Abstract
Background
Diabetes-induced neuropathic pain is manifested as a lowering of nerve transmission rate, increased discomfort, sensual loss, and axonal degradation, and is the most prevalent secondary consequence of diabetes. Diabetes is a devitalizing disease affecting people from diverse groups in both developing and industrialized countries. The inflammation pathway and oxidative stress both contribute considerably to diabetic peripheral neuropathy via the activation of inflammatory cytokines. Hyperglycemia-mediated neural oxidative stress and damage activates a number of metabolic pathways, causing diabetic neuropathy. The current study investigated the neuroprotective potential of methanolic extract of Sphaeranthus indicus Linn (MESI) in ameliorating diabetic neuropathic pain induced by administration of streptozotocin in rats.
Results
Four weeks after intraperitoneal treatment of streptozotocin (STZ), there was a significant decrease in mechano-tactile allodynia and mechanical and thermal hyperalgesia. Furthermore, STZ-induced oxidative stress increases the extent of neural lipid peroxidation (LPO), as evidenced by increased MDA levels, decreases the activities of endogenous antioxidants such as superoxide dismutase (SOD) and glutathione (GSH), and alters sciatic neural histoarchitecture. Chronic administration of methanolic extract of Sphaeranthus indicus Linn (MESI) for 4 weeks significantly and dose-dependently attenuated the decrease in levels of nociceptive thresholds, endogenous antioxidants (SOD and GSH), and increase in LPO. Furthermore, MESI significantly restored sciatic neural histoarchitecture.
Conclusion
The amelioration of streptozotocin-induced diabetic neuropathy by methanolic extract of Sphaeranthus indicus Linn (MESI) could be attributed to its antinociceptive, antioxidant, and neuroprotective properties.
Similar content being viewed by others
Background
Diabetes has progressed into a metabolic endemic, with 366 million people expected to be affected by this disease by 2050. Late systemic and common complications of diabetes that may emerge in approximately 50% of diabetics are retinopathy, neuropathy, nephropathy, and cardiomyopathy [1, 2]. It is among the leading global fatalities, affecting around 6% of the world's population and primarily affecting low- and middle-income countries [3]. The impact on people's well-being, longevity, and lifestyle quality, as well as on healthcare systems, is due to the fast-growing occurrences of prominent global health issues such as pandemics, economic disparities, access to health care, political factors, non-communicable diseases, and environmental factors globally [4].
Neuropathic pain is a prevalent consequence of diabetes mellitus [5]. The patients frequently suffer from severe hyperalgesia and allodynia, which can be debilitating and disturbing [6]. Although the mechanism is incompletely understood, a multifactorial etiology may be considered for developing painful diabetic neuropathy. These mechanistic approaches include an amplified hexosamine shunt, aldose reductase activation, a reduced neural myoinositol content, impaired neurotrophic support, activation of protein kinase C (PKC), and poly (ADPribose) polymerase (PARP), impaired insulin/C peptide action, and the formation of advanced glycation end products (AEG), which synchronize autooxidative glycosylation and polyol pathways, leading to morphological and functional abnormalities of peripheral neurons, spinal neuroglia, and nerve fibers by modulating different interconnecting biochemical pathways [7, 8]. Different cytokines and excitatory neurotransmitters (NT) decrease the pain thresholds of neurons [9].
Streptozotocin (STZ) is a pancreatic islet-cell selective cytotoxic drug that, when taken in large doses, causes full-cell necrosis and diabetes within two days [10]. A rat model for STZ-induced diabetes and other abnormalities has been developed using the STZ-induced diabetic rat model. [11]. In rats, STZ in a single intraperitoneal dose causes a progressive pain syndrome comparable to that experienced in people with agonizing diabetic neuropathy [12]. STZ-induced diabetic neuropathy also causes mechanical hyperalgesia and thermal allodynia [13].
The current treatment regimen of diabetic neuropathy that includes antioxidants, antidepressants, polyphenols, selective serotonin reuptake inhibitors (SSRIs), anti-arrhythmics, opioids, and anticonvulsants has met with limited success in clinical trials. However, these therapies provide relief only to a fraction of patients, and their side-effect profiles limit their use [14, 15]. Gabapentin is renowned for its capacity to manage both static and dynamic allodynia. [16].
