Abstract
Background
Thalictrum foliolosum, a member of the Ranunculus family, is recognized for its therapeutic potential in addressing gastric issues, dyspepsia, tooth pain, abdominal colic pain, and piles. The diverse array of secondary metabolites present in the plant contributes to these therapeutic applications. This study aims to uncover and quantify the bioactive secondary metabolites found in the unexplored leaves, stems, and roots of T. foliolosum. Additionally, we also aimed to evaluate the antibacterial activity and MIC values of these extracts against a panel of pathogenic bacteria, such as pathogenic strains, including Escherichia coli, Pseudomonas aeruginosa, Streptococcus mutant and Staphylococcus aureus.
Result
HPLC analysis suggested all examined compounds were found significantly more in root parts of plant. To determine the potential antimicrobial activity of different plant parts result suggested chloroform fraction of root most effective with variable potency against each examined pathogen at 25–100 µg/ml extracts which indicated rich content of berberine in this fraction. Minimum MIC (121.26 µg/mL) of the chloroform fraction of the root was also supported the results. Fatty acid methyl ester analysis by gas chromatography revealed that the stem contained high levels of fatty acids, such as palmitic acid, stearic acid, and linolenic acid, all of which have antibacterial properties.
Conclusion
The potential antimicrobial activity of extracts of various plant parts strongly supports the T. foliolosum plant's widespread use in folk medicine for the treatment of various chronic diseases and adulterants with various associated medicinal plant species.
Similar content being viewed by others
Background
In nature, plants are the richest resource of bioactive natural products, such as alkaloids, phenolics, flavonoids, carotenoids, triterpenoids and other secondary metabolites for thousands of years [1]. Extensive studies in ancient pharmaceutical practices and contemporary clinical findings consistently affirm that plant-based medicines exhibit a higher safety profile compared to their synthetic counterparts, maintaining a crucial role in healthcare. Derived from botanical sources, these medicinal compounds serve as chemical entities in traditional medicine systems, modern pharmaceuticals, dietary supplements, pharmaceutical intermediates, and synthetic drug formulations [1, 2]. Thalictrum, a genus encompassing approximately 220 species, exhibits a diverse array of secondary metabolites including flavonoids, alkaloids, steroids, triterpenoids, triterpenoid glycosides, carotenoids, lignins, tannins, cardiac glycosides with high molecular weight, fatty acids, phenolic acids and sterol across its various species, highlighting the pharmacological significance of this genus [3,4,5]. While there isn't extensive research specifically on Thalictrum foliolosum's, although this species has been used in traditional medicine for various purposes. Given the relevance of phytopharmaceuticals in today's society, the current study concentrated on Thalictrum foliolosum DC, an endemic herb that has received little attention.
T. foliolosum is a potential medicinal herb belonging to the Ranunculaceae family that is found widely in the Northern Hemisphere region in India and the China Pacific [6,7,8]. Previous studies have shown that T. foliolosum roots are used locally as a tonic, antipyretic, diuretic, laxative, and collyrium for the improvement of eyesight as well as in the treatment of gastric problem, dyspepsia, tooth pain, abdominal colic pain and piles [8, 9]. The combination of the dried root of T. foliolosum with Thymus linearis was very effective in treating colic and gastric problems [10]. Whole-plant extracts of T. foliolosum also showed inhibitory effects against cancer cells as well as the progression of malignant malarial fever [11, 12]. T. foliolosum plants may be regarded as belonging to an elite group of medicinal plants due to the presence of benzylisoquinoline alkaloids (BIQ), which include berberine, protoberberine, magnoflorine palmatine, jatrorrhizine, and other considered isoquinoline alkaloids along with several groups of polyphenolic compounds that demonstrated strong bioactivity [13,14,15]. Previously published reports showed the roots and rhizome parts of T. foliolosum have alleviative properties. Therefore, unrestricted collection of roots and rhizome parts resulted in the decline of their population in their habitats [6, 7]. Generally, it is observed that complete knowledge about the chemical composition and potential medicinal uses of various parts of a plant will enable systematic harvesting and conservation of declining plant populations. Considering the therapeutic importance of T. foliolosum, there were no more reports of bioactive compounds in aerial parts of this plant. The current study used reverse phase HPLC to identify and quantify four therapeutically important BIQ alkaloids, namely magnoflorine, berberine, palmatine, and thalicarpine, in different parts of the plant (leaves, stem, and roots). Additionally, fatty acids were identified using gas–liquid chromatography. Moreover, a comparative study of antimicrobial activity against human pathogenic bacteria, such as E. coli, Pseudomonas aeruginosa, Streptococcus mutans, and Staphylococcus aureus, was conducted using extracts or fractions of various parts of the plant. Thus, the present study was undertaken to analyze the alkaloids content and major fatty acids in T. foliolosum leaves, stem and roots and to understand the relation between with antimicrobial activities.
