Abstract
Background
Chronic lymphocytic leukemia (CLL) is the most common form of adult leukemia. This disease is genetically heterogeneous, and approximately 85% of patients with CLL harbor chromosomal aberrations that are considered effective prognostic biomarkers. The most frequent aberrations include deletions in 13q14, followed by trisomy 12, and deletions in 11q22.3 and 17p13 (TP53). Currently, fluorescence in situ hybridization (FISH) is the most widely used molecular cytogenetic technique to detect these aberrations. However, FISH is laborious, time-consuming, expensive, and has a low throughput. In contrast, multiplex ligation-dependent probe amplification (MLPA) is a reliable, cost-effective, and relatively rapid technique that can be used as a first-line screening tool and complement with FISH analysis. This study aimed to evaluate the contributions of MLPA as a routine standalone screening platform for recurrent chromosomal aberrations in CLL in comparison to other procedures. Thirty patients with CLL were screened for the most common genomic aberrations using MLPA with SALSA MLPA probemix P038-B1 CLL and FISH.
Results
In 24 of the 30 cases (80%), the MLPA and FISH results were concordant. Discordant results were attributed to a low percentage of mosaicism. Moreover, the MLPA probemix contains probes that target other genomic areas known to be linked to CLL in addition to those targeting common recurrent CLL aberrations.
Conclusions
The usage of MLPA as the first screening platform followed by FISH technique for only the negative cases is the most appropriate approach for CLL diagnosis and prognosis.
Similar content being viewed by others
Background
Chronic lymphocytic leukemia (CLL) is the most common form of adult leukemia in western countries, accounting for 30% of all leukemia cases. However, it is infrequent in the Eastern world. In Upper Egypt, CLL accounts for around 11.3% of all leukemia cases. This hematopoietic neoplasm arises from B-lymphocytes in the peripheral blood, bone marrow, and/or lymph nodes [1, 2]. Moreover, CLL is a genetically heterogeneous disease, and the clinical course may range from months to decades. Approximately 85% of CLL patients harbor chromosomal aberrations, which are considered effective prognostic biomarkers. The most frequent aberrations involve deletions in 13q14 (50–60%), which are associated with a good prognosis. The next most frequent aberration is trisomy 12 (12–25%), which is associated with intermediate prognosis, followed by 11q22.3 (ATM; 10–20%) and 17p13 (TP53; 5–10%) deletions, which are associated with a poor prognosis. These aberrations are important prognostic biomarkers for treatment decision-making [3].
Currently, fluorescence in situ hybridization (FISH) is the most widespread molecular cytogenetic technique used to detect genetic abnormalities in CLL [4]. However, FISH cannot detect small or intragenic deletions. Moreover, FISH is a laborious, time-consuming, expensive, and low-throughput procedure relative to other molecular genetic procedures used to detect common aberrations. Several other chromosomal aberrations in CLL have been detected using different techniques. However, these aberrations are not usually analyzed in clinical practice [5].
Multiplex ligation-dependent probe amplification (MLPA) was first introduced in 2002 [6]. This multiplex PCR technique can detect abnormal copy numbers in up to 50 different genomic DNA or RNA sequences and can differentiate sequences differing in only one nucleotide. Up to 96 samples can be tested simultaneously by MPLA, and the turn-around time is within 24 h. Consequently, MLPA has considerably increased the detection rates of various genetic disorders [7]. MLPA has also been applied successfully to the detection of copy number abnormalities in various malignant hematopoietic disorders, such as CLL [8]. MLPA is a reliable, cost-effective technique and is more rapid than FISH. Although MLPA cannot detect low-level mosaicism, it remains useful as a first-line screening tool and complement with FISH analysis [9]. The commercially available SALSA MLPA probemix P038 was designed specifically for CLL screening and permits the concurrent evaluation of various risk-linked genomic targets. This kit contains probes for 10q (PTEN), 11q (ATM, RDX, PPP2R1B, CADM1), chromosome 12, 13q14 (RB1, DLEU1/2/7, KCNRG, MIR15A), 14q, 17p (TP53), and chromosome 19.
This study aimed to evaluate the contributions of MLPA as a routine standalone screening platform for recurrent chromosomal aberrations in CLL in comparison to other procedures such as FISH.