In Indian traditional medicine, the therapeutic herb Sphaeranthus indicus Linn has long been used to treat a variety of ailments [17]. In tropical India, it thrives in rice fields, arid waste regions, and cultivated lands. It may be found from sea level to 1200 m above sea level in India, Sri Lanka, Africa, and Australia [18]. Some of the notable biological activities of Sphaeranthus indicus Linn are anxiolytic, neuroleptic, sedative, immunomodulatory, antioxidant, anti-inflammatory, antipyretic, analgesic, mast cell stabilizing, anti-hyperglycemic, hepatoprotective, wound healing, antimicrobial, antibacterial, antifungal, antiviral, antiparasitic, bronchodilator, antihyperlipidemic, renoprotective, and other miscellaneous activities [19]. Sphaeranthus indicus Linn has not been researched for its potential in treating diabetes-related problems, despite its well-known pharmacological benefits. The goal of the current study was to evaluate the effects of long-term administration of a methanolic extract of Sphaeranthus indicus Linn (MESI) in ameliorating diabetic neuropathic pain caused by STZ in albino rats of the Wistar strain by assessing various behavioral parameters, biomarkers of oxidative stress, and histoarchitectural alterations.
Methods
Drugs and chemicals
STZ, 5, 5’-dithiobis-2-nitrobenzoic acid (DTNB), epinephrine, and metformin were purchased from Sigma-Aldrich, St. Louis, USA. All the other analytical reagents, chemicals, and biochemical diagnostic kits were available from the commercial suppliers. The reagents used in the detection of the Ex Vivo antioxidants assay were prepared freshly in the laboratory.
Procurement and authentication of plant
The whole plant of Sphaeranthus indicus Linn was procured from the agricultural lands of Pahine, Trimbakeshwar region, native to Nashik city in the Nashik district of Maharashtra state, from August to September 2021. For future use, the voucher specimen HPTRYK/342/2021–22 was placed at the Post Graduate Botany Department at the Gokhale Education Society’s HPT Arts & RYK Science College in Nashik. The apical parts (leaves, stems, fruits, and flowers) were dried in the shade, ground into a powder, and then sealed in an airtight container for storage.
Preparation of extract
Using a mechanized grinding machine, the dried apical parts of Sphaeranthus indicus Linn were ground into a coarse powder. The powder was then stored in an airtight, moisture-free container. Using 250 g of powder and the Soxhlet apparatus, a methanolic extract of Sphaeranthus indicus Linn (MESI) was prepared. Evaporation of solvent and concentration of extract were done under reduced pressure using a rotary evaporator. The percentage yield of freeze-dried MESI was calculated, and the extract was analyzed for the presence of preliminary phytoconstituents [20].
Animals
Adult Wistar albino rats (150–200 g) and Swiss albino mice (6–8 weeks old, 20–25 g) of either sex were housed at a standard condition temperature (25 °C ± 1 °C), relative humidity (45–55%), and a 12 h light–12 h dark cycle, with ad libitum access to food pellets with filtered water. An acclimatization period (7–10 days) was followed before the implementation of the experimental protocol. The Institutional Animal Ethics Committee (IAEC) of Bhupal Nobles' College of Pharmacy in Udaipur, Rajasthan (870/PO/Re/S/05/CPCSEA), granted permission for the research investigation, which was carried out in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines on animal experimentation [21].
Acute toxicity
Following the Organization for Economic Co-operation and Development (OECD) guideline 425, the acute oral toxicity of MESI was investigated [22]. Mice were given different oral doses of MESI (175, 550, 1750, and 2000 mg/kg) [23] in the form of a suspension prepared in 1% (w/v) carboxy methyl cellulose (CMC). The incidence of death or any indication of toxicity was then monitored in mice for up to 72 h.
Induction and assessment of diabetes
A single intraperitoneal dose of 55 mg/kg STZ in citrate buffer (pH 4.4, 0.1 M) was used for the induction of diabetes. An equal volume of citrate buffer was administered to normal non-diabetic (ND) rats. Through the puncture of the retro-orbital plexus, blood samples were drawn in the heparinized tubes after 48 h of STZ administration to confirm the induction of diabetes. Using the Glucose (Glu) Colorimetric Assay Kit (GOD-POD Method), serum glucose levels were measured. In this study, rats with serum glucose concentrations of more than 250 mg/dL were chosen for the current study.
