Abstract
Background
Marine bacteria have recently attracted increasing attention to be harnessed for the production of valuable enzymes, vitamins, and bioactive compounds. Bacteria associated with the surfaces of marine macroalgae, called epibionts, are particularly interesting from ecological and biotechnological points of view, as they often exhibit antimicrobial activities to compete with pathogenic bacteria for nutrients and spaces. In search for biotechnologically potential genes from marine bacteria, we sequenced and analysed the genome of the epibiont HI03-3b, a polysaccharide-degrading bacterium associated with the surface of the Indonesian brown algae Hydroclathrus sp.
Results
The algal epibiont HI03-3b has a genome of approximately 4,860,704 bp in size with 42.02 mol% G + C content, consisting of 5655 open reading frames (ORFs), 4409 genes coding for proteins (CDSs), 94 genes for tRNAs, and 32 genes for rRNAs. The genome sequence of HI03-3b was most closely related to that of Cytobacillus firmus NCTC10335 with the average amino acid identity (AAI) of 95.0 %, average nucleotide identity (ANI) of 94.1 %, and a recommended DNA-DNA hybridization (DDH) of 57.60 %. These scores are lower than the most frequently used standard for species demarcation (95% ANI cutoff) and the new species threshold (DDH > 70.0% for the same bacterial species). Some differences in genome features and gene composition were observed between HI03-3b and NCTC10335, such as genes encoding carbohydrate active enzymes. These suggest that HI03-3b is unique and likely a novel species within Cytobacillus genus, and we therefore proposed its name as Cytobacillus wakatobiense HI03-3b. Genome sequence analyses indicated the presence of genes involved not only in polysaccharide and protein degradation but also in vitamin and secondary metabolite biosynthesis. Some of them encode enzymes and compounds with biotechnological interest, such as protease, chitinase, subtilisin, pullulanase, and bacillolysin, which are often associated with antimicrobial or antibiofilm activities. This antimicrobial potential is supported by our finding that the extracellular protein fraction of this epibiont inhibited the growth of the bacterial pathogen Staphylococcus aureus.
Conclusion
The epibiont Cytobacillus HI03-3b harbours genes for polysaccharide and protein degradation as well as for natural product biosynthesis, suggesting its potential ecological roles in outcompeting other bacteria during biofilm formation as well as in protecting its algal host from predation. Due to the presence of genes for vitamin biosynthesis, it might also provide the algal host with vitamins for growth and development. Some of these metabolic genes are biotechnologically important, as they could become a platform for bioengineering to generate various seaweed-derived substances sustainably, such as antibiofilm agents and vitamins, which are beneficial for human health.
Similar content being viewed by others

Background
Marine macroalgae, also called seaweeds, are valuable sources of health-promoting secondary metabolites, enzymes, and vitamins for being developed as nutraceuticals, pharmaceuticals, and cosmeceuticals [24, 36, 42]. Particularly, enzymes from seaweeds have attracted increasing attention for biotechnological applications due to their unique features [55]. However, the natural purification of algae-derived enzymes is extremely difficult due to their high content of polysaccharides, polyphenols, and stable cell walls [45]. Furthermore, the limited supply of seaweed-derived bioactive compounds and enzymes represents a big challenge in their development into high-value marketable products, because most existing cultivation techniques developed for producing commoditized biomass may not necessarily be optimized for seaweed bioactive production [24].
Microorganisms associated with seaweeds have recently been recognized as the producers of novel bioactive compounds and enzymes [40]. Their ability to produce bioactives is considered as an ecological strategy to compete for nutrients and space on the surfaces of marine macroalgae [18]. This strategy also helps their algal hosts to chemically defend against the secondary colonization by other microscopic and macroscopic epibiota [17]. A notable example of epibionts that play this crucial ecological role is Pseudoalteromonas species inhabiting seaweed surfaces, as they produce toxic compounds, bacteriolytic substances, and extracellular enzymes for outcompeting other bacteria during biofilm formation [26, 27]. Continuous attempts to isolate potential seaweed-associated bacteria for identifying biotechnologically relevant genes are urgently needed to produce bioactive compounds and enzymes with biotechnological interest in sustainable ways.
In search for biotechnologically potential genes from seaweed epibionts, we initially isolated a novel polysaccharide-degrading bacterial species associated with the Indonesian brown algae Hydroclathrus sp. [70]. We found that the cell-free culture of this epibiont was able to inhibit the bacterial pathogen S. aureus, indicating its potential ability to produce antimicrobial substance extracellularly. This preliminary result encouraged us to sequence the whole genome of this bacterium in order to better understand its ecological role and biotechnological potential. Analysing the HI03-3b genome sequence has enabled us to identify metabolic genes, including those involved in polysaccharide and protein degradation as well as in natural product biosynthesis. Since polysaccharides and proteins represent key components of the extracellular polymeric substances (EPS) of pathogenic microbial biofilms [57], this finding could become a basis for further exploring antimicrobial enzymes and compounds to treat persistent pathogenic biofilms. Subsequent heterologous expression of these genes is necessary to produce useful enzymes or compounds in sustainable ways for biotechnological applications.
Methods
Isolation and bioassay of a potential algae-associated epibiont
A polysaccharide-degrading bacterium, designated as HI03-3b, was isolated from the brown algae Hydroclathrus sp. inhabiting sandy shallow water around Hoga Island, Wakatobi, South Sulawesi, Indonesia, at the site coordinate of 5.28317 S and 123.45377 E. Briefly, the algae sample was collected at the depth of 0.5–1.0 m (Fig. 1A). It was placed in a 50-ml conical tube and stored in a cool box. A total of 5 g seaweed sample was put into a bottle containing sterile seawater and vortexed for approximately 10 min to release the bacterial cells attached on the surface of Hydroclathrus sp. Aliquot of the seaweed mixture was inoculated into solid minimal seawater (MS) media by dilution (10−5–10−8) and then incubated for 3–7 days at 30 °C. The MS medium was made up of 0.5% tryptone, 0.1% yeast extract, 0.1% sodium alginate, and 2% agar dissolved in seawater (30 ppt). The colonies growing on solid MS were isolated and purified. The ability of the bacterial isolates to degrade polysaccharides was assessed by a 24-h incubation and subsequent staining with Lugol’s iodine solution, followed by staining with CaCl2 solution (10%) with 1-h incubation to observe the capability of degrading alginate [70]. The most potential bacterial isolate was subsequently tested for the antimicrobial ability of its extracellular fraction against Staphylococcus aureus ATCC®25923™, an opportunistic bacterial pathogen that can cause various infections and food poisoning [14, 63]. Briefly, the cell-free culture was initially prepared and concentrated through a 10-kDa membrane filtration (Whatman). The concentrated extracellular fraction (50 μl) was applied on a paper disc placed on the solid medium streaked with S. aureus.
