Abstract
Background
Diabetes mellitus is one of the leading causes of morbidity and mortality globally. Although synthetic hypoglycemic agents are commonly used to manage this disorder, such medications, besides being unable to cure the disease, are expensive and associated with side effects. Conversely, medicinal plants have emerged as effective, safe and affordable alternative treatments. Boswellia dalzielii plant has been reported to possess ethnomedicinal properties for the treatment of various health conditions; however, scientific studies exploring this plant as antihyperglycemic agent are still limited. Thus, this study evaluated the antihyperglycemic activity of aqueous stem bark extract (ASBE) of B. dalzielii in alloxan-induced diabetic Wistar albino rats.
Methods
Phytochemical screening of the ASBE of B. dalzielii was conducted. Twenty male Wistar albino rats weighing 100–150 g divided into 4 groups (A–D) of five rats were used for the study. Group A served as the normal control and received neither ASBE of B. dalzielii nor glibenclamide. The treatment for the other three groups was as follows: Group B, 10 mg/kg of glibenclamide (diabetic control); Group C, 500 mg/kg ASBE of B. dalzielii; and Group D, 1000 mg/kg ASBE of B. dalzielii. Treatments were administered orally every 24 h for a period of 2 weeks. Blood glucose level and body weight were evaluated at weeks 0, 1 and 2. Histomorphological features of the rats’ pancreas in all the groups were compared.
Results
The phytochemical analysis revealed the presence of alkaloids, saponins, tannins, cardiac glycosides, flavonoids, carbohydrates, steroids and triterpenes. The two different doses of the plant extract significantly reduced blood glucose level at weeks 1 and 2 (all p < 0.05), with the 1000 mg/kg dose demonstrating a greater reduction compared with glibenclamide at week 2 (p = 0.014). However, only the 500 mg/kg dose led to restoration, albeit slight, of the pancreatic islet cells.
Conclusion
This study suggests that B. dalzielii plant exhibits a potent antihyperglycemic activity evidenced by reduced blood glucose levels and slight restoration of pancreatic islet cells. This plant could be, therefore, considered in the treatment of diabetes mellitus.
Graphical Abstract

Similar content being viewed by others
Background
Diabetes mellitus (DM), a common chronic metabolic disorder characterized by abnormal glucose homeostasis resulting in hyperglycemia (high blood glucose levels [BGLs]), is one of the leading causes of morbidity and mortality globally. According to current estimates, about 573 million adults (aged 20–79 years) are living with DM and this figure is projected to rise to 783 million by 2045 if no effective prevention methods are implemented [1]. In 2021, DM accounted for 6.7 million deaths representing 1 death every 5 seconds [2]. Moreover, the global healthcare expenditures on people with DM were estimated to be 966 billion USD and are projected to reach 1054 billion USD by 2045 [1]. Accordingly, the escalating prevalence, deaths, and healthcare expenditure due to DM inflict a colossal social, financial and health system burden globally [3].
The impact of living with DM is concerning, particularly in low- and middle-income countries as the prevalence is growing rapidly and the majority of DM-related deaths occur in these regions owing to the higher number of undiagnosed cases in addition to factors such as lack of education, low levels of income, difficulty in accessing healthcare and medications, and insufficient health expenditure [4, 5]. This is especially true to Sub-Saharan African countries like Nigeria with an estimated DM prevalence of 11.2 million people (5.8%) [6], and a mortality rate of 30.2 per 100,000 population [7] suggesting that effective preventive and treatment strategies are highly needed.
Although DM is commonly treated with the use of synthetic hypoglycemic agents such as insulin secretagogues (sulfonylureas, meglitinides), insulin sensitizers (biguanides, metformin, thiazolidinediones) and α-glucosidase inhibitors (miglitol, acarbose), these medications are unable to cure the disease since they cannot restore normal glucose homeostasis [8]. Additionally, they are often associated with side effects [9], besides being inaccessible and unaffordable for most people, especially in low-resource or rural settings [10]. Conversely, medicinal plants have been employed by humans since ancient times to prevent or cure diseases including DM [11]. Indeed, traditional medicinal practitioners from various parts of the world claim to cure DM or at least lessen its major symptoms and progression owing to the potent antidiabetic properties of medicinal plants [12]. Consequently, herbal diabetic medicines have emerged as effective, safe and affordable alternative treatments for DM.