Experimental protocol
Following a baseline recording of the nociceptive response at week 4 after STZ injection, the control and diabetic rats are randomly chosen and divided into six subgroups of six animals each. The following is the treatment schedule:
-
[A]
Non-diabetic animals.
Group 1 Normal non-diabetic (ND) rats were administered a single injection of citrate buffer (vehicle) and an oral feeding of 1% (w/v) carboxy methyl cellulose (CMC).
-
[B]
Diabetic animals.
-
Group 2 Diabetic (STZ, 55 mg/kg, i.p.) control rats were administered oral feeding of 1% (w/v) carboxy methyl cellulose (CMC).
-
Group 3 Diabetic (STZ) + MESI (100) rats were given an oral feeding of Sphaeranthus indicus Linn Methanolic extract (100 mg/kg, p.o.) in a suspension prepared in 1% (w/v) carboxy methyl cellulose (CMC).
-
Group 4 Diabetic (STZ) + MESI (200) rats were given an oral feeding of Sphaeranthus indicus Linn Methanolic extract (200 mg/kg, p.o.) in a suspension prepared in 1% (w/v) carboxy methyl cellulose (CMC).
-
Group 5 Diabetic (STZ) + MESI (400): rats were given an oral feeding of Sphaeranthus indicus Linn Methanolic extract (400 mg/kg, p.o.) in a suspension prepared in 1% (w/v) carboxy methyl cellulose (CMC).
-
Group 6 Diabetic (STZ) + Metformin (500) rats were given an oral feeding of metformin (500 mg/kg, p.o.) in a suspension prepared in 1% (w/v) carboxy methyl cellulose (CMC).
-
Starting at week 5 following STZ injection, three different dosages (100, 200, and 400 mg/kg) of MESI were given for four weeks. The sciatic nerves were promptly isolated when the rats were slaughtered under deep anesthesia after 8 weeks, and the neural tissue homogenate was made in 0.1 M Tris–HCl buffer (pH 7, 4) for biochemical markers of oxidative stress like lipid peroxidation (LPO), reduced glutathione (GSH), and superoxide dismutase (SOD) [24].
Assessment of diabetic neuropathy
Body mass, food and water ingestion, and urine production
At the end of week 8 of the experimental procedure, the change in body mass (g) of animals was measured. Food consumption (g) was calculated based on the number of pellets consumed per day by experimental animals, whereas calibrated water bottles were used for the estimation of water consumption (ml). The volume of urine produced (ml) was measured by housing the animals in metabolic cages and collecting the urine in a calibrated 250 ml attached container [25].
Serum glucose and glycosylated hemoglobin (HbA1c) levels
Blood was drawn from the retro-orbital plexus by puncturing in Eppendorf tubes containing disodium salt of ethylenediaminetetraacetic acid (EDTA) as an anticoagulant. To separate the serum, a cold centrifuge (2,500 rpm for 15 min at 4 °C) was used. A separate serum sample was kept at 20 °C for further analysis. Using the commercially available GOD-POD kit from Accurex, India, and the Hemoglobin HbA1c (glycated) kit from Sigma-Aldrich, USA, serum glucose and glycosylated hemoglobin (HbA1c) levels were determined with a biochemical analyzer. [26]
Assessment of behavioral parameters
Behavioral parameters such as mechanical hyperalgesia, mechano-tactile allodynia, and thermal hyperalgesia were assessed on day 0, and at the end of the 4th to 8th week.
Randall–Selitto paw pressure test
A traditional approach [27] was used to measure the mechanical nociceptive threshold, a measure of mechanical hyperalgesia. Using the Randall–Selitto apparatus (UGO Basile, SRL Biological Research Apparatus, Italy), the flexor withdrawal reflex was measured. Withdrawal of the hind paw was used to assess the nociceptive threshold.
