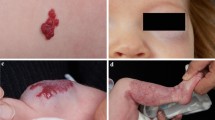
Chang et al. described that treatment duration depends on patient’s response rather than the age at which therapy was initiated [20]; they noted that a longer duration of treatment is usually required for those patients who experience partial regression of the lesion and those in which the recurrence rate is frequently high and the best time to stop treatment is when complete regression achieved [20]. Analysis of our data showed that there was a significant inverse correlation between the age of the patients at the initiation of therapy and duration of treatment (R2 linear = 0.234 at 0.01 level, with statistically significant Pearson correlation P = 0.009) (Fig. 4), the younger ages at starting treatment required longer duration of treatment. Patients who were treated earlier were probably in the earlier period of the proliferative phase and therefore they required longer treatment. This is also could be attributed to the fact that, starting to treat large size hemangiomas in a young infant showed a dramatic response which encourages the parents to continue the therapy until nearly complete disappearance of the lesion (as in Figs. 6 and 7). In contrast, when treatment starts late, the reduction in the surface area is not remarkable which discourages the parent to continue treatment.
Rebound growth in IH after stopping propranolol therapy occurs in 6–25% of cases [21]. Shah et al. noted that in addition to hemangioma size, depth, gender, and location, the risk of rebound growth was linked to the duration of treatment and the age of discontinuation of therapy [22]. In this series, two patients (7.14%) developed rebound growth following cessation of treatment (Table 1). In one case, the patient was female with right labial hemangioma which start treatment at the age of 6 months and then the parents stopped the treatment abruptly at the age of 14 months without gradual tapering of the dose, which required resuming propranolol therapy for 4 weeks followed by gradual tapering for another two weeks. In the other case, the patient developed mild rebound growth of perianal hemangioma at age of 12 months in spite of 8 months duration of treatment and gradual tapering of propranolol therapy it was just mild regrowth and did not require repeating the propranolol treatment. Our finding of the recurrence rate is consistent with the study of Chang et al. who stated that rapid rebound growth occurs with sudden termination of therapy and that those patients with complete regression need 2 weeks of gradual tapering of treatment [20].
The most common reported adverse effects of propranolol are transient bradycardia and hypotension. Other reported adverse effects include ECG changes (prolong PQ duration), sleep disturbance, hypoglycemia, bronchospasm, diarrhea, hyperkalemia, and gastroesophageal reflux [23,24,25,26]. We encountered simple adverse effect of propranolol in two cases. One case was a female patient aged 7 months experienced sleep disturbances after 3 months from initiation of therapy, and it was temporary for 3 days only and disappeared without any intervention or modification of the propranolol dose. The second patient was an 8-month-old male who developed exacerbation of a wheezy chest following chest infection for which we temporarily stopped the treatment for 5 days only according to the report of consensus conference in 2013 by Drolet et al. [24].
Conclusion
In addition to the safety and efficacy of propranolol therapy in IH, a higher response rate can be gained when the treatment initiated early and with a prolonged course of therapy. Propranolol therapy should continue until there is no more response for two consecutive months regardless of the age at which therapy initiated and the duration of treatment.
Availability of data and materials
All data and materials are available on a reasonable request.
Abbreviations
- bFGF:
-
Basic fibroblast growth factors
- EGV:
-
Endothelial growth factor
- IH:
-
Infantile hemangioma
- SPSS:
-
Statistical Packages for Social Sciences
- US:
-
United States
References
Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170:907–13 https://doi.org/10.1111/bjd.12804.
Kilcline C, Frieden IJ. Infantile hemangiomas: how common are they? A systematic review of the medical literature. Pediatr Dermatol. 2008;25(2):168–73 https://doi.org/10.1111/j.1525-1470.2008.00626.x.
Chang LC, Haggstrom AN, Drolet BA, et al. Hemangioma Investigator Group. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics. 2008;122(2):360–7 https://doi.org/10.1542/peds.2007-2767.
Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358(24):2649–51.
Leaute-Labreze C, Voisard JJ, Moore N. Oral propranolol for infantile hemangioma. N Engl J Med. 2015;373(3):284–5.
FDA Oks Propranolol hydrochloride for infantile hemangioma. Available at https://www.medscape.com/viewarticle/8221155. [Accessed on 4 May 2018].
Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. (2012); volume 2012; article ID 645678. https://doi.org/10.1155/2012/645678
Lamy S, Lachambre MP, Lord-Dufour S, et al. Propranolol suppresses angiogenesis in vitro: inhibition of proliferation, migration, and differentiation of endothelial cells. Vascul Pharmacol (2010); 53:200–208. [PubMed: 20732454]
Bingham MM, Saltzman B, Vo NJ, Perkins JA. Propranolol reduces infantile hemangioma volume and vessel density. Otolaryngol Head Neck Surg. (2012); 147:338–344. [PubMed: 22691693]
Shapi’i A, Sulaiman R, Hasan MK, et al. Application of computer aided design (CAD) in Medical Imaging. Int J Adv Sci Eng Inform Technol. 2011;1(6):698–701 https://doi.org/10.18517/ijaseit.1.6.140.
