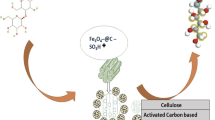
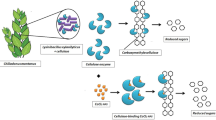
Abstract
Background
Cellulase is an important bioprocessing enzyme used in various industries. This study was conducted with the aim of improving the biodegradation activity of cellulase obtained from the Bacillus subtilis AG-PQ strain. For this purpose, AgO and FeO NPs were fabricated using AgNO3 and FeSO4·7H2O salt respectively through a hydro-thermal method based on five major steps; selection of research-grade materials, optimization of temperature, pH, centrifuge, sample washed with distilled water, dry completely in the oven at the optimized temperature and finally ground for characterization. The synthesized NPs were characterized by scanning electron microscope (SEM), energy dispersive X-ray (EDX), and X-ray diffraction (XRD) to confirm the morphology, elemental composition, and structure of the sample respectively. The diameter of the NPs was recorded through SEM which lay in the range of 70–95 nm.
Results
Cultural parameters were optimized to achieve better cellulase production, where incubation time of 56 h, inoculum size of 5%, 1% coconut cake, 0.43% ammonium nitrate, pH 8, and 37 °C temperature were found optimal. The enhancing effect of AgO NPs was observed on cellulase activity (57.804 U/ml/min) at 50 ppm concentration while FeO NPs exhibited an inhibitory effect on cellulase activity at all concentrations. Molecular docking analysis was also performed to understand the underlying mechanism of improved enzymatic activity by nanocatalysts.
Conclusion
This study authenticates AgO NPs as better nanocatalysts for improved thermostable cellulase biodegradation activity with the extraordinary capability to be potentially utilized in bioethanol production.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Carboxymethyl cellulase (CMCase) belongs to a class of cellulase enzymes known as endo -1, 4- β-glucanase, which can convert cellulase into soluble glucose [1]. Microbial cellulase has a number of applications in various industries, including the pulp and paper industry, textile industry, bioethanol industry, wine and brewery industry, food processing industry, animal feed industry, detergent industry, waste management, and agriculture industry [2]. Cellulase is an inducible enzyme that is synthesized by a wide variety of microorganisms including fungi and bacteria, and they can be anaerobic, aerobic, thermophilic, or mesophilic [3]. The costs of these enzymes produced by fungi are very high and therefore bacteria are preferred because they have a short generation time and high growth rate as compared to fungi [4, 5]. Bacteria are successful candidates for the industrial production of cellulase enzymes because they have the capability to produce cellulases that can tolerate extreme conditions such as acid, alkali, and thermostability [6]. The majority of these bacteria are found and isolated from animal waste, compost, soil, and sugar cane [7]. Various bacteria are reported for the production of cellulase such as Bacillus sp. including B. brevis, B. cereus, B. amyoliquefaciens DL-3, B. subtilis YJ1, B. vallismortis RG-07, B. megaterium, B. pumilus, and B. circulans [1, 8] Gram positive B. subtilitis has been extensively studied in past years [9]. They are well characterized and have remarkable fermentation properties and a high yield of production. They also produce non-toxic by-products and have high adaptability to environmental changes [10, 11]. Previous studies have reported the production of CMCase from Bacillus subtilitis [12,13,14].
Cellulosic biomass is composed of lignin, cellulose, and hemicellulose, which is one of the most abundant renewable resources [15]. Cellulosic biomass can be transformed into a variety of products, including paper and pulp, animal feed and textiles, using various technologies, and in particular Biotechnology. The abundant and renewable cellulosic biomass also represents a potential alternative resource to fossil fuels, with rising global demands for energy [16]. Cellulase (EC 3.2.1.4) first hydrolyzed cellulosic biomass to glucose for conversion into other products, and then different biological or chemical processes could be performed. The degradation of cellulosic material has therefore received considerable attention for improved cleaning process [17]. Different methods for hydrolyzing cellulase have been proposed, including steam explosion, acid-activated montmorillonite catalysts, alkaline, acid, enzymatic hydrolysis, and microbiological methods [18, 19]. In nature, the enzyme-mediated cellulase breakdown requires various types of cellulolytic enzymes, the major ones being β-1,4-exoglucanase (EC 3.2.1.91), β-1,4-endoglucanase (EC 3.2.1.4), and β-glucosidase (EC 3.2.1.21) [20]. The challenges to industrial applications of these enzymes are high cost and low production yield that need to be overcome with improved cellulase production [5, 21]. One of the limiting factors for biofuel production from cellulose feedstocks is the inefficient transformation of cellulase into fermented sugars. New ways of enhancing the kinetics and stability of cellulases are crucial to the economic feasibility for production of biofuel [22].
Metal ions have the ability to interact with a carboxylic acid or amine group of amino acids and in this way, they activate or inhibit the activity of the enzyme [1, 5]. Several studies have shown the role of metal ions in the activation or inhibition of microbial cellulases [23,24,25]. Nanoparticles can play a significant role in improving the pH and thermal stability of the cellulase enzymes due to various unique chemical and physical characteristics [26] such as high surface reaction activity, large surface-to-volume ratio, strong adsorption ability, and high catalytic efficiency [18, 19]. In industry, nanoparticles have proven to be beneficial catalysts [26]. Several studies have reported the use of nanoparticles to enhance the production, activity, pH, and thermal stability of cellulose [18, 19]. Biosynthesized silver nanoparticles were used as nanocatalysts and showed a two-fold increase in the cellulose degradation activity of cellulase [26]. Carboxymethyl cellulose (CMC) assay revealed an increase in the activity of carboxymethyl cellulase (CMCase) in the presence of CaCl2 nanoparticles [27].