Of the various medicinal plants discovered to possess potent ethnomedicinal properties is the Boswellia dalzielii–commonly known as “Frankincense tree” belonging to the family Burseraceae. The plant is a tall tree growing up to 13 m high and produce aromatic white flowers [13]. It is widely spread in many African countries such as Burkina Faso, Togo, Cameroon, Benin, Ghana, Ivory Coast, Nigeria and Central African Republic [13, 14], and has been traditionally used as a medicament for several diseases and ailments. In northern Nigeria, this plant is locally called Hannu or Ararrabi in the Hausa language (meaning to prevent bad luck) [15].
Although B. dalzielii plant, particularly the stem back extract, has been widely reported as antibacterial, antifungal, antiinflammatory, antiarthritic and antispasmodic agents [13, 16,17,18,19,20], scientific studies exploring this plant as antihyperglycemic agent are still limited [21]. Thus, the purpose of this study was to evaluate the antihyperglycemic activity of aqueous stem bark extract (ASBE) of B. dalzielii in alloxan-induced diabetic Wistar rats for potential use in the treatment of DM.
Methods
Material and reagents
Alloxan monohydrate was purchased from Sigma St. Louis (Missouri, USA). Ketamine (Ketamine®) was purchased from Panpharma Gmbh (Rotexmedica, Germany). Diazepam (Valium®) was purchased from Hoffman-La Roche Ltd. (Ontario, Canada). BGLs were measured using digital glucometer (Accu-check advantage, Roche Diagnostic, Germany). Body weight of the rats was measured using a digital electronic lab weighing scale (KERRO, BL-2000, China).
Collection of plant materials
Fresh stem bark of B. dalzielii was collected from Kufena village, Zaria, Kaduna State, Nigeria, and botanical authentication of the plant parts took place at the herbarium unit of Department of Biological Sciences, Ahmadu Bello University (ABU), Zaria, Kaduna State, Nigeria. The voucher sample (0900121) and photographs were deposited at the institute for future reference.
Experimental animals
Wistar strain male albino rats weighing between 100 and 150 g from the Animal House, Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmaceutical Sciences, ABU, Zaria, Kaduna State, Nigeria were obtained and housed under standard environmental conditions. The animals were allowed free access to food (grower and starter mash) and water for a period of two weeks to get acclimatize before the commencement of the experiment. All animal procedures and experimental protocol were strictly followed in accordance with the National Research Council, Guide for the Care and Use of Laboratory Animals [22]. The study was approved by the Research and Ethical Committee on the use of laboratory animals of Nigerian Defence Academy, Kaduna, Kaduna State, Nigeria.
Preparation of plant extract
The fresh stem bark was cleaned, air-dried and pounded into a coarse powder using a mortar and pestle. The powder obtained was extracted with water using a soxhlet apparatus, and the solvent was removed in a vacuum and evaporated using a rotary evaporator at 60 °C to obtain extraction yield of 121.13 g of the plant extract. The extracted yield was then stored in a refrigerator at 4 °C till usage. To prepare the different dosages of the plant extract (500 and 1000 mg/kg) for the study experiment, 5 g/5000 mg and 10 g/1000 mg of the plant extract were each dissolve in 10 ml of distilled water. Dosage administered to each animal was calculated using the formula: volume = dose/stock × animal weight (kg).
Phytochemical screening
Phytochemical screening of the ASBE of B. dalzielii was conducted at the Pharmacognosy Department, Faculty of Pharmaceutical Sciences, ABU, Zaria, Kaduna State, Nigeria following standardized protocols as reported in the literature [23,24,25]. The extract was screened for alkaloids using Mayer’s test and Wagner’s test, saponins using Frothing test, tannins using Ferichloride test, anthracene derivatives using Bontrager's test, cardiac glycosides using Keller–Killiani test and NaOH test, flavonoids using Shinoda test and NaOH test, carbohydrates using Mollish test, and steroids and triterpenes using Liebermann–Burchard test.