Basoya S, Vinod VC, Nath P, et al. Estimation of Age by Pulp-Tooth Area Ratio Using Three Computer Aided Software’s. Qual Prim Care. 2016;24(4):161–6.
Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011;128(2):e259–66.
Ma X, Zhao T, Xiao Y, et al. Preliminary experience on treatment of infantile hemangioma with low-dose propranolol in China. Eur J Pediatr. 2013;172(5):653–9 https://doi.org/10.1007/s00431-012-1928-9.
Fuchsmann C, Quintal M-C, Giguere C, et al. Propranolol as first-line treatment of head and neck hemangiomas. Arch Otolaryngol Head Neck Surg. 2011;137(5):471–8 https://doi.org/10.1001/archoto.2011.55.
Smithson SL, Rademaker M, Adams S, et al. Consensus statement for the treatment of infantile haemangiomas with propranolol. Australas J Dermatol. 2017;58:155–9.
Buckmiller L, Dyamenahalli U, Richter GT. Propranolol for airway hemangiomaa: case report of novel treatment. Laryngoscope. 2009;119:2051–4.
Denoyelle F, Leboulanger N, Enjorlas O, et al. Role of propranolol in the therapeutic strategy of infantile laryngotracheal hemangioma. Int J Pediatr Otorhinolaryngol. 2009;73:1168–72.
Theletsane T, Redfern A, Raynham O, et al. Life threatening infantile hemangioma: a dramatic response to propranolol. J Eur Acad Dermatol Venereol 2009; 23(12): 1465-1466 https://doi:https://doi.org/10.1111/j.1468-3083.2009.03261.
Özcan R, Aktemur S, Emre Ş, et al. Oral Propranolol Administration in Treatment of Hemangiomas: An Update. İstanbul Med J. 2017;18:227–31.
Chang L, Gu Y, Yu Z, et al. When to stop propranolol for infantile hemangioma. Sci Rep. 2017;7:43292 https://doi.org/10.1038/srep43292.
Lee D, Boscolo E, Durham JT, et al. Propranolol targets the contractility of infantile haemangioma-derived pericytes. Br J Dermatol. 2014;171(5):1129–37.
Shah SD, Baselega E, McCuaig C, et al. Rebound growth of infantile hemangiomas after propranolol therapy. Pediatrics. 2016;137(4):e20151754.
Xiao Q, Li Q, Zhang B, et al. propranolol therapy of infantile hemangioma: efficacy, adverse effects, and recurrence. Pediatr Surg Int. 2013;29(6):575–81 https://doi.org/10.1007/s00383-013-3283-y.
Drolet BA, Frommelt PC, Chamlin SL, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics. 2013;131(1):128–40 https://doi.org/10.1542/peds.2012-1691.
Pavlakovic H, Kietz S, Lauerer P, et al. Hyperkalemia complicating propranolol treatment of an infantile hemangioma. Pediatrics. 2010;126(6) available at: www.pediatrics.org/cgi/content/full/126/6/e1589.
Holland KE, Freiden IJ, Frommlet PC, et al. Hypoglycemia in children taking propranolol for the treatment of infantile hemangioma. Arch Dermatol. 2010;146(7):775–8.
Acknowledgements
Not applicable
Confirmation of approved submission of this manuscript by all authors
Confirmation that the content of this manuscript has not been published or submitted for publication elsewhere. we believe that the manuscript represents valid work. Neither this manuscript nor one with substantially similar content under my authorship has been published or is being considered for publication elsewhere, except as described in the covering letter.
Funding
None.
Author information
Authors and Affiliations
Contributions
AF and AE analyze and interpreted the patient data regarding infantile hemangioma cases. OE drafted the work and was the major contributor in writing the manuscript and revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate and publishing
The study is approved by the local scientific ethical committee. Name of the ethic committee: “Scientific-Ethical Committee” in “Mustanisiriyah University-College of Medicine.” Committee reference number “239” on 13 June 2018.
Written informed consent was obtained from parents/guardians of patients for participation in the study and also they approved using images and data of their children for publishing.
Consent for publication
We hereby transfer, assign, or otherwise convey all copyright ownership, including any and all rights incidental thereto, exclusively to the Annals of Pediatric Surgery, in the event that such work is published by the Annals of Pediatric Surgery. The Annals of Pediatric Surgery shall own the work, including copyright; the right to grant permission to republish the article in whole or in part, with or without fee; the right to produce preprints or reprints and translate into languages other than English for sale or free distribution; and the right to republish the work in a collection of articles in any other mechanical or electronic format. Written informed consent was obtained from parents/guardians of patients for using images and data of their children for publishing.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Al-Mayoof, A.F., Joda, A.E. & Almushhadany, O.E. Propranolol therapy in infantile hemangioma: correlation of age and duration of treatment to the outcomes. Ann Pediatr Surg 15, 8 (2019). https://doi.org/10.1186/s43159-019-0007-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43159-019-0007-7