By considering the industrial importance of cellulase, the presented study was conducted for the production and characterization of cellulase Bacillus subtilis AG-PQ. Cultural parameters were optimized to achieve maximum cellulase yield by Bacillus subtilis AG-PQ strain using solid-state fermentation and submerged fermentation. Further, the study explored the silver and iron NPs effect on the biodegradation activity of cellulase. This study will help understand NPs’ interaction with cellulase for its beneficial and effective role in various industries such as paper and pulp, food, and textile industries.
Methods
This study was conducted in the Applied Microbiology and Biotechnology Lab (AMBL) at the International Islamic University, Islamabad, Pakistan. All the chemicals used in this study were of analytical grade.
Synthesis of silver oxide and iron oxide nanoparticles by hydrothermal method
A hydrothermal technique was adopted to synthesize silver oxide and iron oxide nanoparticles (NPs) for our desired morphology at the nanoscale. The advantage of such a technique over others was its ability to make the desired NPs in a pure crystalline phase in large quantities. Such NPs in pure and controlled size were obtained by adjusting the factors that affect the morphology of NPs during the experimental process like temperature, pressure, and reactants, also research-grade equipment played a vital role that was used during the hydrothermal method; Furnace, Oven, Teflon, Autoclave, Centrifuge Machine, digital balance, magnetic stirrer and mortar, and pestle [28, 29]. To synthesize pure crystalline of required NPs, the hydrothermal technique is based upon six major steps; (i) research-based materials selection; (ii) optimization of molarity and pH of the solution; (iii) precursors were mixed uniformly through magnetic stirrer; (iv) adjust temperature for the reaction; (v) reaction proceed and pressure sustained in the autoclave; and (vi) centrifuge the sample and completely dried in an oven [30, 31]. To study and improve the biodegradation activity of cellulose we synthesized silver oxide and iron oxide NPs using a hydrothermal technique. For such a project, research-grade salt 1 M of AgNO3 was selected and dissolved in 50 ml distilled water. The precursor AgO was prepared by the addition of 2 M aqueous solution of NaOH dropwise with vigorous constant stirring (1300 rpm) on a hot plate at 60 °C for 1 h. Then continuous precipitate of AgO was synthesized till the reaction stopped at pH = 11.4. The resulting colloidal mixture was put into Teflon. The Teflon was shifted and placed into the stainless-steel autoclave. Then the autoclave was kept in a conventional oven for 24 h at 180 °C. Subsequently, the colloidal were shifted to the test tube to centrifuge for 2 h. After centrifuge, the sample (supernatant) was washed with distilled water a couple of times and also washed a minimum of two times with ethanol to remove the impurities from the NPs. The resultant product was dried in an oven for 5 h at 100 °C. The product was then ground for 30 min. At the end of grinding the sample was ready for characterization [32]. The iron oxide NPs were synthesized in a similar manner as explained above for silver oxide NPs. For such NPs, the research-grade salt 1 M FeSO4·7H2O was selected and dissolved into 50 ml distilled water. The AgO and FeO NPs were characterized by scanning electron microscope (SEM), Energy Dispersive X-ray (EDX), and X-ray diffraction (XRD). The morphology and elemental compositional were determined by using Hitachi SU6600 scanning electron microscope (SEM), and energy dispersive X-ray spectroscopy (EDX). The structural composition was confirmed from Panalytical X-pert pro MPD X-ray diffraction (XRD).
Sampling and isolation of microorganisms
Healthy soil samples were collected from the Agriculture region (Latitude 33o 64′ 98′, Longitude 73° 03′ 02′) of Islamabad, Pakistan in sterilized glass bottles and transported aseptically to a laboratory for further processing. The samples were diluted up to 10−10 and suspended on an agar plate supplemented with 0.5% peptone, 0.5% yeast extract, Tris–HCl buffer of pH 9, and NaCl with 2% agar [30]. The inoculated plates were incubated at 37 °C. Each distinct colony was picked and sub-cultured again on above mentioned supplemented agar plates to obtain a pure culture. Morphological and biochemical characterizations were performed to identify the isolated bacterium and further screened for cellulase activity by using submerged fermentation [15].
Morphological, biochemical, and molecular identification
The isolated strain was observed under the light microscope (LM) with 50–100 × resolution. Morphological and physiological characteristics of bacterial strain were examined based on shape, color, respiration, pH, and optimum temperature for proper identification [31]. Different biochemical tests including oxidase test, catalase test, Simmon citrate test, urease test, Voges Proskauer test, methyl red test, indole test, hydrogen sulfide, acid formation from sugars, and OF (oxidation/fermentation) test were performed to identify bacterial strain [31,32,33]. For molecular identification, the genomic DNA was isolated by phenol–chloroform method and the 16S rRNA region was amplified by PCR using universal 27f and 1492r primer pair [34]. The amplified region was sequenced commercially (Macrogen Korea). Subsequently, the raw sequenced data were assembled and analyzed with BioEdit version 7.1.9 [35]. The sequence was submitted to the NCBI database under the accession number MG662180. Phylogenetic analysis was carried out by using the obtained sequence with previously published 16S rRNA sequences of Bacillus sp. retrieved from GenBank (n = 31). The multiple sequence alignment was analyzed to construct a maximum likelihood tree with 1000 bootstrap replicates using the software MEGA-7 [36].
Enzyme and protein assay
The spectrophotometric assay performed to quantify the cellulase activity with a cellulose substrate using the method given by Miller [37]. The reaction mixture consisted of 0.5 ml of a crude enzyme, 0.5 ml of 0.5% cellulose, and 1 ml of 50 mM sodium phosphate (pH) 8.0 and was subjected to incubation at 50 °C for 20 min. After the incubation period was over, 1 ml of Dinitrosalicylic acid (DNS) was added to terminate the reaction. The mixture was boiled for 5 min and the optical density (OD) was measured at 540 nm with a spectrophotometer (UV-1700 APC). Bradford method [38] was used to assess the protein content and the obtained results were accessed by measuring absorbance in a spectrophotometer (UV-1700 APC) at 590 nm.