Von Frey hair test
Mechano-tactile allodynia was evaluated using a method that has already been described by Chaplan et al., 1994 [28]. Punctuated mechanical stimuli of various intensities were delivered using von Frey hairs (IITC, Woodland Hills, USA). The threshold value was defined as the weight of the filament that would cause the paw to be drawn five times out of ten times, or a 50% response.
Eddy’s hot plate test
The Eddy's hot plate test was used to evaluate a condition of altered perception of temperature (thermal hyperalgesia). The animals were placed on a heated plate that was kept at 55 °C ± 0.5 °C (Orchid Scientific, India). The amount of time it took for the first response to be made was called reaction latency (licking, jumping, or flickering of the hind paw). To avoid tissue injury, 15 s of cutoff time was followed [29].
Tail-Flick test
Acute nociception was elicited using a tail-flick device (TFA-01) from Orchid Scientific in India [30]. To put it briefly, the distal portion of the animal's tail was focused on using an intensity-controlled laser beam in order to determine the tail-flick latency of the animal. Tail-flick latency, i.e., interval between the start of stimulation and the abrupt tail withdrawal was measured in seconds. Two to three recordings were made for each animal, spaced by 15 min, and the mean value was used for statistical analysis.
Biochemical estimations
At the end of week 8 of the treatment plan and following the assessment of behavioral parameters, all test animals were killed under deep anesthesia. Then, using a probe homogenizer (Polytron PT 2500E, Kinematica, Switzerland), sciatic nerves were sensibly separated and homogenized in ice-cold phosphate buffer (0.1 M, pH 7.4). By measuring the quantity of thiobarbiturate acid reactive substances (TBARS) in 10% of the homogenate supernatant, malondialdehyde (MDA) production was quantitatively estimated [31, 32]. An index of oxidative degradation of lipids (lipid peroxidation, LPO) was determined by determining the response of MDA production to thiobarbituric acid. The profiles of enzymatic antioxidants like superoxide dismutase (SOD) [33] and reduced glutathione (GSH) [34] were also assessed.
Histoarchitectural examination
The sciatic nerves were cautiously detached and well-preserved in neutral formalin buffer and embedded in paraffin wax. The Leica Biosystems Microtome, Germany, was used to cut 5-m-thick sections, and Mayer's hematoxylin and eosin (H & E) stain were used to color the sections. Pathological alterations were checked on the slides. Under 400 light microscopy, on the neural specimen, the signs of neural lesions, chromatolysis, leukocytic infiltration, neuronal swelling and degeneration, myelin and axon fragmentation, inflammation, the proliferation of Schwann cells and glial cells were recognized.
Statistical analysis
GraphPad Prism 9.0 software (GraphPad, San Diego, USA) was used to analyze the experimental data, which were expressed as mean ± standard error mean (SEM). Behavioral biomarkers were statistically analyzed using a two-way analysis of variance (ANOVA) followed by Bonferroni's multiple range post hoc test. Oxidative biomarkers were analyzed using one-way ANOVA followed by a post hoc Tukey's multiple range test. A value of P < 0.05 was considered to be statistically significant.
Results
Preliminary phytoconstituent analysis
Preliminary phytoconstituent analysis showed the presence of various compounds such as alkaloids (Mayer’s test, Dragendroff's test, Hager's test, and Wagner's test), flavonoids (Shinoda's test), phenols, tannins (Foam test), and steroids (Liebermann–Burchard reaction). The percentage yield of freeze-dried MESI was found to be 13.50% (w/w).
Acute toxicity
In an acute toxicity study, administration of oral graded doses of MESI (175, 550, 1750, and 2000 mg/kg) in the form of a suspension prepared in 1% (w/v) CMC did not produce an incidence of mortality or any sign of toxicity up to 72 h in mice. Hence, the dose of 2000 mg/kg of MESI was considered the maximum tolerable dose as per OECD guidelines, and the doses of 100, 200, and 400 mg/kg were selected for exploring antinociceptive, antioxidant, and neuroprotective properties in treating STZ-induced diabetic neuropathic pain.