Acute toxicity test
The median lethal doses (LD50) of the ASBE of B. dalzielii were determined in 12 rats in accordance with the method described by Lorke [26], which involves two phases. In the first phase, the rats were divided into three groups with each group consisting of 3 animals. They were then treated with the extract at doses of 10, 100, and 1000 mg/kg body weight orally and observed for 24 h for signs of toxicity. In the second phase, 3 rats were divided into three groups with each group consisting of 1 animal. They were also treated with the same extract but at doses of 1600, 2900, and 5000 mg/kg body weight orally. The median lethal dose (LD50) was obtained using the second phase.
Induction of diabetes mellitus
Twenty male Wistar strain albino normoglycemic rats (weighing 100–150 g) were used for the study. They were fasted overnight for the duration of 12 h but allowed water ad libitum. DM was induced in fasted rats by a single intraperitoneal injection of Alloxan Monohydrates at the dose of 150 mg/kg body weight [27]. The animals were allowed free access to 5% glucose solution to overcome the drug-induced hypoglycemia [28]. BGLs of these rats was estimated 72 h after Alloxan administration, and diabetes was confirmed by blood samples collected from the tip of the tail using Accu-check Advantage digital glucometer. Animals with BGLs equivalent to or more 200 mg/dl were declared diabetic [29] and were used in the entire experimental group.
Experimental design
The study animal rats were divided into four groups (A–D) of five rats (n = 5) as follows:
-
Group A: represents normal control group and received 10 ml/kg of sterile distilled water orally.
-
Group B: represents diabetic control group and received a 10 mg/kg of glibenclamide orally.
-
Group C: represents experimental group and received oral dose of 500 mg/kg ASBE of B. dalzielii.
-
Group D: represents experimental group and received oral dose of 1000 mg/kg ASBE of B. dalzielii.
All animals fasted overnight for 12 h prior to baseline determination of BGLs and treatments (oral feeds). All treatments were administered on daily basis for 2 weeks. Both BGLs (measured by collecting blood samples from the tip of their tail artery using the digital glucometer expressed in mg/dl) and body weight (by measuring with the KERRO, BL-2000 digital electronic lab weighing scale expressed in grams [g]) were measured at week 0 (first day of treatment), week 1 (7th day of treatment) and week 2 (14th day of treatment).
Histopathological investigation of pancreas
At the end of the study, 1 animal selected randomly from each of the study groups was sacrificed using Ketamine and Diazepam injections intramuscularly at a dose of 75 mg/kg and 5 mg/kg, respectively. An incision was made in the abdomen and the pancreas was removed, washed with cold saline and preserved in 10% buffered formalin. Section of the pancreas was stained in hematoxylin and eosin and then observed with a light microscope at × 250 magnification. The histology was conducted at the Gross Anatomy Research Laboratory, Department of Human Anatomy, Faculty of Basic Medical Sciences, ABU, Zaria, Kaduna State, Nigeria.
Statistical analysis
Descriptive statistics of mean and standard error of measurement (mean ± SEM) were used to summarize the data. To analyze the effect of treatment on body weight and BGLs, a within- and between-subjects design analysis of variance (ANOVA) with group (normal control, diabetic control, 500 mg/kg ASBE of B. dalzielii, 1000 mg/kg ASBE of B. dalzielii) as between-subjects factor and time (week 0, week 1, week 2) as within-subjects factor was applied using the General Linear Model. Post-hoc test with Least Significant Difference (LSD) was used for multiple pairwise comparisons in case of any significant ANOVA. All statistical analyses were performed using SPSS version 23.0 (IBM Co., Armonk, NY, USA) with the level of significance set at p < 0.05.
Results
Phytochemical screening
The results of the phytochemical screening revealed the presence of all the expected constituents (alkaloids, carbohydrates, cardiac glycosides, flavonoids, saponins, steroids, tannins and triterpenes) in the ASBE of B. dalzielii except anthraquinones (Table 1).
Acute toxicity test
The results of the acute toxicity test in the first and the second phase revealed no mortality. Therefore, the LD50 was determined to be above 5000 mg/kg.
