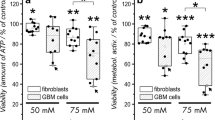
Abstract
Background
Nutrient stress (NS), one of the hallmarks of the tumour microenvironment, can render cancer cells tolerant to cytotoxicity. Fibroblasts, on the other hand, have cancer cell-like traits, such as plasticity and resiliency. Hence, this study aimed to evaluate the cytotoxicity of the drug on reseeded human U87 glioblastoma (GBM) cells as well as on mouse L929 fibroblasts in the form of monolayer and colonies that grew after NS induction.
Results
No treatment for 48 h showed a statistically significant difference in U87 cell viability when compared to the 50% hypothetical value. However, temozolomide (TMZ) (151.0 µg/ml) and azithromycin (AZI) (92.0 µg/ml) significantly diminished the number of U87 cell colonies compared to the untreated control, and AZI also outperformed doxycycline (DOXY) (147.0 µg/ml). L929 fibroblasts survived NS, but the cytotoxicity of AZI, DOXY, and AZI + DOXY (92.0 + 147.0 µg/ml) substantially increased than in L929 fibroblasts without NS induction.
Conclusions
The present findings suggest that NS does not inevitably contribute to cytotoxic drug tolerance in GBM cells. In addition, although fibroblasts can withstand NS, they can also become susceptible to cytotoxic drug-induced death; nevertheless, the type of drug may play a role.
Graphical Abstract

Similar content being viewed by others
Background
High-grade glioma, notably glioblastoma (GBM), is one of the most challenging cancers to treat, partly due to its high degree of heterogeneity. Despite the discovery of drugs that can interfere with GBM growth, such as temozolomide (TMZ), most patients succumbed to death. The major issues are that not all patients respond to the same effective drugs, and even if they do, patients ultimately develop resistance. Although much work is currently being expended on immune-based therapies [1], alternative effective agents are in constant demand.
Azithromycin (AZI) and doxycycline (DOXY) are intriguing candidates of antibiotics actively being studied for their anticancer attributes. Both drugs have exhibited substantial anti-proliferative and pro-apoptotic effects in different human cancer cells [2,3,4,5]. They are also capable of modulating various therapeutic targets associated with cancer hallmarks, including angiogenesis and pro-survival autophagy [2, 4, 6, 7]. Furthermore, they are effective against drug-tolerant persister cells of a wide range of origins, such as cancer stem-like cells, in part by inhibiting mitochondrial oxidative phosphorylation (OXPHOS) [3, 8,9,10].
The tumour microenvironment (TME) is composed of a complex network of cells (e.g., malignant/stromal cells) and non-cellular components (e.g., extracellular matrix proteins) that surround and interact with one another, which mutually contribute to tumour growth and development. The metabolism of tumours, including GBM, is malleable to factors in the environment in which they emerge. Metabolism is the chemical process that allows cells to use available nutrient resources to sustain their survival and propagation. The availability and quantity of nutrients, as well as other elements that enable metabolism, such as oxygen, and the presence of other cells (such as fibroblasts, endothelial and immune cells) that compete for or fuel such nutrients, are all intrinsic factors that can influence tumour behaviours [11].
Nutrient scarcity is one of the hallmarks of TME, and the Warburg effect (a glucose-dependent glycolytic aerobic process) is an atypical metabolic phenomenon of cancer cells. As a result, cancer cells not only reprogram their metabolism but also develop various mechanisms to meet their energy demands and sustain survival. For instance, they switch metabolism towards mitochondrial OXPHOS and phenotype to a quiescent state in response to glucose stress [12, 13]. Secondly, cancer cells secrete molecules that promote angiogenesis to improve blood flow and delivery of oxygen and nutrients, as well as attract other cells (e.g., fibroblasts) that can provide additional resources [14, 15]. Indeed, the ability of cancer cells to adapt, survive, and propagate under such an intricate milieu promotes evolution, driving them towards a more aggressive phenotype/behaviour and resulting in drug resistance [11, 13, 16, 17].