Effect of MESI on body mass, serum glucose, and glycosylated hemoglobin (HbA1c) levels
Four weeks following intraperitoneal injection of STZ, a significant decrease in body mass (149.17 ± 3.00 g; P < 0.001), an increase in serum glucose level (460.10 ± 5.62 mg/dL; P < 0.001), and glycosylated hemoglobin (HbA1c) level (12.35 ± 0.58%; P < 0.001) were observed in diabetic (STZ) control rats in contrast to body mass (241.67 ± 5.58 g), serum glucose level (163.13 ± 6.75 mg/dL), and HbA1c level (5.04 ± 0.39%) of normal non-diabetic rats. Chronic administration of MESI (200 and 400 mg/kg) for four weeks showed significant and dose-dependent improvement in decreased body mass (170.17 ± 3.00 g; P < 0.01, and 173.17 ± 3.00 g; P < 0.001, respectively) as well as ameliorated increased serum glucose level (424.80 ± 5.62 mg/dL; P < 0.05, and 418.8 ± 5.62 mg/dL; P < 0.01, respectively) and HbA1c level (10.47 ± 0.47%; P < 0.05, and 9.61 ± 0.44%; P < 0.01, respectively) when compared to diabetic (STZ) control rats. Furthermore, in diabetic animals treated with standard oral hypoglycemic, metformin significantly improved decreased body mass (175.67 ± 5.58 g; P < 0.001), increased serum glucose level (333.13 ± 6.75 mg/dL; P < 0.001), and HbA1c levels (9.41 ± 0.44%; P < 0.001) as compared to diabetic (STZ) control rats (Table 1).
Effect of MESI on food and water ingestion, and urine production
Four weeks after STZ injection, food and water ingestion, and urine production (67.00 ± 3.75 g, 137.17 ± 5.15 mL, and 51.33 ± 2.25 mL, respectively, P < 0.001) was significantly enhanced in diabetic (STZ) control rats in contrast to food and water ingestion, and urine production (22.00 ± 1.69 g, 43.00 ± 1.45 mL, and 8.00 ± 1.32 mL, respectively) of normal non-diabetic rats. Chronic treatment with MESI (200 and 400 mg/kg) for 4 weeks significantly and dose-dependently declined food ingestion (59.00 ± 1.65 g; P < 0.05, and 55.67 ± 1.43 g; P < 0.01, respectively), water ingestion (124.83 ± 3.23 mL; P < 0.05, and 120.50 ± 1.84 mL; P < 0.01, respectively), and urine production (43.83 ± 1.83 mL; P < 0.05 and 42.50 ± 1.69 mL; P < 0.01, respectively) as compared to diabetic (STZ) control rats. In comparison with diabetic (STZ) control rats, metformin-treated rats showed significant retardation in food and water ingestion (48.67 ± 1.67 g; P < 0.001 and 87.33 ± 1.61 mL; P < 0.001), and urine production (36.17 ± 1.19 mL; P < 0.001) (Table 1).
Effect of MESI on behavioral parameters
Randall–Selitto paw pressure test
On day 0, there was no noticeable variance in the mean paw withdrawal threshold of diabetic (STZ) control rats (263.00 ± 5.12 g) and normal non-diabetic rats (268.00 ± 4.61 g). During 8 weeks, no significant variation in the mean paw withdrawal threshold of normal non-diabetic rats was observed. After 4 weeks of STZ injection, in contrast to normal non-diabetic rats (263.00 ± 6.42 g), a significant decrease in the mean paw withdrawal threshold was produced in the diabetic (STZ) control rats (43.00 ± 4.61 g; P < 0.001). In rats administered MESI (200 and 400 mg/kg), a significant and dose-related increase in mean paw withdrawal threshold was observed (75.00 ± 3.87 g; P < 0.05 and 103.00 ± 4.61 g; P < 0.01, respectively, at the end of week 7, and 100.00 ± 6.32 g; P < 0.05 and 133.00 ± 4.61 g; P < 0.01, respectively, at the end of week 8) in contrast to diabetic (STZ) control rats (50.00 ± 5.00 gat the end of week 7, and 50.00 ± 6.32 g at the end of week 8, respectively). Besides, diminution of reduced mean paw withdrawal threshold by metformin (175.00 ± 6.32 g and 183 ± 8.14 g, at the end of week 7 and week 8, respectively) was more significant (P < 0.001) in contrast to diabetic (STZ) control rats (Fig. 1).