Methods
Sample collection of T. foliolosum plants and processing
T. foliolosum, a perennial herb, was obtained in September month from the forest areas of the Nanital district (2290-m asl), Kumaon region of Uttarakhand state in India (Fig. 1). Nanital is located at the GPS coordinates of 29° 22′ 49.0944'' N and 79° 27′ 48.8520'' E. This region has a humid subtropical climate with an average annual temperature of 17.1 °C (62.7 °F) and 1903 mm (74.9 in) of rainfall. The plants were identified by Dr. Harsh Singh (Taxonomist scholar) and a voucher specimen number 257717(LWG) was deposited at CSIR-National Botanical Research Institute (India). The collected plant materials (leaves, stem and roots) were initially carefully washed with saline water, then the samples were placed under running tap water for further washing. After some time, plant materials are rinsed again with autoclave water for half an hour and then put on blotting paper for 48 h to dry. The plant materials were blotted dry before being chopped into small pieces and dried in the shade for 15–20 days. Finally, the dried plant material was ground into a fine powder using an electric grinder (Mill CT 293 Cyclotec TM) and stored in sterilized glass bottles [5, 6].
Chemicals
All the standards of BIQ alkaloids (Magnoflorine chloride, berberine chloride, palmatine chloride and thalicarpine) were procured from Sigma-Aldrich (USA). Solvent such as methanol, hexane, ethyl acetate, chloroform and acetonitrile and other chemicals were purchased from Merck (India).
Extraction of plant materials for HPLC analysis
For quantitative analysis, fine powders of different plant materials (1 g) were extracted with 25 ml of acidic (1% HCl) methanol using ultrasonication method (Aczet, Ultrasonic cleaner CUB) at room temperature for 40 min. After ultrasonication, plant materials in extracted solvents were kept at room temperature for overnight. The next day, all sonicated extracts were filtered through a Whatman No 1 filter paper in a round bottle flask, and fresh extracted solvent was added to the same plant materials for additional ultra-sonification. Three times, the same procedure was followed. Later all extracted materials were pooled in round bottom flask and evaporated at 50 °C using a rotary evaporator (Buchi, USA) under low pressure. Finally, the dried extracted plant materials were dissolved in methanol and filtered through a 0.45 µm filter and stored at 4 °C for subsequent HPLC analysis [6, 7]. The acidic-methanol extract of T. foliolosum had a yield of 27.42%, 21.10% and 16.24% of the extracted root, leaves and stem respectively. Reference standard solutions (i.e. berberine, palmatine, magnoflorine and thalicarpine) (Sigma-Aldrich) were prepared in methanol. All of the extracted solutions as well as the standard solutions were maintained at 4 °C in the refrigerator.
HPLC method
Plant samples were extracted and subjected to further analysis using High-Performance Liquid Chromatography (HPLC) with a gradient method for identifying bioactive compounds, following the protocol established by Mishra et al. [6, 7]. The analysis was performed using a Shimadzu (Japan) HPLC Prominence system comprising a 20 μL sample loop, a PDA SPD M 20 A photodiode array system, an LC-20AD dual pump system, and a SIL-20 AC Autoinjector with a cooler. Compounds were separated on a Shimadzu RP-C18 column (250 × 4.6, 5 μm pore size) with a guard column of the same packing material. A gradient mobile phase was employed consisting of component A (0.3% formic acid + 0.3% triethylamine) and component B (acetonitrile). The mobile phase gradient program was as follows: 0–25 min, 5–25% B; 25–35 min, 25%-35% B; 35–45 min, 35–45% B; 45–60 min, 45–100% B, at a flow rate of 1 mL/min. Data integration and compound identification were performed using Shimadzu Lab Solution software at a wavelength of 265 nm [6]. Results were compared against standards obtained from Sigma-Aldrich, USA. Quantitative analysis was conducted by averaging the results of three independent analyses of the same sample.