Aside from that, tumour behaviours are greatly influenced by activated fibroblasts, one of the most abundant stromal cells in the TME. They are renowned as cancer-associated fibroblasts and are heterogeneous, with distinct functions that include tumour growth, survival, and metabolism, as well as mediating drug resistance [14, 15]. Ironically, fibroblasts have cancer cell-like traits, such as plasticity and resiliency, and they are associated with all stages of tumour progression [15]. Tumours have even been dubbed “wounds that do not heal” [18].
To that end, it is of interest to expound the impact of nutrient stress (NS) on drug cytotoxicity in GBM cells and fibroblasts. Our earlier findings showed that the concentrations of TMZ, AZI, and DOXY that caused 50% of U87 GBM cell monolayer to be affected (IC50) under nutrient-favourable conditions for 48 h were 151.0, 92.0, and 147.0 µg/ml, respectively [19]. This study investigated their effects on U87 cell viability and colonies, as well as L929 fibroblast viability after the induction of NS.
Methods
Drugs and reagents
TMZ (Sigma-Aldrich, St. Louis, MO, USA), AZI (BioVision, Milpitas, CA, USA), DOXY (Sigma-Aldrich, St. Louis, MO, USA), 1 × phosphate buffer saline (PBS) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), 10.0% neutral buffered formalin (NBF) (Sigma-Aldrich, St. Louis, MO, USA), and gram crystal violet reagent (GCC Diagnostics, UK). Note that AZI (Sigma-Aldrich, St. Louis, MO, USA) was used to treat NIH3T3 and human dermal fibroblasts (HDF), but the manufacturer discontinued the drug in the middle of the study.
Culture of GBM cells and fibroblasts
The human U87 GBM cell line (HTB-14) was obtained from the American Type Culture Collection (ATCC) (Rockville, MD, USA) and maintained at 37 °C with 5% CO2 and 95% humidity in Dulbecco’s Modified Eagle Medium (DMEM) (4.5 g/l glucose with L-glutamine and sodium pyruvate) (Nacalai Tesque, Kyoto, Japan), supplemented with 10% foetal bovine serum (FBS) (Tico Europe, Amstelveen, Netherlands) and 1% non-essential amino acid (Nacalai Tesque, Kyoto, Japan). Mouse connective tissue L929 and embryonic NIH3T3 fibroblasts (ATCC), and HDF (RIKEN Cell Bank, Tsukuba, Ibaraki, Japan) were cultured in the same manner as GBM cells.
NS induction
To induce NS in GBM cells, U87 cells were seeded in T75 flasks at a density of 1.0 × 103 in a final volume of 12 ml and maintained under standard growth conditions. In brief, the medium was refreshed on days 30 and 33, cells were then subcultured on day 36, and harvested on day 38, followed by seeding. To induce NS in normal cells, L929 fibroblasts were seeded in T75 flasks at a density of 1.0 × 103 in a final volume of 12 ml and maintained under standard growth conditions. In brief, the medium was refreshed on day 48, fibroblasts were then subcultured on day 54, and harvested on day 56, followed by seeding.
The cells were sustained in culture for a period until some adherent (spindle-shaped) cells were observed (Fig. 1), day 30 and day 48 for GBM cells and fibroblasts, respectively. GBM cells were fully repopulated on day 36, while fibroblasts were on day 54. This field is based on the principle that metabolic activities in cancer cells differ from normal cells, with cancer cells exhibiting rapid abnormal growth and higher metabolic demands.
Determination of U87 cell viability
A total of 1.0 × 103 cells in a final volume of 100 µl was seeded in 96-well plates and cultured for 72 h before being treated. After 48 h of cytotoxic drug treatments, the MTT assay was carried out in accordance with the manufacturer’s protocol, and the percentage of cell viability was calculated using the following equation:
Determination of U87 cell colonies
Cells were seeded in 6-well plates at a density of 1.0 × 103 in a final volume of three ml and maintained under standard growth conditions. On day 21, the medium was refreshed, and cells were further incubated until day 25, after which they were treated for 48 h. On day 27, cells were fixed with two ml of 10.0% NBF for 20 min, rinsed once with two ml of 1 × PBS, and stained in two ml of 0.01% crystal violet for 20 min. In each well, eight regions were randomly captured under a visual field (5 ×) using a phase-contrast inverted microscope (Zeiss, Germany), and the number of colonies with at least 50 cells was counted using ImageJ software (NIH Image, Bethesda MD, USA).