Isolation of the potential algae-associated epibiont HI03-3b. The Indonesian brown algae Hydroclathrus sp. (photo taken by S. N. Ethica) where HI03-3b was isolated (A) and colony morphology of HI03-3b (B). Bioassays of HI03-3b showing a clearing zone surrounding colonies (duplo) on an alginate-containing MS plate after Lugol staining (C) and inhibition zone by the extracellular culture against the lawn bacterial pathogen S. aureus (D)
Whole-genome sequencing
The genome of Cytobacillus sp. HI03-3b was sequenced using Oxford Nanopore Technology (ONT) carried out at the PT Genetika Science Indonesia, a certified service provider of ONT in Indonesia. Briefly, the HI03-3b genomic DNA was initially extracted using QIAGEN Genomic-tip 500/G followed by size selection with Agencourt AMPure XP beads and Circulomics Short Read Eliminator Kit. The libraries of HI03-3b genomic DNA for sequencing were subsequently constructed using Ligation Sequencing Kit (SQK-LSK109) according to the instructions of the manufacturer Oxford Nanopore Ltd., UK. In principle, the size-selected DNA fragments were end prepped using Ultra II End-prep enzyme mix and ligated with barcode adapter. With adapter-ligated DNA as the template, a barcoding PCR reaction was set up in LongAmp Taq 2× master mix. The resulting pooled barcoded libraries were then end/nick repaired and dA tailed using the NEBNext End Repair/dA-tailing module. The DNA concentration in each library preparation step was measured using Qubit fluorometer based on Qubit dsDNA HS (High Sensitivity) assay (Invitrogen™). The product of each reaction step was washed with 70% EtOH with the help of magnetic separator. The prepared library was loaded into the MinION™ Flow Cells on a ONT MinION sequencing device (Oxford Nanopore Ltd., UK). V14 kit chemistry in combination with the new R10.4.1 nanopore was used to provide Q20+ (≥ 99%) raw read accuracy with high sequencing yield. FastQC version 0.11.9 (written by Simon Andrews of Babraham Bioinformatics) was subsequently run to do control checks per base sequence quality data.
Genome sequence assembly
Genome sequence assembly was carried out according to the main steps summarized in Fig. S1A. Bacterial genome sequence datasets were initially assembled using Flye Assembler Version 2.9, a de novo assembler for single-molecule sequencing reads [31, 56]. The Flye assemblies were subsequently polished with one-round Medaka (https://nanoporetech.com) for error correction to prepare high-quality genome sequences (available online: https://github.com/nanoporetech/medaka). Quality parameters of the assembled HI03-3b genome sequence using QUAST [22] (Galaxy Version 5.2.0+galaxy1). The parameters were set up with the minimum IDY% considered as proper alignment of 95.0 and the lower threshold for a contig length (in bp) of 500. The GC% content and read count of HI03-3b genomic sequence were determined using RSeQC (v 2.6.4) [67]. Taxonomic distribution analysis was conducted using MyTaxa Scan result from MiGA to determine the degree of affiliation or novelty of sequences based on the genome-aggregate average amino acid identity [38, 52]. The order and direction of contigs generated after genome sequence assembly were determined based on blastn pairwise alignment [2] and subsequently verified by average nucleotide identity (ANI) analysis [29] on PROKSEE with CGView Server [58, 59] using the complete genome sequences of closely related taxa as the references.
Taxa novelty analysis
HI03-3b genomic sequence was subjected to MiGA (Microbial Genomes Atlas) [52] (version v1.0.0 — prima 14 April 2021) against all taxonomically classified taxa with available genome sequence data for determining its taxonomic classification (http://microbial-genomes.org/). This was based on average nucleotide identity and amino acid identity (ANI/AAI) concepts. ANI is a whole-genome similarity metric, which can facilitate high-resolution taxonomic analysis. In taxonomic studies, the standard for species demarcation is the 95% ANI cutoff [29]. HI03-3b genome sequence was compared to the genome sequences of closely related taxa using Mauve [13] (version snapshot 2015 February 25 build 0 (c) 2003–2015). Genome-to-Genome Distance Calculator (GGDC) 3.0 using a generalized linear model (GLM) [41] was run to confirm similarity level between the genome sequences of HI03-3b and the most closely related species. This analysis outcome was based on DNA-DNA hybridization (DDH) values to determine relatedness between bacterial species [21]. Phylogenetic analysis was conducted in MEGA X [32] based on the unweighted pair-group method with arithmetic mean (UPGMA) as the distance analysis method for constructing a tree [43].
Genome sequence annotation
The assembled HI03-3b genome was visualized using PROKSEE on the CGView Server [58, 59] and subsequently annotated with Prokka version 1.1.0 [54], allowing the prediction of the numbers of CDSs (coding sequences) as well as genes for tRNAs and rRNAs (5S, 16S and 23S). These were verified using tRNAscan-SE 2.0 [9, 37] and NCBI record (Ref. Seq.: NZ_JAKDDU000000000.1). To predict genomic islands, the entire HI03-3b genome sequence was aligned against the complete genome sequence of the closely related taxon Cytobacillus oceanisediminis YPW-V2 [Accession Number: CP015506.1] using IslandViewer 4 [5] with the default parameters described in this link: www.pathogenomics.sfu.ca/islandviewer/about/. To predict HI03-3b primary metabolisms, all CDSs resulted from GeneMarkS analysis [6] were analysed using KofamKOALA [3] against KOfam, a customized HMM database of KEGG Orthologs (KOs) [3] combined with BLASTx analysis [2]. Carbohydrate-active enzyme (CAZy) database [8] was used as the reference to identify genes encoding carbohydrate active enzymes on HI03-3b genome sequence. By referring to gene position on contigs based on GeneMarkS analysis [6], biotechnologically potential genes were annotated on the circular HI03-3b genome map using PROKSEE on the CGView Server [58, 59]. Further analysis using AntiSMASH version 6.0 [7] was performed to identify natural product biosynthetic gene clusters (BGCs).
Results
Polysaccharide-degrading epibiont with antimicrobial activity
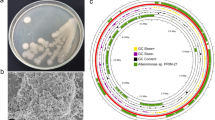
In search for biotechnologically potential genes from marine sources, we screened bacterial isolates from the surface of the Indonesian brown algae Hydroclathrus sp. (Fig. 1A). This led to the isolation of a polysaccharide-degrading bacterium, designated as HI03-3b, as indicated by the presence of a clearing zone around its colonies after staining with Lugol’s iodine solution and CaCl2 solution (10%) (Fig. 1B and C). Interestingly, the cell-free culture of this algae-associated epibiont exhibited inhibition against S. aureus (Fig. 1D), indicating its potential ability to produce antimicrobial protein or enzyme extracellularly. This subsequently encouraged us to sequence the genome of HI03-3b to identify useful genes that might encode proteins or enzymes with potential antimicrobial properties.
Genome sequence and taxa novelty analyses
The FastQC analysis (written by Simon Andrews of Babraham Bioinformatics) indicated that the resulting HI03-3b sequencing data contained 1,097,444 sequence reads with the sequence length range of 139 to 14972 bp. All of these sequence reads were assembled into a circular genome of 4,860,704 bp with 42.02 mol% G + C content (Fig. S1B and C). The assembled HI03-3b genome sequence consisted of 11 contigs with the largest size of 2,127,197 bp. The assembly quality was checked using QUAST [22], showing good quality indicated by low values of L50 and L75. Taxonomic distribution analysis based on MyTaxa scan result from MiGA [52] showed the majority of light blue colour (Fig. S2), indicating the high quality of H03-3b genome sequence with minor contamination.