Fatty acid analysis using gas liquid chromatography
500 mg dry weight of plant samples (leaf, stem and roots) were used for FAME preparation. Fatty acid methyl esters (FAMES) were prepared as per method followed by Bureau of Indian Standards IS: 548 (Part-III), 1976 reaffirmed 1994. Prepared FAME dissolved in hexane were analyzed using GC system (7890B GC System, Agilent Technologies) equipped with a flame ionization detector (FID). FAMEs were separated on DB-225 column (30 m × 0.25 mm ID × 0.25 µm film thickness). Nitrogen was used as carrier gas and hydrogen and air as ignition gases. The conditions used for GC analysis were: injector temperature of 230 °C, detector (FID) temperature of 260 °C with split mode injection (1:20). The oven temperature program was started from 90 (2 min hold) to 130 °C at ramping rate of 3 °C min−1. Temperature from 130° to 230° (5 min hold) was achieved with ramp of 2 °C min−1. The peaks were identified by comparing with standards and the results are presented as mean of triplicates. Data were integrated by Open LAB CDS Chem Station Edition software. Reference standards of methyl palmitate, methyl stearate, methyl oleate, methyl linoleate, methyl arachidate and methyl behenate, methyl linoleate were procured by M/s Sigma-Aldrich.
Extracts preparation for antimicrobial assay
For antimicrobial activity various plant parts materials (20 g) were extracted in aqueous methanol (50%) by sonication method. Then extracted solvents were further fractionated by various organic solvents, such as ethyl acetate, chloroform and hexane [5]. All extracted samples were filtered using Whatman filter paper No. 1. The filtrate was then dried using a low-pressure rotary evaporator (Buchi, USA) and lyophilized. All samples were stored at 4 °C in an airtight tube until further analysis was completed.
Microorganism used in study
In vitro antimicrobial activity was determined against four different human pathogenic bacteria i.e. E. coli (ATCC 25922), S. mutans (ATCC 25175), S. aureus (ATCC 4944) and P. aeruginosa (MTCC 424). E. coli is a gram-negative, rod-shaped bacterium that is commonly found in the lower intestine and most of these strains are harmless. However, pathogenic E. coli varieties cause serious food poisoning, septic shock, meningitis, or urinary tract infections in humans [16]. S. mutans is a gram-positive coccus bacterium, primarily found in mouth, pharynx, and intestine and significantly involved in tooth decay [17]. Staphylococcus aureus is a gram positive, round shape, facultative anaerobe bacterium. S. aureus are frequently found in the upper respiratory tract and on the skin and being a common cause of respiratory infections including skin infections [18]. P. aeruginosa is encapsulated rod-shaped, gram-negative bacterium. The important characteristic of this bacteria is multidrug resistance. Usually, P. aeruginosa found in medical equipment’s hence these bacteria associated hospital acquired infections, i.e. ventilator associated pneumonia and other sepsis syndrome the general symptoms of these bacteria are inflammation and sepsis but if their colonization occurs in lungs, kidneys and urinary tract it may be fatal [19].
Screening of antimicrobial activity
In vitro antimicrobial activity was determined against four different human pathogenic bacteria using disk-diffusion method with slight modification [20]. In short, 18–24 h old bacteria culture (0.5 OD600 nm) was spreading over the entire surface of nutrient agar plates (90 mm size) using autoclaved steel spreader and sterile paper disks (approx. 6 mm diameter) were placed on them. Then different concentrations of test samples (25, 50 and 100 µg) were loaded on disks. Streptomycin (25 µg) was utilized as a positive control and respective solvents to which extract dissolved was used as a negative control. Then, plates were allowed to incubate at 37 ± 1 °C for 18–24 h. The diameter of the inhibition zone (ZOI) was measured after 24 h of incubation to assess antibacterial activity (measured in mm including disk size). All experiments were carried out in triplicate, and the observed ZOI values are expressed as a mean with standard error of the mean (SEM).