Effect of aqueous stem bark extract of B. dalzielii on body weight
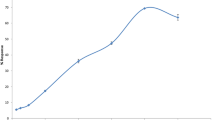
As shown in Table 2, within-subjects ANOVA revealed that there was a statistically significant change in body weight for the normal control (p = 0.008) and diabetic control (p = 0.021) groups across the weeks. Post-hoc analysis with LSD showed a significant increase in body weight from week 0 to week 1 (p < 0.05) and from week 0 to week 2 (p < 0.05) but from week 1 to week 2 (p > 0.05) for the normal control and diabetic control groups. However, no statistically significant change in body weight was observed for the two experimental groups across the weeks (p > 0.05) even though there was a trend indicating a reduction in body weight (Table 2).
Between-subjects ANOVA revealed that there was no statistically significant difference (p > 0.05) in body weight between the groups at all time points (Table 2). Figure 1 shows the trend of body weight scores among the groups across the weeks.
Effect of aqueous stem bark extract of B. dalzielii on blood glucose level
As shown in Table 3, there was a statistically significant change in BGLs in the two experimental groups (500 mg/kg ASBE of B. dalzielii, p = 0.016; and 1000 mg/kg ASBE of B. dalzielii, p = 0.001) across the weeks as revealed by within-subjects ANOVA. Post-hoc analysis with LSD showed a significant reduction in BGLs between week 0 and 1 (p < 0.05) and between week 0 and week 2 (p < 0.05) but between week 1 and week 2 (p > 0.05). For the normal and diabetic control groups, no statistically significant change in BGLs was observed across the weeks (p > 0.05).
Between-subjects ANOVA revealed statistically significant difference (p > 0.05) in BGLs between the groups across the weeks (Table 3). At week 0, post-hoc analysis with LSD showed that the diabetic control group and the two experimental groups were comparable (p > 0.05) and had higher BGLs compared to the normal control group (p < 0.05). At week 1, post-hoc analysis with LSD showed that the normal control group had lower BGLs compared with the diabetic control group and the two experimental groups (p < 0.05), but the experimental group treated with 1000 mg/kg ASBE of B. dalzielii had lower BGLs (p = 0.014) compared with the diabetic control group. At week 2, post-hoc analysis showed that the normal control group had lower BGL compared with the diabetic control group and the two experimental groups (p < 0.05), but the experimental group treated with 1000 mg/kg ASBE of B. dalzielii had lower BGL compared with the diabetic control group (p = 0.001) and the experimental group treated with 500 mg/kg ASBE of B. dalzielii (p = 0.016). Figure 2 shows the trend of BGLs among the groups across the weeks.
Histomorphological investigation of pancreas
The histology study of the pancreatic islet cells of the animals in all the groups are shown in Fig. 3.
In the normal control group (NC), the histology demonstrated a normal pancreas. The islet cells were full of centrally placed beta cells, appeared very compact, and surrounded by seroacinar cells. The nuclei capillaries were also normal (Fig. 3). In the diabetic control group (DC), the histology demonstrated a lack of restoration of the islet cells. A lymphatic infiltration which results in inflammation is demonstrated. These extensive necrotic changes are accompanied by fibrosis and atrophy. In the group treated with 500 mg/kg ASBE of B. dalzielii (500-BD), the histology demonstrated a slight restoration of the islet cells with evidence of destruction and atrophy (Fig. 3). In the group treated with 1000 mg/kg ASBE of B. dalzielii (1000-BD), the histology demonstrated a lack of islet cells with accompanying necrotic changes in the pancreas (Fig. 3).
Discussion
The rising prevalence of DM and the costs, as well as side effects associated with current synthetic medications, necessitate the discovery of affordable and safe alternative therapies to battle this troublesome disease. Indeed, the World Health Organization Expert Committee [30] has so far recommended medicinal plants for the management of DM as well as studies into exploring traditional medicines for this refractory disease. In the quest to search for natural remedies for DM, the present study was undertaken to evaluate the antihyperglycemic activity of ASBE of B. dalzielii in alloxan-induced diabetic Wistar rats for potential use in the treatment of DM. The findings of the present study revealed that the ASBE of B. dalzielii exhibits a potent hypoglycemic activity.