Effect of chronic treatment of MESI and metformin on diabetes-induced mechanical hyperalgesia in Randall–Selitto paw pressure test. Data are expressed as mean ± SEM (n = 6) and analyzed by two-way ANOVA followed by Bonferroni's multiple range post hoc test.*P < 0.05 and **P < 0.01for MESI, and ***P < 0.001 for metformin-treated groups compared to the diabetic (STZ) control group. ###P < 0.001 for the diabetic (STZ) control group compared to the normal non-diabetic group
Von Frey hair test
On day 0, before the induction of diabetic neuropathy, there was no significant variation was observed in the mean paw withdrawal threshold of diabetic (STZ) control rats (69.13 ± 1.72 g) and normal non-diabetic rats (68.67 ± 1.41 g). A significant reduction in mean paw withdrawal threshold (i.e., mechanical allodynia) was observed in diabetic (STZ) control rats (29.30 ± 1.14 g; P < 0.001) after 4 weeks of STZ injection, in response to Von Frey hair stimulation, as compared to normal non-diabetic rats (66.17 ± 1.66 g). As compared to diabetic (STZ) control rats, 4-week chronic treatment of MESI (200 and 400 mg/kg) showed significant and dose-dependent amelioration of the reduced mean paw withdrawal threshold at the end of week 7 (35.58 ± 1.03 g and 41.77 ± 1.60 g; P < 0.05, respectively) and week 8 (42.57 ± 1.78 g; P < 0.05and 47.15 ± 1.77 g; P < 0.01, respectively). Moreover, in comparison with diabetic (STZ) control rats, metformin-treated diabetic rats significantly attenuated the reduced paw withdrawal threshold (59.55 ± 1.89 g, P < 0.001) at the end of week 8 (Fig. 2).
Effect of chronic treatment of MESI and metformin on mechanical allodynia in von Frey hair test. Data are expressed as mean ± SEM (n = 6) and analyzed by two-way ANOVA followed by Bonferroni's multiple range post hoc test.*P < 0.05 and **P < 0.01for MESI, and ***P < 0.001 for metformin-treated groups compared to the diabetic (STZ) control group. ###P < 0.001 for the diabetic (STZ) control group compared to the normal non-diabetic group
Eddy’s hot plate test
Prior to the induction of diabetic neuropathy, on day 0, not any significant variability in the response latency (flickering and licking of hind paw) in diabetic (STZ) control rats (10.52 ± 0.36 s) and normal non-diabetic rats (11.10 ± 0.37 s) was noted. A noteworthy reduction (P < 0.001) in mean response latency was produced after 4 weeks of STZ injection in the diabetic (STZ) control rats (4.53 ± 0.43 s) as compared to normal non-diabetic rats (11.05 ± 0.47 s). More significant and dose-dependent attenuation of decreased mean response latency was observed at the end of week 8 in the rats administered MESI (200 and 400 mg/kg) for 4 weeks (6.90 ± 0.37 s; P < 0.05 and 7.40 ± 0.29 s; P < 0.01, respectively) as compared to diabetic (STZ) control rats. Furthermore, compared to diabetic (STZ) control rats, metformin significantly attenuates the decreased mean response latency (9.82 ± 0.22 s; P < 0.001) at the end of week 8 (Fig. 3).
Effect of chronic treatment of MESI and metformin on diabetes-induced thermal hyperalgesia in Eddy’s hot plate test. Data are expressed as mean ± SEM (n = 6) and analyzed by two-way ANOVA followed by Bonferroni's multiple range post hoc test.*P < 0.05 and **P < 0.01 for MESI, and ***P < 0.001 for metformin-treated groups compared to the diabetic (STZ) control group. ###P < 0.001 for the diabetic (STZ) control group compared to the normal non-diabetic group
Tail-flick test
Before the induction of diabetic neuropathy (day 0), there was no significant difference in the tail-flick latency in diabetic (STZ) control rats (13.27 ± 0.30 s) and normal non-diabetic rats (13.10 ± 0.31 s). After 4 weeks of STZ injection, a significant decrease in mean tail-flick latency was produced in the diabetic (STZ) control rats (3.88 ± 0.36 s; P < 0.001) as compared to normal non-diabetic rats (13.15 ± 0.24 s). After week 8, MESI (200 and 400 mg/kg) treatment for 4 weeks showed significant and dose-related attenuation of the reduced mean tail-flick latency (5.30 ± 0.24; P < 0.05 and 5.50 ± 0.19 s; P < 0.01, respectively) as compared to diabetic (STZ) control rats. However, at the end of week 8, metformin was more significant (8.93 ± 0.32 s; P < 0.001) in attenuating the reduction of mean tail-flick latency in comparison with diabetic (STZ) control rats (Fig. 4).