Blastn search of the entire 16S rRNA gene sequence of HI03-3b showed homology with those from the family Bacillaceae. Further phylogenetic analysis of the HI03-3b’s 16S rDNA sequence with those from some representative genera within Bacillaceae (Bacillus, Cytobacillus, Mesobacillus, Neobacillus, and Peribacillus) showed that HI03-3b was most closely related to Cytobacillus especially within the C. firmus clade (Fig. 2). Based on this outcome, we performed genome sequence multiple alignment between HI03-3b and three closely related species (C. firmus NCTC10335, C. oceanisedirmins YPW-V2, and C. oceanisedirmins 2691) using Mauve [13]. It was found some differences in genomic composition among them, as visualized in Fig. S3.
The phylogenetic analysis of HI03-3b 16S-rRNA gene sequence using the UPGMA method [43]. The bootstrap consensus tree inferred from 1000 replicates represents the evolutionary history of the taxa analyzed [19]. The evolutionary distances were calculated according to the maximum composite likelihood method [62]. Evolutionary analyses were performed in MEGA X [32], which involved 45 16S-rRNA gene sequences from representative members of some genera within Bacillaceae family. There were a total of 1593 positions in the final dataset. The phylogenetic tree covers representative members of some genera within Bacillaceae family (Bacillus, Cytobacillus, Mesobacillus, Neobacillus, and Peribacillus). Neobacillus clade is as follows: 1, N. bataviensis NBRC 102449; 2, N. drentensis IDA1967; 3, N. novalis NBRC 102450; 4, N. niacini NBRC 15566; and 5, N. pocheonensis Gsoil 420. Mesobacillus clade is as follows: 1, M. foraminis CV53; 2, M. zeae JJ-247; 3, M. campisalis SA2-6; 4, M. stamsii BoGlc83; 5, M. thioparans BMP-1; 6, M. boroniphilus T-15Z; 7, M. subterraneus COOI3B; and 8, M. jeotgali YKJ-10. Peribacillus clade is as follows: 1, P. kribbensis BT080; 2, P. cavernae L5; 3, P. asahii A001; 4, P. psychrosaccharolyticus 23296; 5, P. muralis LMG 20238; 6, P. frigoritolerans DSM 8801; 7, P. implex NBRC 15720; and P. simplex LMG 11160. Bacillus clade is as follows: 1, B. aerophilus 28K; 2, B. stratosphericus 1KF2a; 3, B. licheniformis DSM 13; 4, B. licheniformis ATCC 14580; 5, B. velezensis FZB42; 6, B. vallismortis DSM 11031; 7, B. subtilis IAM 12118; 8, B. inaquosorum BGSC 3A28; 9, B. halotolerans DSM 8802; and 10, B. niacini IFO15566
Furthermore, based on MiGA [52], HI03-3b genome sequence showed the most similar ANI (average nucleotide identity) score of 94.1 % and AAI (average amino acid identity) score of 95 % to those of C. firmus NCTC10335 (GenBank assembly accession: GCA900445365). The next top hits were Sporosarcina globispora DSM 4 [GenBank assembly accession: GCA001274725.1] and C. oceanisediminis CGMCC 1.10115 [GCA007830235] with the AAI/ANI scores of 88.1%/86.7 and 82.81%/81.46%, respectively. To determine the order and direction of the 11 contigs of HI03-3b genome sequence, we run Blastn [2] for pairwise DNA-DNA sequence comparison using the genome sequences of C. oceanisedirmins 2691 and C. firmus NCTC10335 as the subject sequences (Table S1). To validate the order and direction of HI03-3b contigs, we then carried out ANI analysis [29] on PROKSEE with CGView Server [58, 59] using C. oceanisedirmins 2691 and C. firmus NCTC10335 as the references, respectively (Fig. 3).
Pairwise genome sequence comparison to determine the order and direction of HI03-3b contigs. A HI03-3b contigs were mapped onto with the genome sequence of C. firmus NCTC10335 (Acc. Nu. NZ_UFTC01000001.1) with the ANI score of 94.15 %. B HI03-3b contigs were compared with the complete genome sequence of C. oceanisediminis 2691 (NCBI Acc. Nu. GCA_000294775.2) with the ANI score of 88.6 %
Finally, we run Genome-to-Genome Distance Calculator (GGDC) 3.0 [41] to confirm the similarity level of the genome sequences between HI03-3b and C. firmus NCTC10335 as the closest taxa. The GGDC analysis revealed a recommended a DNA-DNA hybridization (DDH) of 57.60 % to C. firmus NCTC10335. This value is lower than the new species threshold (DDH > 70.0% for the same species and DDH > 79.0% for the same subspecies). The difference in % G+C between the two genome sequences was 0.30. The genome comparative and phylogenetic studies suggest that HI03-3b is a novel species within Cytobacillus, and therefore, we propose the name as Cytobacillus wakatobiense HI03-3b (HI refers to Hoga Island, the place where it was derived from).
Genome properties and primary metabolisms
By referring to Blastn comparison and ANI analysis in Fig. 3, the 11 contigs of the assembled HI03-3b genome sequence were visualized in the right order and direction using PROKSEE on the CGView Server [58, 59] with Prokka annotation [54]. The results showed HI03-3b genomic features, such as open reading frames (ORFs), CDSs (coding sequences), 94 genes for tRNAs, and 32 genes for rRNAs (5S, 16S, and 23S) (Fig. 4A). The sequenced HI03-3b genome harboured 5655 ORFs based on GeneMark.hmm PROKARYOTIC Version 3.26 analysis [6] and 4409 CDSs based on the NCBI record (Table S2). Furthermore, tRNAscan-SE 2.0 [9, 37]. showed 24 types of tRNAs encoded on HI03-3b genome, which were dominated by tRNAs for Gly, Glu, and Leu (Table S3). Based on KofamKOALA [3], 12 of 32 rRNA genes belong to 16S rRNA genes (Table S4). By aligning with the reference C. oceanisediminis YPW-V2 on IslandViewer 4 [5], we found 13 genomic islands in HI03-3b, including polysaccharide biosynthesis gene cluster, based on the integrated methods of SIGI-HMM [66] and IslandPath-DIMOB [28] (Fig. S4B). However, no pathogenic islands/genes were detected within the HI03-3b genome [4].
Visualization of HI03-3b genome features. A Identification of CDSs, rRNA genes, and tRNA genes (indicated by blue, purple, and green colours) using PROKSEE on the CGView Server [58, 59] with Prokka annotation [54]. The order and direction of contigs generated after genome sequence assembly were determined based on blastn pairwise alignment [2] using the genome sequences of C. oceanisediminis 2691 and C. firmus NCTC10335 as the references. B Prediction of genomic islands using IslandViewer 4 based on three different methods (indicated by red, orange, and blue colours) [5] with the genome sequence of C. oceanisediminis YPW-V2 as the reference. Note: CDS, coding sequence; GI, genomic island
The sequences of all ORFs or total genes resulted from GeneMarkS analysis [6] were analysed further using KofamKOALA [3] against KOfam, a customized HMM database of KEGG Orthologs (KOs) to predict HI03-3b primary metabolisms. The results showed the presence of genes encoding enzymes involved in carbohydrate metabolisms, such as glycolysis, gluconeogenesis, citrate cycle, pentose phosphate pathway, Entner-Doudoroff pathway, and glycogen degradation [3] (Table S5 for the detail). Other metabolisms identified in this genome based on KofamKOALA [3] include carbon fixation, sulphur metabolism, fatty acid metabolism, purine metabolism, metabolisms of amino acids, shikimate pathway, lipopolysaccharide metabolism, polyamine biosynthesis, and cofactor and vitamin metabolism (Tables S6–S12 for the detail) [3].