Optimization of the time course, medium, and inoculum size for cellulase production
One factor at a time approach was used for the optimization of cellulase production from Bacillus subtilis AG-PQ. Four different inoculum concentrations (2.5%, 5%, 7.5%, and 9%) were prepared and added into a 250-ml Erlenmeyer flask containing 100 ml production media. Five different types of media were tested to optimize the best fermentation medium for the maximum yield of cellulase. All these media were inoculated with optimized culture conditions. All experiments were performed in triplicate and bars displayed mean and S.D.
Effects of culture conditions on cellulase production
Economic carbon sources such as agricultural waste were utilized to make the cellulose production process cost-effective. The effect of economic carbon sources such as rice husk, corn cob, cellulose, wheat bran, and coconut cake at 2% concentration was examined for improved cellulase production [39]. Different nitrogen sources such as urea, ammonium sulfate, diammonium phosphate, ammonium chloride, and ammonium nitrate were added to the fermentation medium to observe their effect on cellulase production [40]. The pH of the production medium was adjusted to 3–8 by using 0.1 N sodium phosphate buffer and 0.1 N citric acid Vyas et al. [41] to investigate the influence of pH on cellulose yield. In order to examine the effect of temperature, fermentation was carried out at temperatures ranging from 25 to 40 °C to optimize the best-suited temperature for cellulase production.
To evaluate the effect of silver (Ag) and iron (Fe) nanoparticles on CMCase production from isolated strains, various concentrations of these nanoparticles (10 to 100 ppm) were added to the fermentation media. The enzyme assay and protein estimation were performed after every 8 h interval for quantification of cellulase activity and protein content.
Partial purification and characterization of cellulase enzyme
The ammonium sulfate precipitation (APS) method was used for the partial purification of the enzyme according to the method described by Gomori, [42]. The crude extract was treated with different concentrations of APS (20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%) to obtain the purified fraction of cellulase [43]. The precipitates were dissolved in 10 mM phosphate buffer (pH 8) and suspended in a dialyzing membrane. This membrane dialyzed against the same and was kept in a refrigerator at 4 ℃ for 24 h. Various concentrations of cellulose (substrate) were added to the reaction mixture to examine the effect of substrate concentration on cellulase activity [43, 44]. The effect of pH and temperature on cellulase activity was determined at various pH values (4–9) and temperatures (30–65 °C). To investigate the effect of silver oxide (AgO) and iron oxide (FeO) nanoparticles on cellulase activity, various parts per million (ppm) concentrations (10–70 ppm) of these nanoparticles were added to the reaction mixture [45]. Lineweaver–Burk plot was plotted to calculate the Km and Vmax values.
Molecular docking sides of Ag and Fe nanoparticles with cellulase activity
To predict the preferred binding sites and binding mode of Ag and Fe with cellulase enzyme Auto Dock Vina tool was used by Trott and Olson [46]. The structures of Ag and Fe were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov). The 3D structure of CMCase (PDB ID: 3PZU) was obtained from Protein Data Bank https://www.rcsb.org/structure/3PZU. Before docking analysis, water molecules were removed from the protein. To determine the binding sites on protein, Ag, and Fe molecules were allowed to move within the whole protein region. The output from AutoDock Vina was further analyzed with PyMOL (Ahmad et al. [5] used for the structural representation of figures.
Results
Synthesis and characterization of silver and iron oxide NPs
The morphology and elemental composition of synthesized silver and iron oxide nanoparticles (NPs) examined through a scanning electron microscope (SEM) and energy dispersive X-ray analysis (EDX) are shown in Fig. 1, where surface morphology confirming the hexagonal and circular shape of silver oxide and iron oxide nanoparticles is evident (Fig. 1a, b). The impurities-free NPs with smooth uniform grain dispersions and surfaces can be seen. The diameter recorded for these NPs was in the range of 70–95 nm. The elemental composition of these nanoparticles was confirmed through EDX as shown in Fig. 1c, d. The different peaks are also shown in Fig. 1c, d because the gold was sputtered on top of NPs as a conducting material before SEM characterization.
Morphological, biochemical, and molecular identification of bacterial strain
The isolated bacterium was identified as Bacillus subtilis based on morphological, biochemical, and molecular characterization, and the strain was designated as AG-PQ. The data of morphological and biochemical tests for the strain Bacillus subtilis AG-PQ (MG662180) is presented in Table 1.
The amplified 16S rRNA region of Bacillus subtilis genomic DNA, ~ 1000 base pairs. The data presented in the NJ tree (Fig. 2) based on the 16S rRNA gene shows the dispersion of isolated bacterial strains throughout the clades corresponding to other bacterial strains. The tree indicated that all the bacterial strains form a well-supported group.
Neighbor-joining consensus tree based on partial 16S rDNA gene sequences showing the evolutionary relationships between Bacillus subtilis AG-PQ of the present study (Specified in red) with reference strains from GenBank. The significance of each branch is indicated by the bootstrap value calculated for 1000 replicates (only values higher than 50% are indicated)
Effects of cultural parameters on cellulase production
The effect of critical cultural parameters was optimized that can influence the cellulase production by B. subtilis AG-PQ using submerged fermentation, the optimization of culture media composition according to the Ahmad et al. [5] design of experimental methodology. The production medium for cellulase allowed incubating for 72 h to optimize incubation time. The maximum cellulase production was achieved after 56 h of incubation time (Fig. 3B). Therefore, 56 h is designated as the optimal time for cellulase production for subsequent experimentation. The results are presented in Fig. 3. It is evident from the results that the optimal inoculum size for cellulase production was 5% at which the maximum 315.0 U/ml/min enzyme activity was observed. The cellulase activity declined to (312.0 U/ml/min) at 7.5% inoculum concentration.
Four different media were used for the optimization of the fermentation process to achieve better enzyme activity. The basal media was found optimal for efficient cellulase activity (302.5 U/ml/min) at 37 °C and 56 h of incubation time (Fig. 3).