Methods
This study was conducted at the National Research Centre, Egypt, and was approved by its Medical Ethical Committee. Informed written consent was obtained from the study participants. Thirty CLL patients (16 males, 14 females) were included in this study. The average age at the time of sampling was 65 years (range, 36–88 years). All participants attended the National Cancer Institute, Cairo University, Egypt. The diagnosis of CLL was established according to the World Health Organization classification of hematolymphoid tumors [10]. CLL was diagnosed by the presence of at least 5000 monoclonal B-lymphocytes/μl with a CLL immune phenotype in the peripheral blood (PB) for at least 3 months. Typically, CLL lymphocytes are small and mature-looking, with scanty cytoplasm and a dense nucleus containing partially aggregated chromatin. PB samples were collected on heparin to enable blood culture and on K2-EDTA in a vacutainer tube to allow DNA extraction.
FISH analysis
Peripheral heparinized blood samples were cultured without mitogens and incubated at 37°C for 24 h. Cell harvesting and slide preparation were performed using the standard conventional cytogenetic methods.
FISH analysis was performed according to the manufacturer’s instructions and Pinkel et al. [11], using FISH probes for the most common genomic aberrations associated with CLL, including trisomy 12 and deletions at the 13q14, 11q22, and 17p13 loci. All FISH probes were commercially available (Cytocell, UK). The slides were examined using a suitable filter set on an optimally performing fluorescence microscope with an applied imaging system. A total of 200 interphase cells were examined per patient.
MLPA assay
DNA was extracted from the PB lymphocytes of all 30 cases and reference samples (one reference sample per seven patient samples, with a minimum of three references per test) using the QIAamp DNA Mini Kit (Germany) according to the manufacturer’s instructions. The quality and quantity of the DNA samples were determined using a NanoDrop spectrophotometer.
The MLPA assay was performed using SALSA MLPA probemix P038-B1 CLL according to the manufacturer’s instructions (MRC-Holland, Netherlands). This probemix comprises multiple probes specific for chromosomal regions and genes associated with recurrent copy number aberrations in B-lymphocyte CLL, including 10q23.31 (PTEN), 11q 22 (ATM, RDX, PPP2R1B, CADM1), chromosome 12, 13q14 (RB1, DLEU1/2/7, KCNRG, MIR15A), 14q, 17p (TP53), and chromosome 19. Moreover, the P038 probemix contains three probes to detect the NOTCH1 7541-7542delCT, SF3B1 K700E, and MYD88 L265P mutations, which only produce a signal when the precise mutation is present. The assay kit included SD009 sample DNA as a positive control for the mutation-specific probes and data binning in the fragment analysis.
The DNA denaturation and overnight MLPA probemix hybridization steps were followed by probe ligation and amplification on the following day. The amplified products were separated using an ABI 3500 Genetic Analyzer (Applied Biosystems, USA). The results were interpreted using the Coffalyser.Net software (MRC, Holland). Ratios of <0.75, 0.75–1.30, and >1.3 were considered to indicate deletion, normal, and duplication, respectively.
Results
Samples from 30 patients with CLL were studied. The FISH and MLPA results are summarized in Table 1.
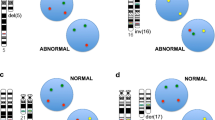
FISH detected aberrations in 21 cases (70%), whereas no abnormalities were detected in nine cases (30%). The most common defect was trisomy 12, which was present in 12 patients (40%). A 13q14 deletion was detected in 10 cases, while an 11q22 deletion was observed in four cases, and a 17p13 deletion was detected in three cases (Table 2, Fig. 1).
FISH analysis showing a trisomy 12 denoted by the presence of three red signals, b mosaic positive 11q23 del denoted by the presence of one red signal and two control green signals for centromere 11, c mosaic positive 13q14 del denoted by the presence of one red signal and two control green signals for the 13q34 region, and d mosaic positive 17p13 del denoted by the presence of one red signal and two control green signals for centromere 17
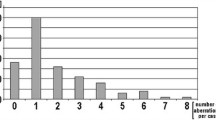
MLPA detected aberrations in 20 cases (66.7%) and no abnormalities in the remaining 10 cases (33.3%). The most common abnormality was trisomy 12, which was present in nine cases (30%). A 13q14 deletion was detected in nine cases, while the RB1 gene was not included in the deleted area in four cases. The 17p13 deletion and 11q22 deletion were detected in three cases each, and the 14q deletion and trisomy 19 were observed in one patient each (Table 2). NOTCH1 7541-7542delCT, SF3B1 K700E, and MYD88 L265P mutations were not detected in any of the patients (Fig. 2).