Determination of L929 fibroblast viability
A total of 5.0 × 103 cells in a final volume of 100 µl was seeded in 96-well plates and cultured for 24 h before being treated, followed by an MTT assay. The effects of cytotoxic drugs on the viability of fibroblasts, such as L929, NIH3T3, and HDF without NS induction were also assessed after 48 h of treatment.
Statistical analysis
Data passed the Shapiro–Wilk normality test (p > 0.05). One-sample t-test with 50% as a standard value of interest or the hypothetical value was performed using the SPSS version 26 software (IBM Corporation, NY, USA). While one-way analysis of variance (ANOVA) with Tukey’s post hoc test and two-way ANOVA with Sidak’s post hoc test were computed using the GraphPad Prism version 6.01 software (San Diego, CA, USA). All analyses were two-tailed and p value less than 0.05 was considered significantly different.
Results
Drug cytotoxicity on U87 cell viability
None of the treatments showed a statistically significant difference in U87 cell viability (Table 1). In other words, the drug cytotoxicity does not substantially differ from the hypothesised value of 50%. Besides, one-way ANOVA revealed no statistically significant difference between TMZ (151.0 µg/ml) (positive control group), AZI (92.0 µg/ml), DOXY (147.0 µg/ml), and AZI + DOXY (92.0 + 147.0 µg/ml) treatments (F (3, 8) = 0.726, p = 0.565) (Fig. 2). All in all, there is insufficient evidence to reject the null hypothesis in favour of an alternative hypothesis. It could not be statistically corroborated that NS induction renders GBM cells tolerant to cytotoxic drugs under the present test conditions.
Drug cytotoxicity on U87 cell colonies
The cytotoxicity of tested drugs was further evaluated on U87 colonies that grew after the NS induction. One-way ANOVA discovered a statistically significant difference between groups (F (4, 40) = 7.455, p = 0.0001) (NC (61.89 (7.66), TMZ (47.00 (9.19), AZI (38.44 (7.02), DOXY (57.89 (15.68), and AZI + DOXY (51.33 (8.59)). Based on Tukey’s post hoc test, TMZ and AZI significantly diminished the number of U87 cell colonies compared to the untreated group or negative control (NC) (Fig. 3). AZI also markedly outperformed DOXY.
Drug cytotoxicity on L929 fibroblasts
To compare the cytotoxicity of studied drugs, fibroblasts were treated at the IC50 levels in GBM cells. Based on the comparison made to the 50% hypothetical value, only AZI + DOXY treatment significantly decreased the viability of L929 fibroblasts (Table 2). It is worth noting that the mean difference in L929 fibroblast viability across treatments was lower than the hypothetical value, contrasting to U87 cells (Table 1).
Further to that, a two-way ANOVA showed a non-significant difference between treatment groups (F (3, 16) = 1.303, p = 0.308) and a significant difference between treatment groups before (TMZ (40.27 (2.42), AZI (52.43 (7.42), DOXY (46.97 (10.26), and AZI + DOXY (46.40 (6.24)) and after NS induction (F (1, 16) = 36.56, p < 0.0001); however, the interaction between before and after NS was not significant (F (3, 16) = 2.418, p = 0.104). A Sidak’s post hoc test demonstrated that AZI, DOXY, and AZI + DOXY treatments exhibited an increased cytotoxic effect on L929 fibroblasts after the NS induction (Fig. 4). These data indicate that the induction of NS altered their sensitivity to tested drugs, except for TMZ, which had no meaningful difference between before and after NS. In fact, the lack of interaction between before and after NS suggests that the NS induction does not affect the cytotoxicity of the drug in fibroblasts; nevertheless, the type of drug may play a role.