Genes encoding enzymes with antimicrobial potential
Based on the carbohydrate-active enzyme (CAZy) database [8], it was found that the HI03-3B genome harbours some genes encoding carbohydrate active enzymes (Table S13), including glycoside hydrolases (GHs) and glycosyltransferases (GTs). Some of these genes encoding enzymes that might be related to the degradation of polysaccharides, such as putative type 1 pullulanase (GH13 family), chitinase (GH18 family), and α-glucosidase (GH65 family) based on KEGG Orthology search [3] (Table 1). KEGG Orthology search [3] combined with BLASTx analysis [2] also showed the presence of other genes encoding biotechnologically potential enzymes, such as arginase, cyanophycinase, protease, bacillolysin, xylose isomerase, and alcohol dehydrogenase [35] (Table 1 and see also Table S14 with notes describing their potential applications). Using PROKSEE on the CGView Server [58, 59], we annotated some biotechnologically potential genes on the circular HI03-3b genome map by referring to their position on contigs from the outcome of GeneMarkS analysis [6] (Fig. 5A).
The position of biotechnologically potential genes on the HI03-3b genome map. A Certain loci encoding biotechnologically potential enzymes and secondary metabolite biosynthesis were determined based on PROKSEE analysis on the CGView Server [58, 59]. B Natural product BGCs were identified in the HI03-3b genome sequence based on antiSMASH analysis [7] supported by BLASTx [2], showing the presence of core and additional biosynthetic genes, transport-related genes, regulatory genes, and other genes. The core biosynthetic genes of RiPP-like terpene BGC (from left to right) are predicted to code for squalene-hopene cyclase, leader peptide (SagB-type dehydrogenase domain), YcaO cyclodehydratase, and thiazole-containing bacteriocin maturation protein. PKS type III BGC contains a chalcone synthase gene (indicated by red colour) as the core biosynthetic gene. The siderophore BGC harbours the core biosynthetic genes encoding lucA/lucC family proteins. Based on RiPPMiner prediction [1] the core lassopeptide BGC encodes a precursor peptide that consists of a leader (VKAPGSTGEGHWKLGNLSAEEKSGIPRVAVKCVEMWRNTSGE) and core peptide sequence (GDSLVCN). A crosslink may occur between serine (S) and cysteine (C) residues in the core peptide [1]. Note: PKS, polyketide synthase; BGC, biosynthetic gene cluster; RiPP, ribosomally synthesized and post-translationally modified peptides
Biosynthetic genes of secondary metabolites
We also found putative genes involved in secondary metabolite biosynthesis (Fig. 5), such as those coding for enzymes responsible for terpenoid backbone biosynthesis predicted using KEGG Orthology [3]. Further analysis using AntiSMASH version 6.0 [7] allowed us to identify the presence of a biosynthetic gene cluster (BGC) of ribosomally synthesized and post-translationally modified peptides (RiPP)-like terpene in the contig 12 of HI03-3b genome (Fig. 5, Fig. S5 for the detail), which was verified by Bagel4 analysis [15]. Other BGCs identified in this sequenced genome code for polyketide ketosynthase (PKS) type 3 in the contig 13 (Fig. 5, Fig. S6 for the detail), siderophore/petrobactin BGC (Fig. 5, Fig. S7), and a lassopeptide BGC in the contig 8 (Fig. S8). Further research is required to isolate secondary metabolites and enzymes from this HI03-3b strain in order to test them against biofilm-forming pathogens.
Genome sequence data and strain availability
We deposited our HI03-3b genome sequence in the GenBank database, which was publicly available under the accession number JAKDDU000000000.1 (BioProject ID: PRJNA785558, and BioSample: SAMN23566444). Some of the biotechnologically potential genes in HI03-3b have been annotated by GenBank with the following accession numbers: WP_248347211.1 (chitinase/glycosyl hydrolase family 18 protein), WP_248347527.1 (bacillolysin/M4 family metallopeptidase), WP_248347905.1 (serine protease/S8 family peptidase), WP_248348545.1 (L-asparaginase), WP_248348709.1 (pullulanase), WP_248348651.1 (subtilisin/S8 family serine peptidase), WP_248349705.1 (L-rhamnose isomerase), and WP_248349198.1 (nucleoside hydrolase). This HI03-3b strain was registered in National Center for Biotechnology Information (NCBI) with the taxonomy ID 2862822. Authors maintain this HI03-3b strain at the Microbiology Laboratory of Universitas Muhammadiyah Semarang and at National Research and Innovation Agency (BRIN), Indonesia.
Discussion
We had isolated a biotechnologically potential bacterial epibiont, designated as HI03-3b, from the surface of the Indonesian brown algae Hydroclathrus sp. The ability of this epibiont to degrade complex polysaccharide and to inhibit a pathogenic gram-positive bacterium had motivated us to sequence the whole genome. HI03-3b genome sequence showed the highest AAI and ANI scores to C. firmus NCTC10335, which was supported by 16S rRNA gene phylogenetic analysis. Further genome comparison between HI03-3b and three closely related Cytobacillus species, including NCTC10335 strain, suggested some differences in genomic composition among them. Further GGDC analysis indicated that a recommended DNA-DNA hybridization (DDH) between the two strains is lower than the new species threshold (DDH > 70.0% for the same species). The genome features, such as the numbers of protein-coding genes (CDSs), tRNAs, and rRNAs, were different between HI03-3b and C. firmus NCTC10335 (Table S2). Taken all together, we propose HI03-3b as a novel species within Cytobacillus genus, and therefore, we named it Cytobacillus wakatobiense HI03-3b.
The genome sequence analysis provided insights into metabolic pathways, which helped us to understand the ecological role and biotechnological importance of HI03-3b. For example, some genes identified in this HI03-3b genome are involved in the biosynthesis of vitamins, such as thiamine (B1), riboflavin (B2), pantothenate (B5), pyridoxine (B6), biotin (B7), lipoic acid, cobalamin (B12), and menaquinone (K2) (see Table S11). This indicates that HI03-3b may play a crucial ecological role in providing the algal host with vitamins for growth and development [16]. From biotechnological perspective, the presence of these vitamin biosynthetic pathways suggests that HI03-3b could be harnessed as a natural alternative for industrial vitamin production.