Ratio charts of MLPA results for some CLL patients using SALSA MLPA probemix P038-B1 CLL. Probe ratios below 0.7 (bottom line) or above 1.3 (top line) are usually regarded as indicative of a deletion or duplication, respectively. a is a patient having trisomy 12, b is a patient having 11q deletion and trisomy 12, c is a patient having 13q deletion, d is a patient having 13q deletion (this patient is having 30% mosaic 13q deletion by FISH), and e is a patient having 17p deletion
Discussion
Many authors have recommended the use of MLPA as an initial diagnostic test [12, 13]. In this study, we aimed to determine the usefulness of the MLPA probemix P038-B1 as a routine standalone screening platform for the detection of clinically relevant chromosome abnormalities in CLL.
Samples from six of the 30 studied patients (20%) yielded discordant MLPA and FISH results. Two cases had a 14q deletion and trisomy 19 respectively, which were not evaluated by FISH in this study. Five cases harbored abnormalities that were identified by FISH but not by MLPA. So, MLPA results were consistent with the FISH results in 24 of 30 patients (80%). Fabris et al. [8] detected a 95% concordance rate between MLPA and FISH results in CLL. The discordant results in these cases may be related to a low level of mosaicism. However, the definition of low mosaicism, or the level at which abnormalities could not be detected by MLPA, has differed between studies and remains controversial. For example, the reported mosaicism thresholds have ranged from 36% in a study by Al Zaabi et al. [9] to 20% in a study by Abdool et al. [14]. However, false-negative MLPA results were reported in samples with an aberrant cell percentage <25% [15, 16]. In our study, abnormalities could be detected by MLPA in a sample containing 27% mosaicism.
Interstitial deletion at 13q14 is the most common chromosomal aberration in CLL and is detected in approximately 50% of cases. The deletion of 13q as the sole abnormality is strongly associated with a favorable disease outcome and a better prognosis. Studies suggest that the clinical course of CLL is accelerated in patients with a large 13q14 deletion that includes the RB1 gene. Moreover, reciprocal translocations involving 13q14 [t(13q)] and many different chromosomes have been reported. However, the lack of recurrent other abnormalities suggests that the consequence of these translocations is possibly due to the loss of a tumor suppressor gene rather than the generation of a fusion gene [17]. In our study, five of the nine cases in which a 13q deletion was detected by MLPA were affected by large deletions that included the RB1 gene.
With sufficient accumulated genotoxic damage, CLL cells are directed to undergo cell cycle arrest or apoptosis. ATM and TP53 genes govern the cellular response to DNA damage through the ATM-CHK2-p53 signaling pathway. Alterations of these genes lead to genomic instability and chemoresistance and are associated with adverse prognosis with significantly shorter overall survival [18,19,20].
11q deletion, which causes a loss of the ATM gene at 11q22.3, is detected in 25% of CLL cases. This is the most frequently detected unfavorable genetic anomaly in patients with CLL. Larger 11q deletions also occur and may affect the tumor suppressor genes PPP2R1B, CADM, and RDX [21, 22]. In our study, all patients in whom MLPA detected an 11q deletion were affected by large deletions that included these tumor suppressor genes.
While 17p deletion causes a loss of the tumor suppressor gene TP53 at 17p13.1 and is associated with a rapid disease progression, poor outcome, drug resistance, and reduced survival duration, in the literature, the incidence of 17p deletion varies widely from 3.4 to 16.8% [23, 24]. In our study, both MLPA and FISH detected 17p deletions in three cases (10%).
Trisomy 12 is the third most common chromosomal aberration detected in patients with CLL. This abnormality is identified in 10–20% of patients [25]. In our study, MLPA and FISH detected trisomy 12 in nine and 12 cases, respectively. This discordance was attributed to the previously discussed low level of mosaicism in three cases.