Additionally, a two-way ANOVA showed a significant difference between treatment groups (F (3, 16) = 18.35, p < 0.0001) and a significant difference between fibroblast types (NIH3T3 vs. HDF) (F (1, 16) = 59.28, p < 0.0001) (TMZ (66.27 (11.23) vs. 99.17 (2.29), AZI (44.80 (2.12) vs. 56.83 (7.58), DOXY (48.40 (7.63) vs. 78.60 (3.26), and AZI + DOXY (61.23 (5.70) vs. 83.30 (13.55)); though, the interaction between fibroblast types was not significant (F (3, 16) = 2.21, p = 0.127). A Sidak’s post hoc test demonstrated that drug cytotoxicity differed across fibroblast types (Fig. 5), except for AZI. Despite this, the lack of interaction between fibroblast types suggests that the drug types may affect the effects.
Discussion
In a previous study, AZI and DOXY have shown promising anticancer effects in GBM cells [19]. Nevertheless, the role of NS in driving tumour cells to adaptive growth and survival, as well as its involvement in the development of drug resistance, is a matter of ongoing research.
The main finding herein is that NS-induced U87 cells essentially do not develop into a drug-tolerant phenotype. The mean differences in U87 cell viability across treatments were not statistically significant. Moreover, TMZ and AZI retained their effectiveness in reducing U87 cell colonies. In these situations, different drug mechanisms and targets that certainly influence the findings cannot be ruled out. Indeed, the probable divergence of biological activities and oncogenic signalling pathways between reseeded monolayer and grown aggregates warrants further exploration. In particular, NS promotes chemoresistance [13, 16]. As per Mondal and colleagues, NS induction leads to phenotypic reprogramming, driving U87 cells into a glioma stem cell-like phenotype. Explicitly, NS-induced U87 cells demonstrated increased drug efflux capacity, which is consistent with the MTT cell viability assay, as the cytotoxicity of TMZ, cisplatin, and paclitaxel decreased on these cells [16].
On the other hand, glucose deprivation caused a marked sensitisation of U87 cells to TMZ and carboplatin, nearly doubling the rate of apoptosis [13]. Nevertheless, further analysis revealed that it drove U87 and U251 GBM cells to exit the cell cycle and enter quiescence, and augmented autophagy, allowing them to withstand TMZ- and carboplatin-induced cytotoxicity. Indeed, autophagy is responsible not only in chemoresistance but also tumour survival by acting as a self-degradation channel that maintains metabolism and homeostasis, as well as an intracellular recycling platform that supplies the nutrients needed for growth [13, 14]. Likewise, when glucose was substituted with galactose, PANC1 pancreatic cancer cells switched to autophagy-dependent mitochondrial OXPHOS [12].
In contrast to the aforementioned, we previously found that 21-day survived U87 cells without medium replenishment were completely susceptible to AZI and DOXY treatments [19]. Notably, AZI exhibited preferential cytotoxicity in CAL27 oral squamous and Detroit 562 pharyngeal cancer cells under amino acid-deprived conditions by blocking autophagic flux [2]. GBM, like other cancers, prioritises glucose to produce adenosine triphosphate via aerobic glycolysis, but switches to mitochondrial respiration when nutrients or glucose are scarce [12, 13, 20]. At its core, AZI and DOXY have superior cytotoxicity to nutritionally stressed cells probably owing, in part, to the fact that they mediate cell death through mitochondrial respiration [3, 8,9,10] and autophagy inhibition [2, 4].
In a different study, tetrathiomolybdate (TM) has been discovered to be more toxic to neuroblastoma cells in low-glucose conditions, regardless of normoxia and hypoxia [21]. This can be partly attributed to the role of TM, which increases glucose consumption and activates glycolysis, making them vulnerable to death in low-glucose settings. Onodera and colleagues, alternatively, delineated that the redox system inhibitors, including penicillic acid (PA), have preferential cytotoxicity to PANC-1 pancreatic cancer cells in a nutrient-deprived condition [22]. In this case, nutrient deprivation raises the reactive oxygen species, while PA form an adduct with glutathione (GSH) and a lack of precursor amino acids required for GSH synthesis drops its level, rendering them defenceless to oxidative stress-mediated apoptotic death.