Minimum inhibitory concentration (MIC)
MIC was determined of various extract of leaves, stems and roots parts of T. foliolosum against the different pathogenic bacteria by using the previously reported serial dilution method using 96-well microtiter plates. In brief, all pathogenic bacteria were grown in nutrient broth for 6 h before being inoculated, followed by 106 cells/mL bacterial culture was inoculated in 200 μL nutrient broth containing tube. Now each extract (leaf, stem, and root) has been added separately in bacterial broth with concentrations ranging from 100 to 800 μg/ml. All tubes were incubated at 37 °C for 24 h and further examined for visible turbidity. The minimum inhibitory concentration (MIC) was determined to be the lowest concentration that inhibited visible growth of the tested bacteria. MIC of berberine, magnoflorine and streptomycin were determined with similar procedure.
Results
Identification and quantification of BIQ alkaloids contents in leaves, stems and roots
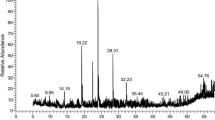
Chromatographic analysis aimed to quantify biologically active BIQ alkaloids, namely magnoflorine, berberine, palmatine, and thalicarpine, in extracts obtained from leaves, stems, and roots. Identification of all significant peaks in the chromatogram was accomplished using authentic reference standards of the respective alkaloids. (Fig. 2A) (Supplementary Fig. 1). In terms of quantitative analysis, the root extracts of T. foliolosum exhibited the highest total alkaloid content (15.03 mg g-1), followed by the leaves (2.75 mg g-1), while the stem extracts contained a comparatively lower amount (Fig. 2B). Examining individual alkaloids in the root part revealed the following order of content: magnoflorine (10.02 mg g-1) > berberine (3.09 mg g-1) > thalicarpine (1.60 mg g-1) > palmatine (0.31 mg g-1). Similar pattern in alkaloid content were observed in the leaves, with stem-derived extracts displaying lower alkaloid levels compared to roots and leaves.
HPLC analysis of antimicrobial responsive alkaloids. a Identified BIQ alkaloids i.e. magnoflorine, thalicarpine, palmataine and berberine peaks in HPLC chromatogram of different parts (leaf, stem and root) of T. foliolosum. b Bar graph represents the comparative observation of identified benzylisoquinoline alkaloids in different plant parts of Thalictrum foliolosum plants. The results are expressed as means ± SD of three replicates
Antimicrobial activity of extracted plant materials (leaf root and stem) and standard compounds berberine and magnoflorine
Various extract fractions obtained from different plant parts exhibited a range of antimicrobial activities against the microbial strains employed in the experiment, as detailed in Table 1. The antimicrobial assay indicated that the chloroform fractions of roots and leaves were particularly effective, demonstrating broad-spectrum growth inhibition across all concentration ranges (25–100 µg/ml) against all pathogenic bacteria. The ethyl acetate fractions of leaves and roots displayed remarkable antimicrobial activity at a concentration of 50 µg/ml against all pathogenic bacterial cultures. However, the ethyl acetate fractions from the stem part did not inhibit the growth of gram-negative P. aeruginosa (Table 1). Additionally, aqueous methanolic extracts of leaves and roots exhibited impressive growth inhibition against E. coli, P. aeruginosa, and S. mutans at concentrations ≥ 100 µg/ml. Hexane extracts of leaves and stems demonstrated antimicrobial activity against all tested microorganisms except P. aeruginosa. However, in our report, hexane root extracts did not exhibit a zone of inhibition against both gram-negative and gram-positive bacteria at concentrations ranging from 25 to 100 µg/ml. Comparatively, when standard compounds berberine chloride and magnoflorine chloride were employed at similar concentrations against the same pathogenic bacterial cultures, berberine emerged as the most effective compound at each concentration. While magnoflorine did not exhibit effective growth inhibition against various pathogens at various concentrations, a slight zone of inhibition was observed against E. coli at 100 µg/ml (Fig. 3).