Five different carbon sources, i.e., rice husk, cellulose, wheat bran, corn cob, and coconut cake, were investigated to opt for the optimal carbon source for cellulase production. The results of this study designated coconut cake as the best carbon source for optimal cellulase production with values of 321.0 U/ml/min in contrast to cellulose (318.0 U/ml/min) and wheat bran (316.0 U/ml/min) (Fig. 4a). Ammonium sulfate, ammonium chloride, urea, ammonium nitrate, and diammonium phosphate were supplemented as nitrogen sources in the production media to achieve the maximum cellulase yield. The results of this study validate ammonium nitrate as the best nitrogen source for cellulase production with the highest values of 329.0 U/ml/min followed by DAP (325.0 U/ml/min) and ammonium sulfate (326.0 U/ml/min) (Fig. 4b).
The pH of the production medium was adjusted to various pH ranges from 5 to 9 to analyze the influence of pH on cellulase production by Bacillus subtilis AG-PQ MG662180. Cellulase production was observed maximum (330.0 U/ml/min) at pH 8. The effect of temperature on the production of cellulase was observed by incubating the production media at different temperatures ranging from 20 to 60 °C. The maximum cellulase production (336.0 U/ml/min) was recorded at 37 °C.
The various parts per million (ppm) of silver oxide and iron oxide nanoparticles were supplemented into an optimized fermentation medium to examine the effect on cellulase production. The maximum cellulase production was noticed at a 50-ppm concentration of AgO NPs (336.0 U/ml/min). It has been also observed that Fe NPs particles pose adverse effects in cellulase production with the lowest units recorded at 100 ppm (320.0 U/ml/min).
Partial purification and characterization of cellulase enzyme
Cellulase was partially purified from the crude extract by ammonium sulfate precipitation (ASP) at 50%. The maximum cellulase activity was observed at 50% ammonium sulfate concentration (360 U/ml/min). Kinetic parameters of partially purified cellulase were determined by incubating the enzyme at various cellulose concentrations (0.1–0.9 mM) to analyze the effect of substrate concentration on enzyme activity under standard assay conditions. Cellulase showed maximum enzyme activity at 0.9 mM substrate concentration (286.4 U/ml/min) in the reaction mixture (Fig. 5).
The dependency on the pH of cellulase activity was investigated by varying pH values ranging from pH 4–9. Cellulase exhibits optimal activity (323.0 U/ml/min) at pH 8. Various temperature ranges from 25 to 65 °C, were evaluated under standard conditions to observe thermal stability and optimal cellulase activity (Fig. 6a, b). The cellulase activity was found maximum at 50 °C.
Effect of AgO and FeO NPs on purified cellulase
The partially purified cellulase was investigated against several concentrations of AgO and FeO nanoparticles (10–70 ppm) to observe their positive or inhibitory effect. Results showed that AgO nanoparticles have positively influenced cellulase activity and improved thermal stability over a period of time (Table 2). The enzyme activity (57.804 U/ml/min) progressively enhanced up to 25 ppm concentration whereas the enzyme activity started decreasing significantly with an increase in AgO NPs onwards.
FeO nanoparticles posed a negative inhibitory effect on cellulase activity. The enzyme activity at 10 ppm concentration was 47.469 U/ml/min and decreased intensely to 32.736 U/ml/min at 70 ppm.
Docking interaction of Ag and Fe nanoparticlas with cellulase
Molecular docking analysis revealed that a list of ligands with their binding energy or binding affinity to understand the possible interaction of different structures with target molecules molecular docking method is very effective. It gives useful information about protein–ligand interaction. In this study, molecular docking was carried out using Auto Dock Vina to find binding sites of AgO and FeO. The results revealed that AgO nanoparticles had − 0.49 kcal/mol binding energy and FeO had − 0.27 kcal/mol binding energy (Fig. 7). Ag nanoparticles had more affinity for surviving compared to Fe nanoparticles because the lower the binding energy more stable the complex.
Discussion
The crystal structure of the synthesized nanoparticles (NPs) of silver and iron oxide were analyzed through the XRD method using the Debye Scherrer formula \(=\frac{{\varvec{k}}{\varvec{\lambda}}}{{{\varvec{\beta}}}_{{\varvec{h}}{\varvec{k}}{\varvec{l}}}{\varvec{cos}}{\varvec{\theta}}}\) that was exposing Cu-Kα (1.5406\(\overset{\circ}{\mathrm{A}}\)) in the radiation of incident X-rays wave length \((\lambda )\). The diffracted intensities of both NPs were measured with respect to 2θ, which range was taken from 20° to 80° as shown in Fig. 8, where \(\theta\) is the Braggs angle, \(k\) indicates the shape factor or correction factor \((k=0.9)\) and \({\beta }_{hkl}\) represent FWHM (full width at half maximum) that was taken in radians [47]. The XRD pattern of Ag NPs was observed at 2θ = 33.07°, 38.07°, 44.44°, 64.75° and 77.47° which correspond to the Miller indices (111), (111), (200), (220), and (311) planes respectively. Such results were confirmed and compared with standard data (JCPDS: 89–3722 and 04–0783) that reflect a face-centered-cubic (FCC) crystal structure corresponding to crystalline in nature [XRD-Ag-1, XRD-Ag-2]. The phase identification of iron oxide (Fe2O3) NPs displaying their peaks at 2θ = 31.12°, 36.14°, 43.19°, 53.09°, 57.02°, 63.22°, and 73.04° are assigned to the planes (220), (311), (400), (422), (511), (440), and (533). The patterns of these NPs were compared with standard data (JCPDS: 82–1533 and 39–1346) [XRD-FeO-1, XRD-FeO-2]. The EDX clearly shows the presence of oxygen with iron confirming the purity crystal of iron oxide NPs (Fig. 1D). Also in both silver and iron oxide NPs, some brooding diffraction peaks were observed that also confirming their small size and crystalline nature with no other phase of impurity in both patterns of XRD.