Herein, it was also discovered that L929 fibroblasts that survived for 48 days in nutrient-deprived conditions (re-enter the cell cycle and proliferate after being subjected to nutrient-favourable conditions) were more susceptible to AZI-, DOXY-, and AZI + DOXY-induced cytotoxicity. In different studies, AZI shows preferential cytotoxicity to senescent fibroblasts [23, 24], and AZI and DOXY both have senotherapeutic potential [24, 25]. These facts likely, in part, explain why the fibroblasts were more susceptible to them after NS induction. Certainly, NS activates autophagy, while autophagy promotes senescence in cancer cells and fibroblasts [14, 26]. Ironically, cancer cells with senescence reversal exhibit more aggressive behaviours [27], whereas autophagic-senescent fibroblasts promote tumour growth and metastasis metabolically through paracrine production of high-energy mitochondrial fuels [14].
Although acknowledging the specificity of drugs of interest is beyond the scope of the present work since fibroblasts were cultured under the same conditions as GBM cells, which may have altered their typical behaviours, data shows that they are adaptable in nature. Fibroblasts are known to have a high degree of plasticity and can change their behaviour depending on the signals and cues from the microenvironment [15]. In a different study, the IC50 value of AZI was reported to be 115 (49) µg/ml in primary human connective tissue-derived fibroblasts cultured in alpha-MEM supplemented with 10% FBS and 1 mM glutamine [28]. DOXY had an IC50 value of 310 µM in primary human skin-derived fibroblasts cultured in DMEM containing growth ingredients, including GlutaMAX, 4.5 g/l glucose, 10% FBS, and 1 mM sodium pyruvate [3]. In the study by Lamb et al., DOXY over the range of 50 to 500 µM showed little or no cytotoxicity in hTERT-BJ1 human foreskin fibroblasts (culture conditions not specified); though at 50 µM, it was significantly cytotoxic against MCF7 and T47D mammospheres [10].
Future recommendations
NS possesses the promise of sensitising cancer cells to chemotherapeutic regimens, which is intricately linked to the mechanism of action of the cytotoxic drugs. To further demonstrate the usefulness of AZI and/or DOXY in the context of cytotoxic preference in NS circumstances, the next envisioned phase entails the utilisation of an in vivo animal model that incorporates dietary restriction, such as through short-term fasting.
Conclusions
In short, this study suggests that NS does not inevitably contribute to cytotoxic drug tolerance in GBM cells. On the other hand, although fibroblasts can withstand NS, they can also become susceptible to cytotoxic drug-induced death; however, the type of drug may play a role.
Availability of data and material
All data generated or analysed during this study are available from the corresponding author on reasonable request.
Abbreviations
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- ATCC:
-
American type culture collection
- AZI:
-
Azithromycin
- ANOVA:
-
One-way analysis of variance
- DOXY:
-
Doxycycline
- DMEM:
-
Dulbecco’s modified eagle medium
- FBS:
-
Foetal bovine serum
- GBM:
-
Glioblastoma
- GSH:
-
Glutathione
- HDF:
-
Human dermal fibroblasts
- IC50 :
-
Half-maximal inhibitory concentration
- NS:
-
Nutrient stress
- NBF:
-
Neutral buffered formalin
- OXPHOS:
-
Oxidative phosphorylation
- PA:
-
Penicillic acid
- TMZ:
-
Temozolomide
- TME:
-
Tumour microenvironment
- TM:
-
Tetrathiomolybdate
References
El Atat O, Naser R, Abdelkhalek M, Habib RA, El Sibai M (2023) Molecular targeted therapy: a new avenue in glioblastoma treatment. Oncol Lett 25(2):1–33. https://doi.org/10.3892/ol.2022.13632