In addition to four probes that target common recurrent CLL aberrations, the MLPA probemix contains probes that target other genomic areas known to be linked to CLL. These areas, namely, 10q (PTEN), 14q, and chromosome 19, are not targeted by the FISH probe panel. PTEN is a tumor suppressor gene. PTEN is impaired in several types of cancers and plays an important role in CLL pathogenesis. Studies have described defective PTEN function in CLL, either through gene mutation/deletion or promoter methylation [26, 27]. While 14q deletions are rare recurrent alterations in CLL frequently associated with trisomy 12, 14q deletions are associated with a short time to treatment. 14q deletions seem to have an adverse prognostic impact when associated with trisomy 12 [28]. Also, trisomy 19 has been detected infrequently in CLL cases and is usually associated with trisomy 12 [29]. In our study, no abnormalities were detected in the 10q (PTEN) region. Only one case harbored a 14q deletion, and one case harbored trisomy 19, and both cases were having associated trisomy 12.
Moreover, the P038 probemix includes probes to detect three mutations: NOTCH1 7541-7542delCT, SF3B1 K700E, and MYD88 L265P. These mutations are recently identified as CLL disease parameters. The presence of these mutations is associated with at least one unfavorable prognostic marker [30,31,32,33]. However, these mutations were not detected in any of our patients.
Conclusions
From our study, both assays have comparable capabilities to detect CLL aberrations. MLPA technique is disadvantaged by its inability to detect targeted abnormalities in a sample with a low level of mosaicism. However, the restricted number of regions that can be evaluated by FISH is considered to be disadvantageous. MLPA is more cost-efficient than FISH and encompasses a broader range of target gene loci. Nevertheless, we recommend the usage of MLPA as the first screening platform followed by FISH technique for only the negative cases as the most appropriate approach for CLL diagnosis and prognosis.
Availability of data and materials
Data and material are available upon request.
Abbreviations
- CLL:
-
Chronic lymphocytic leukemia
- DNA:
-
Deoxyribonucleic acid
- FISH:
-
Fluorescence in situ hybridization
- MLPA:
-
Multiplex ligation-dependent probe amplification
- PB:
-
Peripheral blood
- PCR:
-
Polymerase chain reaction
- RNA:
-
Ribonucleic acid
References
Hussein S, Mohamed D, Hafez R (2019) Risk factors of hematological malignancies in Upper Egypt: a case–control study. Egypt J Internal Med 31(2):171–177. https://doi.org/10.4103/ejim.ejim_81_18
Mosaad ZE, Mohamed ZA, Abdelazeem MA, Hafez R, Hussein S, Elaiw MA (2019) Impact of CD39 expression on CD4+ T lymphocytes and 6q deletion on outcome of patients with chronic lymphocytic leukemia. Hematol Oncol Stem Cell Ther 12(1):26–31. https://doi.org/10.1016/j.hemonc.2018.09.002 Epub 2018 Oct 11. PMID: 30336122
Ayaz A, Tepeli E, Sari I, Cetin O, Eser M, Dogu H, Bagci G (2014) Contribution of MLPA to routine testing to detect the prognostic chromosomal abnormalities in chronic lymphocytic leukemia. Gene Ther Mol Biol 16:1–9
Rahimi H, Sadeghian MH, Keramati MR, Jafarian AH, Shakeri S, Shams SF et al (2017) Cytogenetic abnormalities with interphase FISH method and clinical manifestation in chronic lymphocytic leukemia patients in North-East of Iran. Int J Hematol Oncol Stem Cell Res 11(3):217–224
Durmaz AA, Karaca E, Demkow U, Toruner G, Schoumans J, Cogulu O (2015) Evolution of genetic techniques: past, present, and beyond. Biomed Res Int 2015:461524
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G (2002) Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res 30(12):e57. https://doi.org/10.1093/nar/gnf056
Stuppia L, Antonucci I, Palka G, Gatta V (2012) Use of the MLPA assay in the molecular diagnosis of gene copy number alterations in human genetic diseases. Int J Mol Sci 13(3):3245–3276. https://doi.org/10.3390/ijms13033245
Fabris S, Scarciolla O, Morabito F, Cifarelli RA, Dininno C, Cutrona G, Matis S, Recchia AG, Gentile M, Ciceri G, Ferrarini M, Ciancio A, Mannarella C, Neri A, Fragasso A (2011) Multiplex ligation-dependent probe amplification and fluorescence in situ hybridization to detect chromosomal abnormalities in chronic lymphocytic leukemia: a comparative study. Genes Chromosomes Cancer 50(9):726–734. https://doi.org/10.1002/gcc.20894
Al Zaabi EA, Fernandez LA, Sadek IA, Riddell DC, Greer WL (2010) Multiplex ligation-dependent probe amplification versus multiprobe fluorescence in situ hybridization to detect genomic aberrations in chronic lymphocytic leukemia: a tertiary center experience. J MolDiagn 12(2):197–203
Muller-Hermelink HK, Montserrat E, Catovsky D, Campo E, Harris NL, Stein H (2008) Chronic lymphocytic leukemia/small lymphocytic lymphoma. In: World Health Organization. Classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC Press, Lyon
Pinkel D, Gray JW, Trask B, van den Engh G, Fuscoe J, van Dekken H. Cytogenetic analysis by in situ hybridization with fluorescently labeled nucleic acid probes. Cold Spring HarbSymp Quant Biol. 1986;51Pt 1:151-157. doi:https://doi.org/10.1101/sqb.1986.051.01.018.