The phytochemical screening of the present study revealed the presence of alkaloids, saponins, tannins, cardiac glycosides, flavonoids, carbohydrates, steroids, and triterpenes. These phytochemicals exhibit either cytoprotective function on the pancreatic beta cells or insulino-protective function, resulting in hypoglycemic effects and fewer diabetes complications [31, 32]. More specifically, flavonoids are known to promote pancreatic beta cells proliferation leading to increased insulin secretion and sensitivity [33]. In another vein, flavonoids and tannins may exhibit promising inhibitory potential against α-amylase and α-glucosidase thereby reducing postprandial hyperglycemia [34, 35]. Additionally, alkaloids play a key role against hyperglycemia by promoting glucose consumption and glycogen synthesis through various mechanisms by inhibiting or inducing multiple candidate proteins such as AMP-activated protein kinase, glucose transporters, glycogen synthase kinase-3, sterol regulatory element-binding proteins 1, glucokinase, glucose-6-phosphatase, and acetyl-CoA carboxylase [36]. The presence of these promising compounds in the ASBE of B. dalzielii may, thus, account for the antidiabetic effect observed among the diabetic Wistar rats in the present study. Similar to the findings of the present study, the study by Balogun et al. [21] found the presence of saponins, tannins and flavonoids in the ASBE of B. dalzielii. Additionally, the study by Mamza et al. [37] found the presence of similar phyto-compounds in addition to alkaloids, cardiac glycosides and carbohydrates. In contrast, the study by Nwinyi et al. [17] found the presence of only tannins in the ASBE of this plant. In a study [38] on the complete pharmacognostic evaluation of the leaves, stem bark and root of B. dalzielii, the presence of saponins, tannins, flavonoids, cardiac glycosides and terpenes were reported which all illustrate the therapeutic potentials of this plant.
Regarding body weight changes, our study demonstrated a significant increase in body weight in both the normal and diabetic control groups across time. The two experimental groups, however, had a decrease in body weight, albeit these changes were not statistically significant. While between-group analysis revealed no significant difference in the body weight between the groups at any study point, a decrease in body weight observed in the two experimental groups suggests a potential hypoglycemic activity induced by the different doses of ASBE of B. dalzielii. Reduced body weight is associated with low insulin resistance and optimal glycemic control [39]. The improvement in body weight among the experimental animals could be attributed to the activity of the phytochemicals such as flavonoids in the B. dalzielii plant [40]. In line with our study, a previous study also found a significant reduction in body weight among diabetic Wistar rats receiving an aqueous extract of various medicinal plants compared to normal and diabetic controls [41].
As regard to changes in BGLs, our results indicated that the experimental groups had a significant reduction in BGLs after week 1 and 2 of treatment, with the greatest reduction being more evident at week 2 of treatment. The normal and diabetic control groups, however, did not observe a significant reduction in the BGLs at any week of the study. While the different doses of ASBE of B. dalzielii demonstrated potent hypoglycemic activity across the weeks, between-group comparisons indicated that the 1000 mg/kg dose significantly reduced BGLs compared to the diabetic control group at week 2. This finding suggests that the stem bark extract of the B. dalzielii plant exhibit hypoglycemic activity better than a standard oral synthetic drug (glibenclamide) but in a dose-dependent manner. Supporting the results of the present study, the study conducted by Balogun et al. [21] found both 153 mg/kg and 297 mg/kg doses of ASBE of B. dalzielii to be effective at decreasing BGLs similar to that of 400 mg/kg of chlorpropamide (a standard hypoglycemic agent) among hyperglycemic rats. In addition, Yakubu et al. [15] found that a 400 mg/kg dose of various partitioned portions of crude methanol extract of B. dalzielii resulted in significant hypoglycemic activity compared with 2 mg/kg of glibenclamide in alloxan-induced hyperglycemic rats.