Bacillus subtilis AG-PQ of this study appeared in a clade comprising other bacterial strains of the Bacillus genus and was found in the same lineage with previously reported Bacillus subtilis strains as shown in Fig. 2. Morphological and biochemical characterization provides authentic information about the isolated unidentified strain and its extra/intracellular secretions but molecular characterization is a reliable approach to identifying the isolated strain through phylogenetic analysis by using 16S rRNA sequences [48]. The incubation time was observed and cellulase production was decreased by further increasing the incubation period. This might be due to the exhaustion of nutritional components or the production of secondary metabolites in the production medium [39]. Reddy et al. [49] reported that the optimal incubation time for cellulase production by Bacillus subtilis was 60 h. Whereas, Kiran et al. [39] showed that the optimal incubation time for the production of cellulase by Bacillus subtilis was 48 h. The results of Yan et al. [9] revealed that the cellulase production by the Bacillus subtilis Q-3 strain increased with an increase in incubation time and reached its maximum production at 60 h.
Inoculum size plays a crucial role in the optimization of the fermentation process [50]. To optimize the inoculum size concentration, production media was inoculated with different inoculum concentrations (2.5%, 5%, 7.5%, and 9.0%) to observe their effect on cellulase production. Similar results were reported by Shajahan et al. [51] who optimized the 5% inoculum concentration for the highest cellulase production by Bacillus subtilis. Whereas, Singh and Kaur [52] reported that 5% inoculum size was best for maximum cellulase production by Bacillus subtilis Q-3 strain. Similar observations were made by Hussain et al. [32] for media optimization who reported the maximum production of cellulase using basal media production medium by B. subtilis.
Enzyme regulation is majorly influenced by the culture media composition during the fermentation process [39]. Previous literature reported that carbon and nitrogen sources affect cellulase production from microbial sources [6]. Also, Sethi et al. [40] found coconut cake to be the best inducer for the production of cellulase enzymes by B. subtilis. Soeka [53] reported the maximum production of cellulase from B. subtilis A8 by utilizing rice bran and corncob as substrate. Vyas et al. [41] also reported ammonium nitrate as the best source of nitrogen for cellulase production by B. subtilis. Sethi et al. [40] tested various nitrogen sources, among which ammonium sulfate was found to be the best source of nitrogen for cellulase production by B. subtilis. Zhou et al. [54] reported optimized cellulase production from Bacillus subtilis MU S1 by keeping the pH of the medium constant at pH 8. Similarly, Zamani et al. [55] observed maximum cellulase activity by B. subtilis at pH 7. Hussain et al. [32] showed maximum cellulase activity by Bacillus sp. 313SI under stationary conditions at 37 °C. In another study, 37 °C temperature was found to be optimal for cellulase production by Bacillus subtilis Q-3 Yan et al. [9]. Otajevwo and Aluyi [56] demonstrated a significant ability of B. subtilis SBMP4 strain to produce cellulase at 37 °C.
Lineweaver–Burk plot was plotted against the obtained data in order to retrieve Km and Vmax values [57]. Anu et al. [54] optimized pH 8 for the maximum activity of partially purified cellulase. Similar observations were made by Kiran et al. [39] who reported the maximum cellulase activity at 50 °C from Bacillus subtilis. Cellulase exhibits stability over a range of temperatures with moderately stable at alkaline pH ranges.
Kumar et al. [36] investigated the effect of FeO NPs on the soil microbial community and reported iron oxide nanoparticles as enzyme inhibitors. The results are in concurrence with the observations of Salunke et al. [26] who investigated the effect of biosynthesized Ag nanoparticles on fungal cellulase activity to enhance cellulase degradation. Here, a two-fold increase was observed in enzymatic activity with Ag nanoparticles in cellulose degradation. The results of this study confirm that the combination of free thermostable cellulose and AgO NPs is effectively used for significant cellulose degradation.
Another study by Shah et al. [58] and Eisazadeh et al. [59] examined the effect of Ag NPs on hydrolytic enzymes such as lipase and cellulase to be potentially used in the detergent industry. They revealed that Ag NPs affect the secondary structure of lipase and cause the reduction in its catalytic activity whereas loss of anti-microbial activity and stability of Ag NPs was reported upon interaction with the lipase. Cellulase has been influenced by Ag-NPs in comparison to lipase and its catalytic activity was significantly changed suggesting the positive correlation of Ag-NPs with cellulase. Recently, Gupta et al. [60] reported that biosynthesized Ag NPs have a positive influence on fungal cellulase catalytic activity, stability, and biocompatibility. In our study, cellulase activity was significantly enhanced in the presence of Ag NPs (10–50 ppm), and the cellulase activity was boosted two-fold at 0.012 µg/ml/min of Ag NPs concentration. Srivastava et al. [19] evaluated the pH and thermal stability of cellulase in the presence of chemically synthesized Zinc oxide (ZnO) nanoparticles. Cellulase showed alkaline stability at pH 10.5 and retained 53% activity whereas the enzyme remained thermally stable at 65 °C for 10 h. The results of this study signify the importance of nanocatalysts for enhanced enzymatic activity to be potentially used in cellulase degradation industries.
Conclusions
Agricultural soil is an abundant source of useful biocatalysts and other valuable substances. Bacillus subtilis AG-PQ can efficiently produce cellulase of sufficient quantity by consuming coconut cake as a sole carbon source and ammonium nitrate as a nitrogen source with solid-state fermentation (SSF). Other cultural parameters such as pH and temperature were also optimized to obtain the maximum cellulolytic activity. This study has successfully utilized silver oxide nanoparticles for enhancing the production and activity of cellulase from Bacillus subtilis AG-PQ. Improved cellulase production and biodegradation activity were demonstrated by cellulase in the presence of silver oxide nanoparticles. The study indicates that Bacillus subtilis AG-PQ could be a better candidate for industrial processes owing to its thermophilic nature and thermostable cellulase production.
Availability of data and materials
Not applicable.