Hömig-Hölzel C, Savola S (2012) Multiplex ligation-dependent probe amplification (MLPA) in tumor diagnostics and prognostics. Diagn Mol Pathol 21(4):189–206
Alhourani E, Rincic M, Othman MA, Pohle B, Schlie C, Glaser A, Liehr T. Comprehensive chronic lymphocytic leukemia diagnostics by combined multiplex ligation dependent probe amplification (MLPA) and interphase fluorescence in situ hybridization (iFISH). Mol Cytogenet. 2014;19;7(1):79.
Abdool A, Donahue AC, Wohlgemuth JG, Yeh CH (2010) Detection, analysis and clinical validation of chromosomal aberrations by multiplex ligation-dependent probe amplification in chronic leukemia. PLoS One 5(10):e15407. https://doi.org/10.1371/journal.pone.0015407
Coll-Mulet L, Santidrián AF, Cosialls AM, Iglesias-Serret D, de Frias M, Grau J, Menoyo A, González-Barca E, Pons G, Domingo A, Gil J (2008) Multiplex ligation-dependent probe amplification for detection of genomic alterations in chronic lymphocytic leukaemia. Br J Haematol 142(5):793–801. https://doi.org/10.1111/j.1365-2141.2008.07268.x
Véronèse L, Tournilhac O, Combes P, Prie N, Pierre-Eymard E, Guièze R, Veyrat-Masson R, Bay JO, Vago P, Tchirkov A (2013) Contribution of MLPA to routine diagnostic testing of recurrent genomic aberrations in chronic lymphocytic leukemia. Cancer Genet 206(1-2):19–25. https://doi.org/10.1016/j.cancergen.2012.12.002
Puiggros A, Venturas M, Salido M, Blanco G, Fernandez-Rodriguez C, Collado R, Valiente A, Ruiz-Xivillé N, Carrió A, Ortuño FJ, Luño E, Calasanz MJ, Ardanaz MT, Piñán MÁ, Talavera E, González MT, Ortega M, Marugán I, Ferrer A, Gimeno E, Bellosillo B, Delgado J, Hernández JÁ, Hernández-Rivas JM, Espinet B; GrupoCooperativoEspañol de CitogenéticaHematológica (GCECGH); GrupoEspañol de LeucemiaLinfáticaCrónica (GELLC). Interstitial 13q14 deletions detected in the karyotype and translocations with concomitant deletion at 13q14 in chronic lymphocytic leukemia: different genetic mechanisms but equivalent poorer clinical outcome. Genes Chromosomes Cancer. 2014;53(9):788-97. doi: https://doi.org/10.1002/gcc.22188. Epub 2014 Jun 10. PMID: 24915757.
Knittel G, Liedgens P, Reinhardt HC. Targeting ATM-deficient CLL through interference with DNA repair pathways. Front Genet. 2015;6:207. doi: https://doi.org/10.3389/fgene.2015.00207. PMID: 26113859; PMCID: PMC4461826.