Hirasawa K, Moriya S, Miyahara K, Kazama H, Hirota A, Takemura J et al (2016) Macrolide antibiotics exhibit cytotoxic effect under amino acid-depleted culture condition by blocking autophagy flux in head and neck squamous cell carcinoma cell lines. PLoS ONE 11(12):e0164529. https://doi.org/10.1371/journal.pone.0164529
Protasoni M, Kroon AM, Taanman J-W (2018) Mitochondria as oncotarget: a comparison between the tetracycline analogs doxycycline and COL-3. Oncotarget 9(73):33818–33831
Zhang L, Xu L, Zhang F, Vlashi E (2017) Doxycycline inhibits the cancer stem cell phenotype and epithelial-to-mesenchymal transition in breast cancer. Cell Cycle 16(8):737–745. https://doi.org/10.1080/15384101.2016.1241929
Ozkan T, Hekmatshoar Y, Karabay AZ, Koc A, Gunes BA, Gurel AK et al (2021) Assessment of azithromycin as an anticancer agent for treatment of imatinib sensitive and resistant CML cells. Leuk Res 102:106523. https://doi.org/10.1016/j.leukres.2021.106523
Alshaman R, Alattar A, El-Sayed RM, Gardouh AR, Elshaer RE, Elkazaz AY et al (2022) Formulation and characterization of doxycycline-loaded polymeric nanoparticles for testing antitumor/antiangiogenic action in experimental colon cancer in mice. Nanomaterials 12(5):857. https://doi.org/10.3390/nano12050857
Li F, Huang J, Ji D, Meng Q, Wang C, Chen S et al (2017) Azithromycin effectively inhibits tumor angiogenesis by suppressing vascular endothelial growth factor receptor 2-mediated signaling pathways in lung cancer. Oncol Lett 14(1):89–96. https://doi.org/10.3892/ol.2017.6103
Fiorillo M, Tóth F, Sotgia F, Lisanti MP (2019) Doxycycline, azithromycin and vitamin C (DAV): a potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs). Aging (Albany NY) 11(8):2202–2216
Lamb R, Fiorillo M, Chadwick A, Ozsvari B, Reeves KJ, Smith DL et al (2015) Doxycycline down-regulates DNA-PK and radiosensitizes tumor initiating cells: Implications for more effective radiation therapy. Oncotarget 6(16):14005–14025
Lamb R, Ozsvari B, Lisanti CL, Tanowitz HB, Howell A, Martinez-Outschoorn UE et al (2015) Antibiotics that target mitochondria effectively eradicate cancer stem cells, across multiple tumor types: treating cancer like an infectious disease. Oncotarget 6(7):4569–4584
Anastasiou D (2017) Tumour microenvironment factors shaping the cancer metabolism landscape. Br J Cancer 116(3):277–286. https://doi.org/10.1038/bjc.2016.412
Shiratori R, Furuichi K, Yamaguchi M, Miyazaki N, Aoki H, Chibana H et al (2019) Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Sci Rep 9:18699. https://doi.org/10.1038/s41598-019-55296-3
Wang L, Shang Z, Zhou Y, Hu X, Chen Y, Fan Y et al (2018) Autophagy mediates glucose starvation-induced glioblastoma cell quiescence and chemoresistance through coordinating cell metabolism, cell cycle, and survival. Cell Death Dis 9:213. https://doi.org/10.1038/s41419-017-0242-x
Capparelli C, Guido C, Whitaker-Menezes D, Bonuccelli G, Balliet R, Pestell TG et al (2012) Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis, via glycolysis and ketone production. Cell Cycle 11(12):2285–2302. https://doi.org/10.4161/cc.20718
Kalluri R (2016) The biology and function of fibroblasts in cancer. Nat Rev Cancer 16(9):582–598. https://doi.org/10.1038/nrc.2016.73
Mondal S, Bhattacharya K, Mandal C (2018) Nutritional stress reprograms dedifferention in glioblastoma multiforme driven by PTEN/Wnt/Hedgehog axis: a stochastic model of cancer stem cells. Cell Death Dis 4:110. https://doi.org/10.1038/s41420-018-0126-6
Miyo M, Konno M, Nishida N, Sueda T, Noguchi K, Matsui H et al (2016) Metabolic adaptation to nutritional stress in human colorectal cancer. Sci Rep 6:38415. https://doi.org/10.1038/srep38415