Minimum inhibitory concentrations (MIC’s) analysis
In our investigation, we determined the Minimum Inhibitory Concentration (MIC) of potent plant extracts using a 96-well microtiter plate. The MIC values for aqueous methanol, ethyl acetate, and chloroform fractions are detailed in Table 2. Notably, the chloroform fraction of the root exhibited the lowest MIC against all tested strains, with a lesser value of 121.26 µg/ml observed against S. aureus (refer to Table 3). The ethyl acetate fraction from various plant parts demonstrated effective MIC values ranging from 158.15 to 448.78 µg/ml against all tested microbial pathogens. Particularly, the ethyl acetate root fraction displayed the minimum MIC against S. mutans. Additionally, the aqueous methanolic extracts showed MIC values ranging from 224.78 to 482.67 µg/ml (refer to Table 3). Furthermore, we analyzed the MIC values of reference alkaloid compounds, as presented in Table 4. The findings suggested that berberine chloride showed less MIC values (123.95 µg/ml) compared to magnoflorine chloride against all pathogenic bacterial strains (refer to Table 4).
Fatty acid analysis
Analysis of fatty acids in various parts of the T. foliolosum plant (leaves, stems, and roots) revealed higher levels of FAME in the stems compared to other plant parts. Trace amounts of FAME were recorded in root, in our observation. Table 4 provides detailed information on the identified fatty acids and their respective quantities in different plant parts. Notably, fatty acid esters such as palmitic acid (16:0) (7.81%), stearic acid (2.98%), oleic acid (C18:1) (3.42%), linoleic acid (C18:2) (2.72%), and linolenic acid (C18:3) (3.27%) were found in significant amounts in the stem parts.
Discussion
Plant extracts have been used for centuries as natural medicines due to the diverse array of bioactive compounds they contain. These compounds can have therapeutic effects on the human body. Herbal remedies are often passed down through generations and used to treat various ailments. Extracts of various plant, i.e. Andrographis paniculata, Withania somnifera, Panax ginseng, Panax quinquefolium, Coptis japonica and Carica papaya have been widely used in natural medicine to treatment of various chronic disorders [5, 21]. Similarly, a lesser explored medicinal herb T. foliolosum widely used as traditional medicine in gastric problem, bloating, toothache, abdominal colic pain and piles (hemorrhoids) [7, 22]. Plants contain a variety of bioactive compounds, such as alkaloids, flavonoids, terpenoids, sterols and polyphenols. These compounds possess medicinal properties and can have antioxidant, anti-inflammatory, antiviral, antibacterial, and antifungal effects. Previous reports and contemporary studies have shown that the therapeutic potential of T. foliolosum plants may be due to the presence of bioactive BIQ alkaloids and other non-alkaloidal compounds such as flavonoids, saponins, polyphenolic compounds and steroids, etc. [23, 24].
Quantitative and qualitative analysis of BIQ alkaloids—specifically, berberine, magnoflorine, palmatine, and thalicarpine—was conducted using High-Performance Liquid Chromatography (HPLC) across different plant parts of T. foliolosum. The order of alkaloid abundance was consistently observed as magnoflorine > berberine > thalicarpine > palmatine in both the underground and leaf components of T. foliolosum plants. Magnoflorine is a quaternary aporphine alkaloid with numerous pharmacological properties, including anti-diabetic, anti-inflammatory, neuropsychopharmacology, immunomodulatory, hypotensive, antioxidant, and antifungal properties [25]. Berberine has long history of medicinal use as folk medicine in Chinese, Indian and Native American. Berberine has potential therapeutic use in metabolic syndrome, type 2 diabetes and dyslipidemia as well as antimicrobial activity [26]. The palmatine alkaloids are also used in traditional Asian medicine for the treatment of jaundice, liver infection, high blood pressure, inflammation and dysentery. Palmatine has recently been shown to be beneficial in the treatment of central nervous system problems [27]. Thalicarpine is a hypotensive alkaloid that has been recognized as being involved in the binding of novel antitumor agents [23, 24, 28].