Kwok M, Davies N, Agathanggelou A, Smith E, Oldreive C, Petermann E, Stewart G, Brown J, Lau A, Pratt G, Parry H, Taylor M, Moss P, Hillmen P, Stankovic T (2016) ATR inhibition induces synthetic lethality and overcomes chemoresistance in TP53- or ATM-defective chronic lymphocytic leukemia cells. Blood. 127(5):582–595. https://doi.org/10.1182/blood-2015-05-644872 Epub 2015 Nov 12 PMID: 26563132
Chauffaille MLLF, Zalcberg I, Barreto WG, Bendit I. Detection of somatic TP53 mutations and 17p deletions in patients with chronic lymphocytic leukemia: a review of the current methods. Hematol Transfus Cell Ther. 2020;42(3):261-268. doi: https://doi.org/10.1016/j.htct.2020.05.005. Epub 2020 Jun 25. PMID: 32660851; PMCID: PMC7417461.
Gunn SR, Hibbard MK, Ismail SH, Lowery-Nordberg M, Mellink CH, Bahler DW et al (2009) Atypical 11q deletions identified by array CGH may be missed by FISH panels for prognostic markers in chronic lymphocytic leukemia. Leukemia 23(5):1011–1017. https://doi.org/10.1038/leu.2008.393
Guarini A, Marinelli M, Tavolaro S, Bellacchio E, Magliozzi M, Chiaretti S, de Propris MS, Peragine N, Santangelo S, Paoloni F, Nanni M, del Giudice I, Mauro FR, Torrente I, Foa R (2012) ATM gene alterations in chronic lymphocytic leukemia patients induce a distinct gene expression profile and predict disease progression. Haematologica 97(1):47–55. https://doi.org/10.3324/haematol.2011.049270
Buccheri V, Barreto WG, Fogliatto LM, Capra M, Marchiani M, Rocha V (2018) Prognostic and therapeutic stratification in CLL: focus on 17p deletion and p53 mutation. Ann Hematol 97(12):2269–2278. https://doi.org/10.1007/s00277-018-3503-6 Epub 2018 Oct 12 PMID: 30315344
Bagacean C, Tempescul A, Ternant D, Banet A, Douet-Guilbert N, Bordron A, Bendaoud B, Saad H, Zdrenghea M, Berthou C, Paintaud G, Renaudineau Y. 17p deletion strongly influences rituximab elimination in chronic lymphocytic leukemia. J Immunother Cancer. 2019;7(1):22. doi: https://doi.org/10.1186/s40425-019-0509-0. PMID: 30696487; PMCID: PMC6352369.
Dőhner H, Stilgenbauer S, Benner A, Leupolt E, Krőber A, Bullinger L et al (2000) Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 343(26):1910–1916. https://doi.org/10.1056/NEJM200012283432602
Zou ZJ, Zhang R, Fan L, Wang L, Fang C, Zhang LN, Yang S, Li YY, Li JY, Xu W (2013) Low expression level of phosphatase and tensin homolog deleted on chromosome ten predicts poor prognosis in chronic lymphocytic leukemia. Leuk Lymphoma 54(6):1159–1164. https://doi.org/10.3109/10428194.2012.733880 Epub 2012 Oct 16 PMID: 23013295
Bernardi R, Ghia P. Reactivating nuclear PTEN to treat CLL. Oncotarget. 2017;8(22):35486-35487. doi: https://doi.org/10.18632/oncotarget.17543. PMID: 28473667; PMCID: PMC5482590.
Cosson A, Chapiro E, Belhouachi N, Cung HA, Keren B, Damm F, Algrin C, Lefebvre C, Fert-Ferrer S, Luquet I, Gachard N, Mugneret F, Terre C, Collonge-Rame MA, Michaux L, Rafdord-Weiss I, Talmant P, Veronese L, Nadal N, Struski S, Barin C, Helias C, Lafage M, Lippert E, Auger N, Eclache V, Roos-Weil D, Leblond V, Settegrana C, Maloum K, Davi F, Merle-Beral H, Lesty C, Nguyen-Khac F; Groupe Francophone de CytogénétiqueHématologique. 14q deletions are associated with trisomy 12, NOTCH1 mutations and unmutated IGHV genes in chronic lymphocytic leukemia and small lymphocytic lymphoma. Genes Chromosomes Cancer. 2014;53(8):657-66. doi: https://doi.org/10.1002/gcc.22176. Epub 2014 Apr 12. PMID: 24729385.