In the present study, alloxan monohydrate (a pyrimidine derivative, 2,4,5,6-tetraoxypyrimidine) [42] at a dose of 150 mg/kg was used to chemically induce type I diabetes in the study mice. The mechanism by which alloxan induces diabetes has been largely ascribed to rapid uptake by the beta cells and the formation of free radicals, to which beta cells have poor defense mechanisms to [43]. Thus, the hypoglycemic activity observed in the ASBE of B. dalzielii in our study could be accounted for by the antioxidant activity of the plant thereby blocking the formation of free radicals.
The results of histomorphological investigation of the pancreatic tissue in the current study revealed normal islet cells in the pancreas of the normal rats. Alloxan resulted in the destruction of the islet cells and we expect that the use of ASBE of B. dalzielii might cause significant restoration of these cells while reducing BGLs. However, slight restoration of the islet cells was observed in the rats receiving 500 mg/kg ASBE of B. dalzielii whereas neither the 1000 mg/kg dose of the plant extract nor the diabetic control group exhibited a discernible restoration of islet cells when compared with the normal control group. Although the lack of restoration of islet cells in the group receiving 1000 mg/kg ASBE of B. dalzielii is quite surprising given that the BGLs of this particular group significantly improved across time compared with the rest of the groups. Nevertheless, considering the duration of treatment in our study, which was relatively small, significant restoration is more likely to occur with longer follow-ups. In contrast to the present study, our previous work [44] showed that 1000 and 2000 mg/kg doses of ASBE of Parinari macrophylla resulted in restoration of the islet cells with the 2000 mg/kg dose being more evident after two weeks of treatment. The variations in results across these studies could be ascribed to the variations in the efficacy of different plants besides the variations in the dosage administered. Thus, it is possible that regeneration of the islet cells could also be achieved with higher doses.
Conclusion
Based on the findings of the present study, it can be concluded that the ASBE of B. dalzielii exhibits a potent anti-diabetic activity in alloxan-induced diabetic Wistar rats owing to its hypoglycemic effect. Moreover, the plant extract led to a slight restoration of pancreatic islet cells. While this plant may potentially be considered in the treatment of DM, future rigorous scientific studies examining multiple doses and with longer follow-up are warranted to establish the most optimal treatment dose of this promising plant for the management of DM.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
Abbreviations
- ABU:
-
Ahmadu Bello University
- ANOVA:
-
Analysis of variance
- ASBE:
-
Aqueous stem bark extract
- BD:
-
Boswellia dalzielii
- BGLs:
-
Blood glucose levels
- DM:
-
Diabetes mellitus
- LD50 :
-
Median lethal dose
- LSD:
-
Least significant difference
- NaOH:
-
Sodium hydroxide
- NC:
-
Normal control
- SD:
-
Standard deviation
- SEM:
-
Standard error of measurement
- WHO:
-
World Health Organization
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ (2022) IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
International Diabetes Federation (2021) IDF Diabetes Atlas, 10th edn. Brussels, Belgium. https://www.diabetesatlas.org. Accessed 23 April 2022
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B (2018) IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 138:271–281
Beran D (2015) The impact of health systems on diabetes care in low and lower middle income countries. Curr Diabetes Rep 15(4):20
Karachaliou F, Simatos G, Simatou A (2020) The challenges in the development of diabetes prevention and care models in low-income settings. Front Endocrinol 11:518
Uloko AE, Musa BM, Ramalan MA, Gezawa ID, Puepet FH, Uloko AT, Borodo MM, Sada KB (2018) Prevalence and risk factors for diabetes mellitus in Nigeria: a systematic review and meta-analysis. Diabetes Ther 9(3):1307–1316