In addition to biochemical analysis, antimicrobial activity showed that the chloroform fractions of root and leaves have significant activity against different pathogenic micro-organism. It was reported that berberine is the most active compound in the chloroform fractions and has been identified as the most important antibacterial component in various Thalictrum species of all the BIQ compounds identified [7, 14, 22, 24]. Similar reports were also found in other species of Thalictrum (T. delavayi, T. minus, T. orientale, T. fortune and T. avanicum) further supporting the antimicrobial potential of berberine [29]. Furthermore, our antimicrobial assay using berberine and magnoflorine standard compounds against pathogenic bacteria provided valuable insights into the potential bioactivity of these compounds. The observed superior effectiveness of berberine compared to other reference compounds highlights its promising antimicrobial properties (Table 4). The potential mechanisms of berberine antimicrobial activity may be mediated by the suppressing cell adhesion and migration as well as inhibiting the microbial enzymes [22]. Besides, magnoflorine an aporphine BIQ (usually soluble in water, methanol, and ethanol, and insoluble in low polar organic solvents such as petroleum ether and chloroform) was occurred significant amount in root and leaves but this alkaloid has been showed poor antibacterial activity against various pathogenic bacteria (Fig. 3). However, it was reported that magnoflorine had potent antifungal activity against Penicillium avellaneum and Candida strain, which may account for the potential antifungal activity in T. foliolosum [25].
Additionally, ethyl-acetate extracts of leaves were found to have impressive antimicrobial activity than other parts (roots and stem) of ethyl acetate extract against E. coli, S. aureus, and S. mutans. It has been reported the ethyl-acetate fraction contained significant amount of polyphenolic or flavonoids compounds, which have strong antimicrobial activity and antioxidant activity [5, 30]. Moreover, aqueous methanolic extracts (50%) of leaves and roots also shows the effective antibacterial activity against the E. coli, P. aeruginosa and S. mutans. Inhibition activity in aqueous methanolic extract fraction may be due to soluble fraction of alkaloids, phenol and flavonoids compounds [14, 22]. Furthermore, the non-polar hexane fraction, particularly from stem parts, demonstrated effective antimicrobial activity against both gram-negative and gram-positive bacteria. Fatty Acid Methyl Ester (FAME) analysis revealed significant amounts of fatty acid esters including palmitic acid, stearic acid, and linoleic acid in T. foliolosum stem parts, contributing to their potential antibacterial activity [31, 32]. The comprehensive analysis of Thalictrum foliolosum extracts highlights the presence of various bioactive compounds with significant antimicrobial potential.
Conclusion
In conclusion, our study provides valuable insights into the medicinal properties of T. foliolosum, a lesser-explored herb with a rich traditional history of use in treating various ailments. Through quantitative and qualitative analysis, we identified a diverse array of bioactive compounds in different plant parts, particularly BIQ alkaloids such as magnoflorine, berberine, palmatine, and thalicarpine, alongside substantial presence of both unsaturated and saturated fatty acids. Our findings underscore the potential therapeutic significance of T. foliolosum extracts, with particular emphasis on their antimicrobial activity against a range of pathogenic microorganisms. Berberine emerged as a key antimicrobial compound, exhibiting potent activity against various bacteria. Additionally, ethyl acetate and aqueous methanolic extracts displayed significant antibacterial effects, likely attributed to their polyphenolic or flavonoid content. Furthermore, our study highlights the importance of understanding the composition and pharmacological properties of medicinal plants to harness their therapeutic potential effectively. T. foliolosum shows promise as a natural remedy for combating microbial infections, and further research into its mechanisms of action and clinical applications is needed.
Availability of data and materials
Yes.