We also found the presence of genes involved in polyamine biosynthesis in HI03-3b, which leads to spermidine formation (see Fig. S4 for spermidine biosynthetic pathway). The human gut bacteria Bacteroides thetaiotaomicron and Fusobacterium varium represent other bacterial species reported to synthesize spermidine both in vivo and in vitro [49]. From medical perspective, spermidine is known as a bioactive metabolite that can extend life span in model organisms, suggesting its potential application in delaying ageing and promoting longevity in human [39]. Spermidine has particularly been shown to increase epithelial renewal and anti-inflammatory macrophage development in the colon, highlighting its importance in the maintenance of intestinal homoeostasis and immunity [46]. From ecological point of view, spermidine was found essential for robust biofilm formation in Bacillus subtilis [25]. In addition to spermidine, TatD DNase can contribute to biofilm formation, as has recently shown in the biofilm formation of the bacterium Trueperella pyogenes [69]. Interestingly, TatD DNase-encoding gene was found in HI03-3b genome. Taken together, we propose that HI03-b may rely on molecules such as spermidine and TatD DNase to promote its colonization and biofilm formation on the macroalgal surface.
Among 4409 protein-coding sequences identified in this strain, some genes encode enzymes for polysaccharide and protein degradation known to exhibit anti-biofilm properties against pathogenic bacteria [57]. Notable examples are protease [50], chitinase [12], a mixture of bacillolysin and subtilisin (Protamex®) [20, 48], L-asparaginase [64, 65], and pullulanase [51] (Table 1). From the ecological point of view, the presence of these genes suggests that HI03-3b may produce antimicrobial or anti-biofilm enzymes in competing for nutrient and space against other epibionts as well as in protecting algal host from predation [17, 18]. This was supported by our finding that the extracellular cell-free supernatant of HI03-3b exhibited antimicrobial activity against the bacterial pathogen S. aureus.
Other biotechnologically potential genes identified in this HI03-3b include those encoding arginase, xylose isomerase, carboxylesterases, phospholipases, and arginine decarboxylase (see Table S14). Arginase for example has been used for the environmentally friendly preparation of L-ornithine as food supplement and nutrition product [34]. Xylose isomerase has widely been used in the production of high-fructose corn syrup (HFCS) and bioethanol [47]. It is potentially explored to produce some value-added chemicals in the food, cosmetics, and pharmaceutical industries [44]. Carboxylesterases (CEs) have been applied in xenobiotic and endobiotic degradations, biocatalysis, and drug metabolism [68]. Phospholipases have been used in scientific and medical research, such as inhibitors for generating anti-inflammatory agents and as diagnostic markers for microbial infections [30, 61]. Arginine decarboxylase catalyses conversion of L-arginine into agmatine, a valuable pharmaceutical intermediate with various potential therapeutic functions in neurotransmitter systems, nitric oxide synthesis, and polyamine metabolism [60].
HI03-3b genome harbors a RiPP-like terpene BGC predicted to encode the biosynthesis of thiazole-containing heterocyclic bacteriocin as a new subfamily of ribosomally synthesized peptides antimicrobial peptides (AMPs) ([23, 33]). This RiPP-like terpene BGC is characterized by the presence of genes encoding proteins involved in heterocycloanthracin biosynthesis, such as toxin precursor, SagB-type dehydrogenase domain (nitroreductase family), serine protease, YcaO cyclodehydratase, thiazole-containing bacteriocin maturation protein, and a transport protein [23] (Fig. S5). Bacteriocins that belong to heterocycloanthracin family are known as antimicrobial and antibiofilm agents [53], best exemplified by sonorensin that exhibited antibiofilm activity against S. aureus and food bio-preservative potential [10, 11]. It was reported that bacteriocins of heterocycloanthracin family are initially synthesized as biologically inactive peptides (protoxins or precursors) with an N-terminal leader peptide. This protoxin subsequently undergoes enzymatic modifications that involve the cleavage of the leader peptide by protease and the formation of a thiazole or oxazole ring by cyclodehydratase and dehydrogenase. The resulting mature toxin is then exported by a transport protein [23, 33]. Validation of the function of this RiPP-like terpene BGC in HI03-3b through gene mutagenesis or heterologous expression and subsequent identification of the bacteriocin produced are necessary to explore the biotechnological potential as an antibiofilm and food preservative agent.
We compared the presence of some biotechnologically potential genes between Cytobacillus HI03-3b and the most closely related strain C. firmus NCTC10335 (Table S15). It was found that several genes encoding carbohydrate active enzymes in HI03-3b, such as β-glucosylceramidase [EC:3.2.1.45], xylose isomerase [EC:5.3.1.5], L-rhamnose isomerase [EC:5.3.1.14], chitinase [EC:3.2.1.14], and arginase [EC:3.5.3.1], were absent in NCTC10335. In contrast, α-amylase [EC:3.2.1.1] identified in NCTC10335 was not found in HI03-3b. Another difference is that HI03-3b contained lassopeptide BGC, while NCTC10335 harboured lanthipeptide class 2. Some potential genes present in both strains occur in different copy numbers (Table S15), such as those encoding oligo-1,6-glucosidase [EC:3.2.1.10], α-glucosidase [EC:3.2.1.20], zinc protease [EC:3.4.24.-], serine protease [EC:3.4.21.-], bacillolysin [EC:3.4.24.28], beta-lactamase class A [EC:3.5.2.6], and acetylornithine deacetylase [EC:3.5.1.16]. The difference in genome features and gene composition between Cytobacillus HI03-3b and its closely related strain strongly suggests the uniqueness of HI03-3b that would enable it to adapt to the algal surface environment. This was supported by the finding of 13 genomic islands in HI03-3b that possibly occur through horizontal gene transfer, which may provide this epibiont with adaptive traits to live and survive on the surface of brown algae [4].
Conclusion
The whole-genome sequence analysis of the brown algae-associated epibiont Cytobacillus sp. HI03-3b showed the presence of biotechnologically potential genes, including those encoding commercially useful enzymes, such as chitinase, pullulanase, protease, bacillolysin, subtilisin, and L-asparaginase, as well as biosynthetic genes for secondary metabolites and vitamins. These might ecologically be important as strategies of the epibiont to outcompete with other bacteria during biofilm formation, protect its algal host from pathogenic infection, and provide the host with necessary vitamins. Some of such metabolic genes could potentially be exploited as a basis for the development of anti-infective agents against pathogenic biofilms. Further functional studies by gene heterologous expression, and mutagenesis or enzyme activity assays, are necessary to validate the function of these genes.
Availability of data and materials
We deposited our HI03-3b genome sequence in the GenBank database, which was publicly available under the accession number JAKDDU000000000.1 (BioProject ID: PRJNA785558 and BioSample: SAMN23566444). Some of the biotechnologically potential genes in HI03-3b have been annotated by GenBank with the following accession numbers: WP_248347211.1 (chitinase/glycosyl hydrolase family 18 protein), WP_248347527.1 (bacillolysin/M4 family metallopeptidase), WP_248347905.1 (serine protease/S8 family peptidase), WP_248348545.1 (L-asparaginase), WP_248348709.1 (pullulanase), and WP_248348651.1 (subtilisin/S8 family serine peptidase). This HI03-3b strain was registered in National Center for Biotechnology Information (NCBI) with the taxonomy ID 2862822. Authors maintain this HI03-3b strain at the Microbiology Laboratory of Universitas Muhammadiyah Semarang and at National Research and Innovation Agency (BRIN), Indonesia.