Abbreviations
- µl:
-
Microliter
- NPs:
-
Nanoparticles
- AgO:
-
Silver oxide
- FeO:
-
Iron oxide
- µ:
-
Micro
- SEM:
-
Scanning electron microscopy
- EDS:
-
Energy-dispersive X-ray spectroscopy
- XRD:
-
X-ray Diffraction Analyzer
- ml:
-
Milliliter
- min:
-
Minutes
- AMBL:
-
Applied Microbiology and Biotechnology Lab
- M:
-
Molar
- NaOH:
-
Sodium hydroxide
- h:
-
Hours
- SSF:
-
Solid state fermentation
- OD:
-
Optimum density
References
Irfan M, Mushtaq Q, Tabssum F, Shakir HA, Qazi JI (2017) Carboxymethyl cellulase production optimization from newly isolated thermophilic Bacillus subtilis K-18 for saccharification using response surface methodology. AMB Express 7(1):29. https://doi.org/10.1186/s13568-017-0331-3
Kuhad RC, Gupta R, Singh A (2011) Microbial cellulases and their industrial applications. Enzyme Res 2011:1–10. https://doi.org/10.4061/2011/280696
Khatiwada P, Ahmed J, Sohag MH, Islam K, Azad AK (2016) Isolation, screening and characterization of cellulase producing bacterial isolates from municipal solid wastes and rice straw wastes. J Bioprocess Biotech 6(280):2. https://doi.org/10.4172/2155-9821.1000280
Amore A, Pepe O, Ventorino V, Birolo L, Giangrande C, Faraco V (2013) Industrial waste based compost as a source of novel cellulolytic strains and enzymes. FEMS Microbiol Lett 339(2):93–101. https://doi.org/10.1111/1574-6968.12057
Ahmad K, Khan S, Yasin MT, Hussain S, Ahmad R, Ahmad N, ... Bokhari SAI (2021) Enhanced starch hydrolysis by α-amylase using copper oxide nanowires. Appl Nanosci 11(7):2059–2071. https://doi.org/10.1007/s13204-021-01931-3
Sadhu S, Saha P, Sen SK, Mayilraj S, Maiti TK (2013) Production, purification and characterization of a novel thermotolerant endoglucanase (CMCase) from Bacillus strain isolated from cow dung. SpringerPlus 2(1):10. http://www.springerplus.com/content/2/1/10
Listyaningrum NP, Sutrisno A, Wardani AK (2018) Characterization of thermostable cellulase produced by Bacillus strains isolated from solid waste of carrageenan. E&ES 131(1):012043. https://doi.org/10.1088/1755-1315/131/1/012043
Souii A, Guesmi A, Ouertani R, Cherif H, Chouchane H, Cherif A, & Neifar M (2018) Carboxymethyl cellulase production by extremotolerant bacteria in low-cost media and application in enzymatic saccharification of Stevia biomass. Waste and Biomass Valorization 1–12. https://doi.org/10.1007/s12649-018-0496-2
Yan S, Sun X, Zhang W, Zhu L (2019) June. Isolation, identification and cellulase-producing condition optimization of Bacillus subtilis Q3 strain. In AIP Conference Proceedings (Vol. 2110, No. 1, p. 020004). AIP Publishing LLC
Yasin MT, Ali Y, Ahmad K, Ghani A, Amanat K, Basheir MM, ... Bokhari SAI (2021) Alkaline lipase production by novel meso-tolerant psychrophilic Exiguobacterium sp. strain (AMBL-20) isolated from glacier of northeastern Pakistan. Archiv Microbiol 203:1309–1320. https://doi.org/10.1007/s00203-020-02133-1
van Dijl J, & Hecker M (2013) Bacillus subtilis: from soil bacterium to super-secreting cell factory. 12(3):1–6. http://www.microbialcellfactories.com/content/12/1/3
Chan KY, Au KS (1987) Studies on cellulase production by a Bacillus subtilis. Antonie Van Leeuwenhoek 53(2):125–136
Deka D, Das SP, Sahoo N, Das D, Jawed M, Goyal D, Goyal A (2013) Enhanced cellulase production from Bacillus subtilis by optimizing physical parameters for bioethanol production. Isrn Biotechnol 2013:1–11. https://doi.org/10.5402/2013/965310
Sreena CP, Sebastian D (2018) Augmented cellulase production by Bacillus subtilis strain MU S1 using different statistical experimental designs. J Genet Eng Biotechnol 16(1):9–16. https://doi.org/10.1016/j.jgeb.2017.12.005
Ma L, Lu Y, Yan H, Wang X, Yi Y, Shan Y,... Lü X, (2020) Screening of cellulolytic bacteria from rotten wood of Qinling (China) for biomass degradation and cloning of cellulases from Bacillus methylotrophicus. BMC Biotechnol 20(1):1–13. https://doi.org/10.1186/s12896-019-0593-8
He B, Jin S, Cao J, Mi L, Wang J (2019) Metatranscriptomics of the Hu sheep rumen microbiome reveals novel cellulases. Biotechnol Biofuels 12(1):153. https://doi.org/10.1186/s13068-019-1498-4
Wan C, Li Y (2010) Microbial pretreatment of corn stover with Ceriporiopsis subvermispora for enzymatic hydrolysis and ethanol production. Bioresour Technol 101(16):6398–6403. https://doi.org/10.1016/j.biortech.2010.03.070
Sun S, Sun S, Cao X, Sun R (2016) The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour Technol 199:49–58. https://doi.org/10.1016/j.biortech.2015.08.0610
Srivastava N, Srivastava M, Mishra PK, Ramteke PW (2016) Application of ZnO nanoparticles for improving the thermal and pH stability of crude cellulase obtained from Aspergillus fumigatus AA001. Frontiers Microbiol 7:514. https://doi.org/10.3389/fmicb.2016.00514
Srivastava N, Singh J, Ramteke PW, Mishra PK, Srivastava M (2015) Improved production of reducing sugars from rice straw using crude cellulase activated with Fe3O4/Alginate nanocomposite. Bioresour Technol 183:262–266. https://doi.org/10.1016/j.biortech.2015.02.059
Blanchette C, Lacayo CI, Fischer NO, Hwang M, Thelen MP (2012) Enhanced cellulose degradation using cellulase-nanosphere complexes. PLoS ONE 7(8):e42116. https://doi.org/10.1371/journal.pone.0042116