Abbreviations
- BIQ:
-
Benzylisoquinoline
- HPLC:
-
High Performance Liquid Chromatography
- FAME:
-
Fatty acid methyl esters
- GC:
-
Gas chromatography
- ZOI:
-
Zone of inhibition
- MIC:
-
Minimum inhibitory concentration
References
D Amenu 2014 Antimicrobial activity of medicinal plant extracts and their synergistic effect on some selected pathogens Am j ethnomed 1 1 18 29
F Moussaoui T Alaoui 2016 Evaluation of antibacterial activity and synergistic effect between antibiotic and the essential oils of some medicinal plants Asian Pac J Trop Biomed 6 32 37 https://doi.org/10.1016/j.apjtb.2015.09.024
PL Schiff Jr 1991 Bisbenzylisoquinoline alkaloids J Nat Prod 54 3 645 749
EA Khamidullina AS Gromova Vi Lutsky 2006 Natural products from medicinal plants: non-alkaloidal natural constituents of the Thalictrum species Nat Prod Rep 23 1 117 129
MK Mishra S Pandey A Niranjan P Misra 2021 Comparative analysis of phenolic compounds from wild and in vitro propagated plant Thalictrum foliolosum and antioxidant activity of various crude extracts Chem Papers 75 9 4873 4885 https://doi.org/10.1007/s11696-021-01708-6
MK Mishra S Pandey P Misra A Niranjan 2020 In vitro propagation, genetic stability and alkaloids analysis of acclimatized plantlets of Thalictrum foliolosum Plant Cell Tissue Organ Cult (PCTOC) 142 2 441 446
MK Mishra S Pandey P Misra A Niranjan A Srivastava 2020 An efficient protocol for clonal regeneration and excised root culture with enhanced alkaloid content in Thalictrum foliolosum DC. —an endemic and important medicinal plant of temperate Himalayan region Ind Crops Prod 152 112504 20
MK Mishra S Pandey A Srivastava 2019 A Study of endemism biodiversity threat and conservation strategies of thalictrum for their sustainable utilization Dynamics of ecosystem and climate change in India Serials publication New Delhi 118 138
S Bahadur AK Shukla 1983 Studies on native medicinal plants, I. The quaternary alkaloids of Thalictrum javanicum J Nat Prod 46 454 457 https://doi.org/10.1021/np50028a002
S Joshi SC Sati 2017 Screening of antibacterial potentiality of Thalictrum foliolosum leaves extracts Biomed Nurs 3 1 98 10
DH Li J Guo W Bin N Zhao KB Wang JY Li ZL Li HM Hua 2016 Two new benzylisoquinoline alkaloids from Thalictrum foliolosum and their antioxidant and in vitro antiproliferative properties Arch Pharm Res 39 7 871 877
NG Das B Rabha PK Talukdar D Goswami S Dhiman 2016 Preliminary in vitro antiplasmodial activity of Aristolochia griffithii and Thalictrum foliolosum DC extracts against malaria parasite Plasmodium falciparum BMC Res Notes 9 51 https://doi.org/10.1186/s13104-016-1862-4
JM Hagel PJ Facchini 2013 Benzylisoquinoline alkaloid metabolism: a century of discovery and a brave new world Plant Cell Physiol 54 647 672 https://doi.org/10.1093/pcp/pct020
R Kumar N Sharma R Rolta UR Lal A Sourirajan K Dev V Kumar 2020 Thalictrum foliolosum DC: an unexplored medicinal herb from north western Himalayas with potential against fungal pathogens and scavenger of reactive oxygen species Biocatal Agric Biotechnol 26 101621https://doi.org/10.1016/j.bcab.2020.101621
DS Bhakuni RS Singh 1982 The alkaloids of Thalictrum foliolosum J Nat Prod 45 252 255 https://doi.org/10.1021/np50021a003
JP Nataro JB Kaper 1998 Diarrheagenic Escherichia coli Clin Microbiol Rev 11 142 201 https://doi.org/10.1128/CMR.11.1.142
JA Lemos SR Palmer L Zeng ZT Wen JK Kajfasz IA Freires J Abranches LJ Brady 2019 The biology of Streptococcus mutans Microbiol Spectr 7 1 7 1
Foster TJ, Geoghegan JA, (2015) Staphylococcus aureus. In: Molecular medical microbiology. Elsevier, 655–674
GP Bodey R Bolivar V Fainstein L Jadeja 1983 Infections caused by Pseudomonas aeruginosa Rev Infect Dis 5 2 279 313