Adeloye D, Ige JO, Aderemi AV, Adeleye N, Amoo EO, Auta A, Oni G (2017) Estimating the prevalence, hospitalisation and mortality from type 2 diabetes mellitus in Nigeria: a systematic review and meta-analysis. BMJ Open 7(5):e015424
Chege IN, Okalebo FA, Guantai AN, Karanja S, Derese S (2015) Management of type 2 diabetes mellitus by traditional medicine practitioners in Kenya-key informant interviews. Pan Afr Med J 22:90
Corathers SD, Peavie S, Salehi M (2013) Complications of diabetes therapy. Endocrinol Metab Clin 42(4):947–970
Moucheraud C, Lenz C, Latkovic M, Wirtz VJ (2019) The costs of diabetes treatment in low- and middle-income countries: a systematic review. BMJ Glob Health 4(1):e001258
Jung M, Park M, Lee HC, Kang YH, Kang ES, Kim SK (2006) Antidiabetic agents from medicinal plants. Curr Med Chem 13(10):1203–1218
Rahmatullah M, Azam MN, Khatun Z, Seraj S, Islam F, Rahman MA, Jahan S, Aziz MS (2012) Medicinal plants used for treatment of diabetes by the Marakh sect of the Garo tribe living in Mymensingh district, Bangladesh. Afr J Tradit Complement Altern Med 9(3):380–385
Mbiantcha M, Almas J, Atsamo AD, Ateufack G, Shabana SU, Bomba Tatsinkou DF, Yousseu Nana W, Nida D (2018) Anti-inflammatory and anti-arthritic effects of methanol extract of the stem bark of Boswellia dalzielii Hutch (Burseraceae) in rats. Inflammopharmacology 26(6):1383–1398
Nazifi A, Danjuma N, Olurishe T, Ya’u J (2017) Behavioural effects of methanol stem bark extract of Boswellia dalzielii Hutch (Burseraceae) in mice. Afr J Biomed Res 20(1):103–108
Yakubu J, Mamza UT, Balami VM, Medugu AN, Inna F, Abdulrahman OAS (2020) Antidiabetic effects of partitioned methanol extract of Boswellia dalzielii (Frankincense tree) on rats. J Phytopharmacol 9(4):224–229
Adelakun EA, Finbar EA, Agina SE, Makinde AA (2001) Antimicrobial activity of Boswellia dalzielii stem bark. Fitoterapia 72(7):822–824
Nwinyi FC, Binda L, Ajoku GA, Aniagu SO, Enwerem NM, Orisandipe A, Kubmarawa D, Gamaniel KS (2004) Evaluation of the aqueous extract of Boswellia dalzielii stem bark for antimicrobial activities and gastrointestinal effects. Afr J Biotechnol 3(5):284–288
Hassan H, Musa A, Usman M, Abdulaziz M (2009) Preliminary phytochemical and antispasmodic studies of the stem bark of Boswellia dalzielii. Nig J Pharm Sci 8(1):1–6
Danlami U, Daniel GJ, David BM, Galadanchi KM (2015) Phytochemical, nutritional and antimicrobial screening of hexane, ethyl acetate and ethanolic extracts of Boswellia dalzielii leaves and bark. Am J Biosci Bioeng 3(5):76–79
Adebisi I, Giaze T (2018) Analgesic effect and anti-inflammatory activity of aqueous extract of Boswellia dalzielii (burseraceae) stem bark. Int J Pharm Pharm Sci 10(4):139–140
Balogun O, Ojerinde SO, Alemika TM (2013) Hypoglycemic effect of the aqueous stem bark extract of Boswellia dalzielii Hutch. Cont J Pharm Sci 7(1):36–41
National Research Council (2011) Guide for the care and use of laboratory animals, 8th edn. The National Academies Press, Washington
Gul R, Jan SU, Faridullah S, Sherani S, Jahan N (2017) Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci World J 2017:5873648
Odebiyi OO, Sofowora EA (1978) Phytochemical screening of Nigerian medicinal plants II. Lloydia 41(3):234–246
Sofowora A (1993) Phytochemical screening of medicinal plants and traditional medicine in Africa. Spectrum Books Limited, Nigeria, pp 150–156
Lorke D (1983) A new approach to practical acute toxicity testing. Arch Toxicol 54(4):275–287
Katsumata K, Katsumata K Jr, Katsumata Y, Ozawa T (1994) Acute and chronic effect of ethanol on the occurrence of alloxan diabetes in rats. Horm Metab Res 26(4):166–168
Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N (2002) Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res 46(3):251–255
Stanely Mainzen Prince P, Menon VP (2001) Antioxidant action of Tinospora cordifolia root extract in alloxan diabetic rats. Phytother Res 15(3):213–218