Dvorak HF (1986) Tumors: wounds that do not heal. N Engl J Med 315(26):1650–1659. https://doi.org/10.1056/nejm198612253152606
Hassan SN, Mohamed Yusoff AA, Idris Z, Mohd Redzwan N, Ahmad F (2021) Exploring the cytotoxicity and anticancer effects of doxycycline and azithromycin on human glioblastoma multiforme cells. Neurol Res 44(3):242–251. https://doi.org/10.1080/01616412.2021.1975225
Kant S, Kesarwani P, Prabhu A, Graham SF, Buelow KL, Nakano I et al (2020) Enhanced fatty acid oxidation provides glioblastoma cells metabolic plasticity to accommodate to its dynamic nutrient microenvironment. Cell Death Dis 11(4):253. https://doi.org/10.1038/s41419-020-2449-5
Navrátilová J, Hankeová T, Beneš P, Šmarda J (2013) Low-glucose conditions of tumor microenvironment enhance cytotoxicity of tetrathiomolybdate to neuroblastoma cells. Nutr Cancer 65(5):702–710. https://doi.org/10.1080/01635581.2013.789118
Onodera T, Momose I, Adachi H, Yamazaki Y, Sawa R, Ohba S-i et al (2020) Human pancreatic cancer cells under nutrient deprivation are vulnerable to redox system inhibition. J Biol Chem 295(49):16678–16690. https://doi.org/10.1074/jbc.RA120.013893
Ozsvari B, Nuttall JR, Sotgia F, Lisanti MP (2018) Azithromycin and Roxithromycin define a new family of “senolytic” drugs that target senescent human fibroblasts. Aging (Albany NY) 10(11):3294–3307
Krempaska K, Barnowski S, Gavini J, Hobi N, Ebener S, Simillion C et al (2020) Azithromycin has enhanced effects on lung fibroblasts from idiopathic pulmonary fibrosis (IPF) patients compared to controls. Respir Res 21:25. https://doi.org/10.1186/s12931-020-1275-8
Li X, Khan D, Rana M, Hänggi D, Muhammad S (2022) Doxycycline attenuated ethanol-induced inflammaging in endothelial cells: implications in alcohol-mediated vascular diseases. Antioxidants 11(12):2413. https://doi.org/10.3390/antiox11122413
Slobodnyuk K, Radic N, Ivanova S, Llado A, Trempolec N, Zorzano A et al (2019) Autophagy-induced senescence is regulated by p38α signaling. Cell Death Dis 10:376. https://doi.org/10.1038/s41419-019-1607-0
Yang L, Fang J, Chen J (2017) Tumor cell senescence response produces aggressive variants. Cell Death Discov 3:17049. https://doi.org/10.1038/cddiscovery.2017.49
Jiang X, Baucom C, Elliott RLJA (2019) Mitochondrial toxicity of azithromycin results in aerobic glycolysis and DNA damage of human mammary epithelia and fibroblasts. Antibiotics 8(3):110. https://doi.org/10.3390/antibiotics8030110
Acknowledgements
The authors would like to thank Postgraduate Funding Grant (311/PPSP/4404811) and Universiti Sains Malaysia Fellowship provided by the Universiti Sains Malaysia.
Funding
This research was funded by the Ministry of Higher Education Malaysia through the FRGS (203/PPSP/6171203).
Author information
Authors and Affiliations
Contributions
SNH: Conceptualisation, data acquisition, analysis, and writing-original draft preparation; FA: Supervision, conceptualisation, methodology, reviewing, and editing; AAMY: Supervision, conceptualisation, and methodology; ZI: Supervision and conceptualisation; NMR: Supervision and methodology. The final manuscript was revised by SNH and FA.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors declare no conflict of interest.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, S.N., Yusoff, A.A.M., Idris, Z. et al. A preliminary study on the impact of nutrient stress induction on drug cytotoxicity in glioblastoma cells and fibroblasts. Futur J Pharm Sci 10, 65 (2024). https://doi.org/10.1186/s43094-024-00637-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00637-x