AL Barry F Garcia LD Thrupp 1970 An Improved single-disk method for testing the antibiotic susceptibility of rapidly-growing pathogens Am J Clin Pathol 53 149 158 https://doi.org/10.1093/ajcp/53.2.149
A Gurunathan P Subramanium S Maran 2013 In vitro antimicrobial activity of leaf, stem and root extracts of the medicinal plant species, Thalictrum javanicum blume against certain human pathogens Int J Pharm Pharm 5 71 75
G Pandey S Khatoon MM Pandey AKS Rawat 2018 Altitudinal variation of berberine, total phenolics and flavonoid content in Thalictrum foliolosum and their correlation with antimicrobial and antioxidant activities J Ayurveda Integr Med 9 169 176 https://doi.org/10.1016/j.jaim.2017.02.010
SK Chattopadhyay AB Ray DJ Slatkin JE Knapp PL Schiff Jr 1981 The alkaloids of Thalictrum foliolosum J Nat Prod 44 45 49 https://doi.org/10.1021/np50013a008
N Sharma V Kumar MP Chopra A Sourirajan K Dev M El-Shazly 2020 Thalictrum foliolosum: a lesser unexplored medicinal herb from the Himalayan region as a source of valuable benzyl isoquinoline alkaloids J Ethnopharmacol 255 112736https://doi.org/10.1016/j.jep.2020.112736
T Xu T Kuang H Du Q Li T Feng Y Zhang G Fan 2020 Magnoflorine: a review of its pharmacology, pharmacokinetics and toxicity Pharmacol Res 152 104632
Schor J, Fabno N, (2012) Clinical Applications for Berberine: potential therapeutic applications in metabolic syndrome, type 2 diabetes, and dyslipidemia. Nat Med J. (https://www.naturalmedicinejournal.com).
D Tarabasz W Kukula-Koch 2020 Palmatine: a review of pharmacological properties and pharmacokinetics Phytother Res 34 33 50
SM Kupchan KK Chakravarti N Yokoyamat 1963 Thalictrum alkaloids I Thalicarpine, a new hypotensive alkaloid from Thalictrum dasycarpum J Pharm Sci 52 10 985 988
FZ Erdemgil MERAL Yilmaz MERIH Kivanc 2004 Antimicrobial activity of thalictrum orientale’s extracts the determination of microfungal biodiversity that contribute infection risk in the interior air of newborn unites in five distinct provinces of turkey view project MARMARA Pharm J 19 23 33
C Cueva MV Moreno-Arribas PJ Martín-Álvarez G Bills MF Vicente A Basilio CL Rivas T Requena JM Rodríguez B Bartolomé 2010 Antimicrobial activity of phenolic acids against commensal, probiotic and pathogenic bacteria Res Microbiol 161 372 382 https://doi.org/10.1016/j.resmic.2010.04.006
F Dilika PD Bremner JJM Meyer 2000 Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: a plant used during circumcision rites Fitoterapia 71 450 452 https://doi.org/10.1016/S0367-326X(00)00150-7
G Casillas-Vargas C Ocasio-Malavé S Medina C Morales-Guzmán RG Valle Del NM Carballeira DJ Sanabria-Ríos 2021 Antibacterial fatty acids: an update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents Prog Lipid Res 82 101093https://doi.org/10.1016/j.plipres.2021.101093
Acknowledgements
The first Author (Manoj K. Mishra) was supported by University Grants Commission (UGC), New Delhi through the award of Dr. Kothari Post-Doctoral Fellowship. We thank the late professor (Dr.) Alka Srivastava (Department of Botany, University of Lucknow) for valuable guidance for the present research.
Funding
Funding The first Author (Manoj K. Mishra) was supported by University Grants Commission (UGC), New Delhi for the award of Dr. Kothari Post-Doctoral Fellowship (BL/15-16/0168).
Author information
Authors and Affiliations
Contributions
Manoj Kumar Mishra; Conception and design the study, performed the experiments, analyzed the data and interpretation of data and wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Mishra, M.K. Phytochemical analysis and evaluation of antibacterial activity of various extracts from leaves, stems and roots of Thalictrum foliolosum. Futur J Pharm Sci 10, 70 (2024). https://doi.org/10.1186/s43094-024-00643-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00643-z