Vasconcellos VM, Tardioli PW, Giordano RLC, Farinas CS (2016) Addition of metal ions to a (hemi) cellulolytic enzymatic cocktail produced in-house improves its activity, thermostability, and efficiency in the saccharification of pretreated sugarcane bagasse. New Biotechnol 33(3):331–337. https://doi.org/10.1016/j.nbt.2015.12.002
Wang G, Zhang X, Wang L, Wang K, Peng F, Wang L (2012) The activity and kinetic properties of cellulases in substrates containing metal ions and acid radicals. Adv Biol Chem 02:390–395. https://doi.org/10.4236/abc.2012.24048
Wang S, Lv M, Yang J, Zhou Y, Xu B (2018) Effects and mechanism of metal ions on enzymatic hydrolysis of wheat straw after pretreatment. BioResources 13(2):2617–2631
Pandurangan M, Kim DH (2015) ZnO nanoparticles augment ALT, AST, ALP and LDH expressions in C2C12 cells. Saudi J Biol Sci 22(6):679–684. https://doi.org/10.1016/j.sjbs.2015.03.013
Salunke BK, Sawant SS, Kang TK, Seo DY, Cha Y, Moon SA., ... Kim BS (2015) Potential of biosynthesized silver nanoparticles as nanocatalyst for enhanced degradation of cellulose by cellulase. J Nanomaterials 2015:1–8. https://doi.org/10.1155/2015/289410
Yousef N, Mawad A, Abeed A (2019) Enhancement the cellulase activity induced by endophytic bacteria using calcium nanoparticles. Curr Microbiol 76(3):346–354. https://doi.org/10.1007/s00284-018-1614-x
Asiri S, Sertkol M, Guner S, Gungunes H, Batoo KM, Saleh TA, ... Baykal A (2018) Hydrothermal synthesis of CoyZnyMn1–2yFe2O4 nanoferrites: magneto-optical investigation. Ceramics International 44(5):5751–5759. https://doi.org/10.1016/j.ceramint.2017.12.233
Zhu L, Li Y, Zeng W (2018) Hydrothermal synthesis of hierarchical flower-like ZnO nanostructure and its enhanced ethanol gas-sensing properties. Appl Surface Sci 427:281–287. https://doi.org/10.1016/j.apsusc.2017.08.229
Vijayaraghavan P, VInCEnT SP (2012) Purification and characterization of carboxymethyl cellulase from Bacillus sp. isolated from a paddy field. Polish J Microbiol 61(1):51–55
Hussain T, Roohi A, Munir S, Ahmed I, Khan J, Edel-Hermann V, ... Anees M (2013) Biochemical characterization and identification of bacterial strains isolated from drinking water sources of Kohat, Pakistan. Afri J Microbiol Res 7(16):1579–1590. https://doi.org/10.5897/AJMR12.2204
Hussain AA, Abdel-Salam MS, Abo-Ghalia HH, Hegazy WK, Hafez SS (2017) Optimization and molecular identification of novel cellulose degrading bacteria isolated from Egyptian environment. J Gene Eng Biotechnol 15(1):77–85. https://doi.org/10.1016/j.jgeb.2017.02.007
Nandimath AP, Kharat KR, Gupta SG, Kharat AS (2016) Optimization of cellulase production for Bacillus sp. and Pseudomonas sp. soil isolates. Afri J Microbiol Res 10(13):410–419. https://doi.org/10.5897/AJMR2016.7954
Sipos R, Székely AJ, Palatinszky M, Révész S, Márialigeti K, Nikolausz M (2007) Effect of primer mismatch, annealing temperature and PCR cycle number on 16S rRNA gene-targetting bacterial community analysis. FEMS Microbiol Ecol 60(2):341–350. https://doi.org/10.1111/j.1574-6941.2007.00283.x
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symp Ser 41:95–98
Kumar D, Talreja N (2019) Nickel nanoparticles-doped rhodamine grafted carbon nanofibers as colorimetric probe: Naked eye detection and highly sensitive measurement of aqueous Cr 3+ and Pb 2+. Korean J Chem Eng 36(1):126–135. https://doi.org/10.1007/s11814-018-0139-0
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Bradford MM (1976) A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Kiran T, Asad W, Ajaz M, Hanif M, Rasool SA (2018) Industrially relevant cellulase production by indigenous thermophilic Bacillus licheniformis TLW-3 strain: Isolation-molecular identification and enzyme yield optimization. Pak J Pharm Sci 31(6)
Sethi S, Datta A, Gupta BL, Gupta S (2013) Optimization of cellulase production from bacteria isolated from soil. International Scholarly Research Notices. 2013 https://doi.org/10.5402/2013/985685
Vyas AK, Putatunda C, Singh J, Vyas D (2016) Cellulase Production by Bacillus subtilis M1 using pretreated groundnut shell based liquid state fermentation. BIOTROPIA-The Southeast Asian J Trop Biol. 23(1):28–34. https://doi.org/10.11598/btb.2016.23.1.472
Gomori G (1955) Preparation of buffers for use in enzyme studies. In 'Methods in Enzymology'. (Eds S. P. Colowick and N. O. Kaplan.). 1:138–46. (Academic Press: New York.) https://doi.org/10.1016/0076-6879(55)01020-3
Bokhari SAI, Latif F, Rajoka MI (2009) Purification and characterization of xylanases from Thermomyces lanuginosus and its mutant derivative possessing novel kinetic and thermodynamic properties. World J Microbiol Biotechnol 25:493–502. https://doi.org/10.1007/s11274-008-9915-z
Hemlata B, Uzma Z, Tukaram K (2016) Substrate kinetics of thiol activated hyperthermostable alkaline lipase of Bacillus sonorensis 4R and its application in bio-detergent formulation. Biocatal Agri Biotechnol 8:104–111. https://doi.org/10.1016/j.bcab.2016.08.0081
Rehman H, Akram M, Kiyani MM, Yaseen T, Ghani A, Saggu JI, Bokhari SAI (2019) Effect of endoxylanase and iron oxide nanoparticles on performance and histopathological features in broilers. Biol Trace Elem Res 192(436):1–12. https://doi.org/10.1007/s12011-019-01737-z