Abbreviations
- ORF:
-
Open reading frame
- CDS:
-
Coding sequence
- MS:
-
Minimal seawater
- ANI:
-
Average nucleotide identity
- AAI:
-
Average amino acid identity
- CAZy:
-
Carbohydrate-active enzyme
- DDH:
-
DNA-DNA hybridization
- BGC:
-
Biosynthetic gene cluster
- NP:
-
Natural products
References
Agrawal P, Khater S, Gupta M, Sain N, Mohanty D (2017) RiPPMiner: a bioinformatics resource for deciphering chemical structures of RiPPs based on prediction of cleavage and cross-links. Nucleic Acids Res 45(W1): W80-W88. https://doi.org/10.1093/nar/gkx408
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Aramaki T, Blanc-Mathieu R, Endo H, Ohkubo K, Kanehisa M, Goto S, Ogata H (2020) KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 36:2251–2252. https://doi.org/10.1093/bioinformatics/btz859
Beatriz, Fernández-Gómez Antonio, Fernàndez-Guerra Emilio O, Casamayor José M, González Carlos, Pedrós-Alió Silvia G, Acinas (2012) Patterns and architecture of genomic islands in marine bacteria. BMC Genomics 13(1) 347. https://doi.org/10.1186/1471-2164-13-347
Bertelli C, Laird MR, Williams KP, Lau BY, Hoad G, Winsor GL, Brinkman FS (2017) IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res 45:W30–W35. https://doi.org/10.1093/nar/gkx343
Besemer J, Lomsadze A, Borodovsky M (2001) GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res 29:2607–2618. https://doi.org/10.1093/nar/29.12.2607
Blin K, Shaw S, Kloosterman AM, Charlop-Powers Z, van Wezel GP, Medema MH, Weber T (2021) antiSMASH 6.0: improving cluster detection and comparison capabilities. Nucleic Acids Res 49:W29–W35. https://doi.org/10.1093/nar/gkab335
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37:D233–D238. https://doi.org/10.1093/nar/gkn663
Chan PP, Lin BY, Mak AJ, Lowe TM (2021) tRNAscan-SE 2.0: improved detection and functional classification of transfer RNA genes. Nucleic Acids Res 49:9077–9096. https://doi.org/10.1093/nar/gkab688
Chopra L, Singh G, Choudhary V, Sahoo DK (2014) Sonorensin: an antimicrobial peptide, belonging to the heterocycloanthracin subfamily of bacteriocins, from a new marine isolate, Bacillus sonorensis MT93. Appl Environ Microbiol 80:2981–2990. https://doi.org/10.1128/AEM.04259-13
Chopra L, Singh G, Kumar Jena K, Sahoo DK (2015) Sonorensin: a new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci Rep 5:13412. https://doi.org/10.1038/srep13412
Chung M-C, Dean S, Marakasova ES, Nwabueze AO, van Hoek ML (2014) Chitinases are negative regulators of Francisella novicida biofilms. PLoS One 9:e93119. https://doi.org/10.1371/journal.pone.0093119
Darling ACE, Mau B, Blattner FR, Perna NT (2004) Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res 14:1394–1403. https://doi.org/10.1101/gr.2289704
David MZ, Daum RS (2010) Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev 23:616–687. https://doi.org/10.1128/CMR.00081-09
de Jong A, van Hijum SAFT, Bijlsma JJE, Kok J, Kuipers OP (2006) BAGEL: a web-based bacteriocin genome mining tool. Nucleic Acids Res 34:W273–W279. https://doi.org/10.1093/nar/gkl237
Droop MR (2007) Vitamins, phytoplankton and bacteria: symbiosis or scavenging? J Plankton Res 29:107–113. https://doi.org/10.1093/plankt/fbm009
Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T (2013) The seaweed holobiont: understanding seaweed–bacteria interactions. FEMS Microbiol Rev 37:462–476. https://doi.org/10.1111/1574-6976.12011
Egan S, Thomas T, Kjelleberg S (2008) Unlocking the diversity and biotechnological potential of marine surface associated microbial communities. Curr Opin Microbiol Ecol Indust Microbiol Tech 11:219–225. https://doi.org/10.1016/j.mib.2008.04.001
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
Fernandes P (2016) Enzymes in Fish and Seafood Processing. Front Bioeng Biotechnol 4:59. https://doi.org/10.3389/fbioe.2016.00059
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM (2007) DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91. https://doi.org/10.1099/ijs.0.64483-0
Gurevich A, Saveliev V, Vyahhi N, Tesler G (2013) QUAST: quality assessment tool for genome assemblies. Bioinformatics 29:1072–1075. https://doi.org/10.1093/bioinformatics/btt086
Haft DH (2009) A strain-variable bacteriocin in Bacillus anthracis and Bacillus cereus with repeated Cys-Xaa-Xaa motifs. Biol Direct 4:15. https://doi.org/10.1186/1745-6150-4-15
Hafting JT, Craigie JS, Stengel DB, Loureiro RR, Buschmann AH, Yarish C, Edwards MD, Critchley AT (2015) Prospects and challenges for industrial production of seaweed bioactives. J Phycol 51:821–837. https://doi.org/10.1111/jpy.12326
Hobley L, Li B, Wood JL, Kim SH, Naidoo J, Ferreira AS, Khomutov M, Khomutov A, Stanley-Wall NR, Michael AJ (2017) Spermidine promotes Bacillus subtilis biofilm formation by activating expression of the matrix regulator slrR. J Biol Chem 292:12041–12053. https://doi.org/10.1074/jbc.M117.789644
Holmström C, Egan S, Franks A, McCloy S, Kjelleberg S (2002) Antifouling activities expressed by marine surface associated Pseudoalteromonas species. FEMS Microbiol Ecol 41:47–58. https://doi.org/10.1111/j.1574-6941.2002.tb00965.x
Holmström C, Kjelleberg S (1999) Marine Pseudoalteromonas species are associated with higher organisms and produce biologically active extracellular agents. FEMS Microbiol Ecol 30:285–293. https://doi.org/10.1111/j.1574-6941.1999.tb00656.x
Hsiao W, Wan I, Jones SJ, Brinkman FSL (2003) IslandPath: aiding detection of genomic islands in prokaryotes. Bioinformatics 19:418–420. https://doi.org/10.1093/bioinformatics/btg004
Jain C, Rodriguez-R LM, Phillippy AM, Konstantinidis KT, Aluru S (2018) High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat Commun 9:5114. https://doi.org/10.1038/s41467-018-07641-9
Karabina S-A, Gora S, Atout R, Ninio E (2010) Extracellular phospholipases in atherosclerosis. Biochimie, Phospholipases A2 Lipid Mediators 92:594–600. https://doi.org/10.1016/j.biochi.2010.02.002
Kolmogorov M, Yuan J, Lin Y, Pevzner PA (2019) Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 37:540–546. https://doi.org/10.1038/s41587-019-0072-8
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lee SW, Mitchell DA, Markley AL, Hensler ME, Gonzalez D, Wohlrab A, Dorrestein PC, Nizet V, Dixon JE (2008) Discovery of a widely distributed toxin biosynthetic gene cluster. Proc Natl Acad Sci U S A 105:5879–5884. https://doi.org/10.1073/pnas.0801338105