Alberti KGMM (1980) The World Health Organisation and diabetes. Diabetologia 19:169–173
Adeneye AA, Ajagbonna OP, Ayodele OW (2007) Hypoglycemic and antidiabetic activities on the stem bark aqueous and ethanol extracts of Musanga cecropioides in normal and alloxan-induced diabetic rats. Fitoterapia 78(7–8):502–505
Bacanli M, Dilsiz SA, Başaran N, Başaran AA (2019) Effects of phytochemicals against diabetes. Adv Food Nutr Res 89:209–238
Mahesh T, Menon VP (2004) Quercetin allievates oxidative stress in streptozotocin-induced diabetic rats. Phytother Res 18(2):123–127
Tadera K, Minami Y, Takamatsu K, Matsuoka T (2006) Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J Nutr Sci Vitaminol 52(2):149–153
Jeyakumaran P, Perera K, Fernando WIT, Jayasinghe L, Ramiah S (2015) α-Glucosidase and α-amylase inhibitory activities of nine Sri Lankan antidiabetic plants. Br J Pharm Res 7:365–374
Muhammad I, Rahman N, Nishan U, Shah M (2021) Antidiabetic activities of alkaloids isolated from medicinal plants. Braz J Pharm Sci 57:e19130
Mamza UT, Yakubu J, Chiroma M, Balami VM, Moses S, Sodipo OA, Abdulrahman FI, Alemika TE, Khan IZ (2021) Phytochemical evaluation and in-vitro antibacterial properties of the methanolic leaf extract of Boswellia dalzielii Hutch. (Burseraceae). Bull Pure Appl Sci 40c:48–56
Trinder P (1969) Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem 6(1):24–27
Knudsen LB (2010) Liraglutide: the therapeutic promise from animal models. Int J Clin Pract Suppl 167:4–11
Keshari AK, Kumar G, Kushwaha PS, Bhardwaj M, Kumar P, Rawat A, Kumar D, Prakash A, Ghosh B, Saha S (2016) Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. J Ethnopharmacol 181:252–262
Gupta R, Sharma A (2017) Anti-hyperglycemic activity of aqueous extracts of some medicinal plants on wistar rats. J Diabetes Metab 8(752):2
Lenzen S (2008) The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 51(2):216–226
Nerup J, Mandrap-Poulsen T, Helqvist S, Andersen HU, Pociot F, Reimers JI, Cuartero BG, Karlsen AE, Bjerre U, Lorenzen T (1994) On the pathogenesis of IDDM. Diabetologia 37(Suppl 2):S82–S89
Ibrahim AA, Abdussalami MS, Appah J, Umar AH, Ibrahim AA, Dauda KD (2021) Antidiabetic effect of aqueous stem bark extract of Parinari macrophylla in alloxan-induced diabetic Wistar rats. Future J Pharm Sci 7(1):164
Acknowledgements
The authors would like to thank the entire staff of the Biological Science Department, Nigerian Defence Academy, Kaduna State, Nigeria, for their cooperation during the conduct of this study. We also wish to extend our sincere gratitude to the entire staff of the Human Physiology Department, Ahmadu Bello University, Zaria, Kaduna State, Nigeria for their maximum cooperation during the study. Finally, our appreciation goes to Prof. M.D. Mukhtar and late Prof. I.I. Indabawa of Faculty of Life Sciences, Bayero University, Kano, Nigeria for their valuable contributions.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AAI, MSA and JA conceptualized and designed the study. AAI and AHU executed the experiments and analyzed the data. AAI and AAIJr were responsible for drafting the final manuscript. MSA and JA supervised the study. AAIJr, AUM and SH were responsible for statistical analysis and reviewing the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Research and Ethical Committee on the use of laboratory animals of Ahmadu Bello University, Zaria, Kaduna State, Nigeria (Ref: NDA/PGS/FS/M/1826/14). All animal procedures and experimental protocol were strictly followed in accordance with the National Research Council, Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Competing interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, A.A., Abdussalami, M.S., Appah, J. et al. Evaluation of antihyperglycemic activity of aqueous stem bark extract of Boswellia dalzielii in alloxan-induced diabetic Wistar rats. Futur J Pharm Sci 9, 7 (2023). https://doi.org/10.1186/s43094-023-00458-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00458-4