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Computational Chem 31(2):455–461
He K, Chen N, Wang C, Wei L, Chen J (2018) Method for determining crystal grain size by x-ray diffraction. Crys Res Technol 53(2):1700157. https://doi.org/10.1002/crat.201700157
Lee LP, Karbul HM, Citartan M, Gopinath SC, Lakshmipriya T, Tang TH (2015) Lipase secreting Bacillus species in an oil-contaminated habitat: promising strains to alleviate oil pollution. Bio Med Res Int 1:1–9. https://doi.org/10.1155/2015/820575
Reddy KV, Lakshmi TV, Reddy AVK, Bindu VH, Narasu ML (2016) Isolation, screening, identification and optimized production of extracellular cellulase from Bacillus subtilis Sub. sps using cellulosic waste as carbon source. Int. J. Curr. Microbiol. App. Sci. 5(4): 442–451. https://doi.org/10.20546/ijcmas.2016.504.052
Tariq R, Ansari I, Qadir F, Ahmed A, Shariq M, Zafar U, ... Sohail M (2018) Optimization of endoglucanase production from thermophilic strain of Bacillus licheniformis RT-17 and its application for saccharification of sugarcane bagasse. Pak J Bot 50(2):807–816
Shajahan S, Moorthy IG, Sivakumar N, Selvakumar G (2017) Statistical modeling and optimization of cellulase production by Bacillus licheniformis NCIM 5556 isolated from the hot spring, Maharashtra. India J King Saud Univ Sci 29(3):302–310. https://doi.org/10.1016/j.jksus.2016.08.001
Singh J, & Kaur P (2012) Optimization of process parameters for cellulase production from Bacillus sp. JS14 in solid substrate fermentation using response surface methodology. Braz Arch Biol Technol 55(4):505–512. https://doi.org/10.1590/S1516-89132012000400004
Soeka YS (2019) August. Production and characterization of cellulase from the newly isolated Bacillus subtilis A8 on rice bran and corncob. In IOP Conference Series: Earth and Environmental Science. 308(1):012033. IOP Publishing
Anu Kumar S, Kumar A, Kumar V, Singh B (2020) Optimization of cellulase production by Bacillus subtilis subsp. subtilis JJBS300 and biocatalytic potential in saccharification of alkaline-pretreated rice straw. Preparative Biochem Biotechnol. 1–8. https://doi.org/10.1080/10826068.2020.1852419
Zamani H, Salehzadeh A, Abdolhosseini M (2018) Isolation and molecular identification of a cellulotic bacterium from muncipal waste and investigation of its cellulase production. Biosci J, 34:3 https://doi.org/10.14393/BJ-v34n3a2018-40007
Otajevwo FD, Aluyi HSA (2011) Cultural conditions necessary for optimal cellulase yield by cellulolytic bacterial organisms as they relate to residual sugars released in broth medium. Mod Appl Sci 5(3):141. https://doi.org/10.5539/mas.v5n3p141
Nimisha P, Moksha S, Gangawane AK (2019) Amylase activity of starch degrading bacteria isolated from soil, Inter J Current Microbiol Appl Sci 8(4):659–671. https://doi.org/10.20546/ijcmas.2019.804.071
Shah MM, Ahmad K, Ahmad B, Shah SM, Masood H, Siddique MAR, Ahmad R (2022) Recent trends in green synthesis of silver, gold, and zinc oxide nanoparticles and their application in nanosciences and toxicity: a review. Nanotechnol Environ Engin 7(4):907–922. https://doi.org/10.1007/s41204-022-00287-5
Eisazadeh B, Mirzajani F, Sefidbakht Y (2020) How is the effect of silver nanoparticles and lipase/Cellulase enzymes on each other? Iran J Sci Technol Trans A Sci 44(1):27–35. https://doi.org/10.1007/s40995-020-00820-8
Gupta S, Bisht SA, Kelkar-Mane V (2019) Assessing the enzyme modulating and antimicrobial efficiency of moringa capped silver nanoparticles for their potential use as fodder supplement. https://doi.org/10.26479/2019.0502.69
Acknowledgements
The authors are grateful to the International Islamic University Islamabad (IIUI) Pakistan for the provision of essential resources to conduct this research work.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SH: methodology, validation, writing investigation, research investigation, writing—review, and editing. MTY, KA: optimization of different parameters for cellulase production. KA, SK: synthesized and characterization of Nanomaterial. MA, RA: writing—review and editing enzyme production. MMS, KA: conceptualization, writing—review, and editing. AG, AH: Bacteria ( Bacillus subtilis ) isolation, molecular sequencing, and phylogenetic analysis for enzyme production. MF: purification of cellulase enzyme. HR, HT: Characterization of cellulase enzyme with different parameters and biochemical identification. SAIB: methodology, supervision, conceptualization, resources, investigation, writing—review and editing. JK helped in preparing the initial draft and revising the manuscript thoroughly. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All the authors of this study declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussain, S., Yasin, M.T., Ahmad, K. et al. Enhancement effect of AgO nanoparticles on fermentative cellulase activity from thermophilic Bacillus subtilis Ag-PQ. J Genet Eng Biotechnol 21, 151 (2023). https://doi.org/10.1186/s43141-023-00619-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43141-023-00619-1