Li M, Qin J, Xiong K, Jiang B, Zhang T (2021) Review of arginase as a promising biocatalyst: characteristics, preparation, applications and future challenges. Crit Rev Biotechnol 0:1–17. https://doi.org/10.1080/07388551.2021.1947962
Liu X, Kokare C (2017) Chapter 11 - Microbial enzymes of use in industry. In: Brahmachari G (ed) Biotechnology of Microbial Enzymes. Academic Press (Elsevier), London, UK, pp 267–298. https://doi.org/10.1016/B978-0-12-803725-6.00011-X
López-Hortas L, Flórez-Fernández N, Torres MD, Ferreira-Anta T, Casas MP, Balboa EM, Falqué E, Domínguez H (2021) Applying seaweed compounds in cosmetics, cosmeceuticals and nutricosmetics. Mar Drugs 19:552. https://doi.org/10.3390/md19100552
Lowe TM, Chan PP (2016) tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res 44:W54–W57. https://doi.org/10.1093/nar/gkw413
Luo C, Rodriguez-R LM, Konstantinidis KT (2014) MyTaxa: an advanced taxonomic classifier for genomic and metagenomic sequences. Nucleic Acids Res 42:e73. https://doi.org/10.1093/nar/gku169
Madeo F, Carmona-Gutierrez D, Kepp O, Kroemer G (2018) Spermidine delays aging in humans. Aging (Albany NY) 10:2209–2211. https://doi.org/10.18632/aging.101517
Martin M, Portetelle D, Michel G, Vandenbol M (2014) Microorganisms living on macroalgae: diversity, interactions, and biotechnological applications. Appl Microbiol Biotechnol 98:2917–2935. https://doi.org/10.1007/s00253-014-5557-2
Meier-Kolthoff JP, Carbasse JS, Peinado-Olarte RL, Göker M (2022) TYGS and LPSN: a database tandem for fast and reliable genome-based classification and nomenclature of prokaryotes. Nucleic Acids Res 50:D801–D807. https://doi.org/10.1093/nar/gkab902
Menaa F, Wijesinghe PAUI, Thiripuranathar G, Uzair B, Iqbal H, Khan BA, Menaa B (2020) Ecological and industrial implications of dynamic seaweed-associated microbiota interactions. Mar Drugs 18:641. https://doi.org/10.3390/md18120641
Michener CD, Sokal RR (1957) A quantitative approach to a problem in classification. Evolution 11:130–162. https://doi.org/10.1111/j.1558-5646.1957.tb02884.x
Miyamoto RY, de Melo RR, de Mesquita Sampaio IL, de Sousa AS, Morais ER, Sargo CR, Zanphorlin LM (2021) Paradigm shift in xylose isomerase usage: a novel scenario with distinct applications. Crit Rev Biotechnol:1–20. https://doi.org/10.1080/07388551.2021.1962241
Mucke M, Taube R, Veltel S (2022) Purification of native enzymes from macroalgae. Wiley Analytical Science Magazine: Separation LC Electrophoresis. Wiley, Weinheim, Germany. https://analyticalscience.wiley.com/do/10.1002/was.0004000219
Nakamura A, Kurihara S, Takahashi D, Ohashi W, Nakamura Y, Kimura S, Onuki M, Kume A, Sasazawa Y, Furusawa Y, Obata Y, Fukuda S, Saiki S, Matsumoto M, Hase K (2021) Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat Commun 12:2105. https://doi.org/10.1038/s41467-021-22212-1
Nam KH (2022) Glucose isomerase: functions, structures, and applications. Appl Sci 12:428. https://doi.org/10.3390/app12010428
Nguyen HTM, Sylla KSB, Randriamahatody Z, Donnay-Moreno C, Moreau J, Tran LT et al (2011) Enzymatic hydrolysis of yellowfin tuna (Thunnus albacares) by-products using protamex protease. Food Technol Biotechnol 49:48–55
Noack J, Dongowski G, Hartmann L, Blaut M (2000) The human gut bacteria Bacteroides thetaiotaomicron and Fusobacterium varium produce putrescine and spermidine in cecum of pectin-fed gnotobiotic rats. J Nutr 130:1225–1231. https://doi.org/10.1093/jn/130.5.1225
Park J-H, Lee J-H, Cho MH, Herzberg M, Lee J (2012) Acceleration of protease effect on Staphylococcus aureus biofilm dispersal. FEMS Microbiol Lett 335:31–38. https://doi.org/10.1111/j.1574-6968.2012.02635.x
Petruzzi B, Briggs RE, Swords WE, De Castro C, Molinaro A, Inzana TJ (2017) Capsular polysaccharide interferes with biofilm formation by Pasteurella multocida serogroup A. mBio 8:e01843–e01817. https://doi.org/10.1128/mBio.01843-17
Rodriguez-R LM, Gunturu S, Harvey WT, Rosselló-Mora R, Tiedje JM, Cole JR, Konstantinidis KT (2018) The Microbial Genomes Atlas (MiGA) webserver: taxonomic and gene diversity analysis of Archaea and Bacteria at the whole genome level. Nucleic Acids Res 46:W282–W288. https://doi.org/10.1093/nar/gky467
Santos VL, Nardi Drummond RM, Dias-Souza MV (2017) 16 - Bacteriocins as antimicrobial and antibiofilm agents. In: Thomaz-Soccol V, Pandey A, Resende RR (eds) Current Developments in Biotechnology and Bioengineering. Elsevier, Amsterdam, pp 403–436. https://doi.org/10.1016/B978-0-444-63660-7.00016-4
Seemann T (2014) Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. https://doi.org/10.1093/bioinformatics/btu153
Sekiguchi J, Matsumiya M, Mochizuki A (1995) Distribution of chitinolytic enzymes in seaweeds. Fish Sci 61:876–881. https://doi.org/10.2331/fishsci.61.876
Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM (2015) BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 31:3210–3212. https://doi.org/10.1093/bioinformatics/btv351
Stiefel P, Mauerhofer S, Schneider J, Maniura-Weber K, Rosenberg U, Ren Q (2016) Enzymes enhance biofilm removal efficiency of cleaners. Antimicrob Agents Chemother. https://doi.org/10.1128/AAC.00400-16
Stothard P, Grant JR, Van Domselaar G (2017) Visualizing and comparing circular genomes using the CGView family of tools. Brief Bioinform 20:1576–1582. https://doi.org/10.1093/bib/bbx081
Stothard P, Wishart DS (2005) Circular genome visualization and exploration using CGView. Bioinformatics 21:537–539. https://doi.org/10.1093/bioinformatics/bti054
Sun A, Song W, Qiao W, Chen X, Liu J, Luo Q, Liu L (2017) Efficient agmatine production using an arginine decarboxylase with substrate-specific activity. J Chem Technol Biotechnol 92:2383–2391. https://doi.org/10.1002/jctb.5245
Sutto-Ortiz P, Camacho-Ruiz M d l A, Kirchmayr MR, Camacho-Ruiz RM, Mateos-Díaz JC, Noiriel A, Carrière F, Abousalham A, Rodríguez JA (2017) Screening of phospholipase A activity and its production by new actinomycete strains cultivated by solid-state fermentation. PeerJ 5:e3524. https://doi.org/10.7717/peerj.3524
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci 101:11030–11035. https://doi.org/10.1073/pnas.0404206101
Treangen TJ, Maybank RA, Enke S, Friss MB, Diviak LF, Karaolis DKR, Koren S, Ondov B, Phillippy AM, Bergman NH, Rosovitz MJ (2014) Complete genome sequence of the quality control strain Staphylococcus aureus subsp. aureus ATCC 25923. Genome Announc 2:e01110–e01114. https://doi.org/10.1128/genomeA.01110-14