Sellmann L, Gesk S, Walter C, Ritgen M, Harder L, Martín-Subero JI, Schroers R, Siemer D, Nückel H, Dyer MJS, Dührsen U, Siebert R, Dürig J, Küppers R (2007) Trisomy 19 is associated with trisomy 12 and mutated IGHV genes in B-chronic lymphocytic leukaemia. Br J Haematol 138(2):217–220. https://doi.org/10.1111/j.1365-2141.2007.06636.x
Wang L, Lawrence MS, Wan Y, Stojanov P, Sougnez C, Stevenson K, Werner L, Sivachenko A, DeLuca DS, Zhang L, Zhang W, Vartanov AR, Fernandes SM, Goldstein NR, Folco EG, Cibulskis K, Tesar B, Sievers QL, Shefler E, Gabriel S, Hacohen N, Reed R, Meyerson M, Golub TR, Lander ES, Neuberg D, Brown JR, Getz G, Wu CJ. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365(26):2497-506. doi: https://doi.org/10.1056/NEJMoa1109016. Epub 2011 Dec 12. PMID: 22150006; PMCID: PMC3685413.
Sutton LA, Ljungström V, Mansouri L, Young E, Cortese D, Navrkalova V, Malcikova J, Muggen AF, Trbusek M, Panagiotidis P, Davi F, Belessi C, Langerak AW, Ghia P, Pospisilova S, Stamatopoulos K, Rosenquist R. Targeted next-generation sequencing in chronic lymphocytic leukemia: a high-throughput yet tailored approach will facilitate implementation in a clinical setting. Haematologica. 2015;100(3):370-6. doi: https://doi.org/10.3324/haematol.2014.109777. Epub 2014 Dec 5. PMID: 25480502; PMCID: PMC4349276.
Vollbrecht C, Mairinger FD, Koitzsch U, Peifer M, Koenig K, Heukamp LC, Crispatzu G, Wilden L, Kreuzer KA, Hallek M, Odenthal M, Herling CD, Buettner R. Comprehensive analysis of disease-related genes in chronic lymphocytic leukemia by multiplex PCR-based next generation sequencing. PLoS One. 2015;10(6):e0129544. doi: https://doi.org/10.1371/journal.pone.0129544. PMID: 26053404; PMCID: PMC4459702.
Srinivasan VK, Naseem S, Varma N, Lad DP, Malhotra P. Genomic alterations in chronic lymphocytic leukemia and their correlation with clinico-hematological parameters and disease progression. Blood Res. 2020;55(3):131-138. doi: https://doi.org/10.5045/br.2020.2020080. PMID: 32747613; PMCID: PMC7536571.
Acknowledgements
We would like to express our gratitude to the National Research Centre, Egypt, for giving us the chance to accomplish this study with the help of its updated equipment and instrumentation.
Funding
The MLPA assay part of this research was supported by In-house Research Project grant, grant number: AR111303, National Research Centre, Egypt.
Author information
Authors and Affiliations
Contributions
OE: providing the idea of this research, preparing the design of the research, participated in conducting the laboratory work, interpretation of the data, and preparing the paper for submission and final approval of the version to be published. RA: participated in conducting the laboratory work and preparing the paper for submission. LF: participated in conducting the laboratory work and preparing the paper for submission. AA: participated in conducting the laboratory work and preparing the paper for submission. AR: participated in clinical evaluation of the patients and preparing the paper for submission. RM: participated in conducting the laboratory work and preparing the paper for submission. ME: participated in performing the laboratory work and preparing the paper for submission. The authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of the National Research Centre (18-047), which is in accordance with the ethical standards of the Declaration of Helsinki. All participants gave informed written consent before their inclusion in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eid, O.M., Abdel Kader, R.M.A., Fathalla, L.A. et al. Evaluation of MLPA as a comprehensive molecular cytogenetic tool to detect cytogenetic markers of chronic lymphocytic leukemia in Egyptian patients. J Genet Eng Biotechnol 19, 98 (2021). https://doi.org/10.1186/s43141-021-00198-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43141-021-00198-z