Vimal A, Kumar A (2021) Antimicrobial potency evaluation of free and immobilized l-asparaginase using chitosan nanoparticles. J Drug Deliv Sci Technol 61:102231. https://doi.org/10.1016/j.jddst.2020.102231
Vimal A, Kumar A (2022) l-asparaginase: need for an expedition from an enzymatic molecule to antimicrobial drug. Int J Pept Res Ther 28:9. https://doi.org/10.1007/s10989-021-10312-x
Waack S, Keller O, Asper R, Brodag T, Damm C, Fricke WF, Surovcik K, Meinicke P, Merkl R (2006) Score-based prediction of genomic islands in prokaryotic genomes using hidden Markov models. BMC Bioinformatics 7:142. https://doi.org/10.1186/1471-2105-7-142
Wang L, Wang S, Li W (2012) RSeQC: quality control of RNA-seq experiments. Bioinformatics 28:2184–2185. https://doi.org/10.1093/bioinformatics/bts356
Wheelock CE, Shan G, Ottea J (2005) Overview of carboxylesterases and their role in the metabolism of insecticides. J Pestic Sci 30:75–83. https://doi.org/10.1584/jpestics.30.75
Zhang Z, Liang Y, Yu L, Chen M, Guo Y, Kang Z, Qu C, Tian C, Zhang D, Liu M (2021) TatD DNases contribute to biofilm formation and virulence in Trueperella pyogenes. Front Microbiol 12:758465. https://doi.org/10.3389/fmicb.2021.758465
Zilda DS, Yulianti Y, Sholihah RF, Subaryono S, Fawzya YN, Irianto HE (2019) A novel Bacillus sp. isolated from rotten seaweed: identification and characterization alginate lyase its produced. Biodivers J Biol Divers 20:1166–1172. https://doi.org/10.13057/biodiv/d200432
Acknowledgements
We acknowledge the Indonesian Ministry of Education, Cultural, Research and Technology (Kemendikbud Ristek) for funding this research project. This work was also supported by National Research and Innovation Agency (BRIN), Republic of Indonesia.
Funding
This work was supported by the Indonesian Ministry of Education, Cultural, Research and Technology (Kemendikbud Ristek) through Basic Science Research Program (PD, Penelitian Dasar) 2021 (Grant ID: 166/E4.l/AK.04.PT/2021) and 2022 (Grant ID: 027/061026/PG/SP2H/TD/2022).
Author information
Authors and Affiliations
Contributions
DSZ and SNE conceived and planned the experiments. SD and AS provided project supervision and discussion. DSZ and GP contributed to seaweed sample collection, isolation of bacterial strains, and culture preparation for whole-genome sequencing. SNE and OO contributed to the initial version of the manuscript draft. DSZ performed the bioactivity tests. MM and SSD contributed to the interpretation of the results. ARU and SNE analysed the genomic sequence data. ARU improved and finalized the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Visualization and quality assessment of whole H103-3b genome assembly. [A] Workflow of HI03-3b genome sequence assembly. [B] Quality parameters of the assembled HI03-3b genome sequence using Quast [22]. [C] GC content of the assembled HI03-3b genome sequence against density of reads using RSeQC [67]. Figure S2. Taxonomic distribution analysis based on MyTaxa Scan result from MiGA ([38, 52]) on the assembled HI03-3b genome sequence, showing that the high quality genome (the major light blue) with minor contamination. Figure S3. Genome comparison of HI03-3b with three closely related Cytobacillus strains using Mauve [13]. Each colored block represents a region of the HI03-3b genome sequence, which align with parts of other genomes. Blocks above the central line show forward orientation relative to the first genome sequence, while blocks below the central line indicate regions aligning in the reverse complement orientation. Inside each block, the height of the similarity profile correlates with the average level of conservation region of the genome. Figure S4. Genes involved in spermidine biosynthesis identified in the HI03-3b genome, which were predicted based on KofamKOALA [3] and BLASTx [2]. Notes: spermidine has been shown to increase epithelial renewal and anti-inflammatory macrophage development in the colon, highlighting its importance in the maintenance of intestinal homoeostasis and immunity [46]. From medical perspective, spermidine has been known to extend life span in model organisms, indicating its potential application in delaying aging and promoting longevity in human [39]. Figure S5. RiPP-like terpene BGC identified in the HI03-3b genome, which were predicted based on antiSMASH [7] and BLASTx [2]. Figure S6. PKS type III BGC identified in the HI03-3b genome, which were predicted based on antiSMASH [7] and BLASTx [2]. Figure S7. Siderophore/petrobactin BGC and lassopeptide BGC identified in the HI03-3b genome, which were predicted based on antiSMASH [7] and BLASTx [2]. Table S1. The order and direction of HI03-3b contigs were validated by comparing with the genome sequences of C. oceanisedirmins 2691 and C. firmus NCTC10335. Table S2. Comparation of genomic features between Cytobacillus HI03-3b (in this work) and C. firmus NCTC10335 (GenBank assembly accession number GCA_900445365.1). Table S3. Estimated numbers of tRNAs in HI03-3b genome sequence analyzed using tRNAscan-SE 2.0. ([9] [37];). Table S4. Numbers of 16S-rRNAs in HI03-3b genome sequence based on KofamKOALA [3]. Table S5. Predicted carbohydrate metabolisms in HI03-3b based on KofamKOALA [3]. Table S6. Energy metabolisms in HI03-3b based on KofamKOALA [3] analysis. Table S7. Lipid metabolisms in HI03-3b based on KofamKOALA [3] analysis. Table S8. Nucleotide metabolisms in HI03-3b based on KofamKOALA [3] analysis. Table S9. Predicted amino acid metabolisms in HI03-3b based on KofamKOALA [3]. Table S10. Predicted glycan metabolism in HI03-3b based on KofamKOALA [3]. Table S11. Carbon and vitamins metabolisms in HI03-3b based on KofamKOALA [3]. Table S12. Predicted terpenoid biosynthesis in HI03-3b based on KofamKOALA [3]. Table S13. Some genes encoding carbohydrate active enzymes. Table S14. Genes on HI03-3b genome encoding biotechnologically potential enzymes. Table S15. Comparation of biosynthetically potential genes between Cytobacillus HI03-3b and C. firmus NCTC10335.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ethica, S.N., Zilda, D.S., Oedjijono, O. et al. Biotechnologically potential genes in a polysaccharide-degrading epibiont of the Indonesian brown algae Hydroclathrus sp.. J Genet Eng Biotechnol 21, 18 (2023). https://doi.org/10.1186/s43141-023-00461-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43141-023-00461-5