Effect of chronic treatment of MESI and metformin on diabetes-induced thermal hyperalgesia in Tail-flick test. Data are expressed as mean ± SEM (n = 6) and analyzed by two-way ANOVA followed by Bonferroni's multiple range post hoc test.*P < 0.05 and **P < 0.01 for MESI, and ***P < 0.001 for metformin-treated groups compared to the diabetic (STZ) control group. # # #P < 0.001 for the diabetic (STZ) control group compared to the normal non-diabetic group
Biochemical estimations
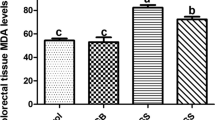
Effect of MESI on diabetes-induced alteration in the index of lipid peroxidation (LPO)
After 8 weeks of STZ injection, in diabetic control rats, levels of neural lipid peroxide (LPO) were substantially higher (9.27 ± 0.28 nM/mg of protein, P < 0.001) than those of healthy non-diabetic rats (2.23 ± 0.22 nM/mg of protein). In comparison with diabetic (STZ) control rats, the lipid peroxide level was considerably and dose-dependently reduced in the MESI (200 and 400 mg/kg)-treated rats (8.24 ± 0.23 nM/mg of protein; P < 0.05, and 8.09 ± 0.34 nM/mg of protein; P < 0.01, respectively). Furthermore, metformin significantly reduced the elevated lipid peroxide level as compared to diabetic (STZ) control groups (6.48 ± 0.16 nM/mg of protein; P < 0.001) (Fig. 5).
Effect of MESI on diabetes-induced alteration in the index of lipid peroxidation (LPO). Data are expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by a post hoc Tukey's multiple range test. *P < 0.05, **P < 0.01 for MESI, and ***P < 0.001 for metformin-treated groups compared to the diabetic (STZ) control group. ###P < 0.001 for the diabetic (STZ) control group compared to the normal non-diabetic group. MESI (100), MESI (200), and MESI (400): Methanolic extract of Sphaeranthus indicus Linn (MESI) 100, 200, and 400 mg/ kg, p.o. treated rats
Effect of MESI on diabetes-induced alterations in the reduced glutathione (GSH) and superoxide dismutase (SOD) levels
Reduced glutathione (GSH) (0.37 ± 0.15 µg/mg protein) and superoxide dismutase (SOD) (3.56 ± 0.74 U/mg of protein) levels in the sciatic nerve of diabetic (STZ) control rats were considerably decreased (P < 0.001) when compared to normal non-diabetic rats' GSH (1.45 ± 0.12 µg/mg protein) and SOD (27.16 ± 1.19 U/mg of protein). As compared to diabetic (STZ) control rats, GSH (0.93 ± 0.11 µg/mg protein; P < 0.05 and 1.04 ± 0.08 µg/mg protein; P < 0.01, respectively) and SOD (10.71 ± 1.80 U/mg of protein; P < 0.05, and 12.38 ± 1.82 U/mg of protein; P < 0.01, respectively) were substantially and dose-dependently elevated in MESI (200 and 400 mg/kg)-treated rats. However, as compared to diabetic (STZ) control rats, animals treated with metformin significantly restored lowered levels of GSH (1.31 ± 0.18 µg/mg protein; P < 0.001) and SOD (19.18 ± 1.05 U/mg of protein; P < 0.001) (Figs. 6 and 7).
Effect of MESIon diabetes-induced alterations in the reduced glutathione (GSH) level. Data are expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by a post hoc Tukey's multiple range test. *P < 0.05, **P < 0.01 for MESI, and ***P < 0.001 for metformin-treated groups compared to the diabetic (STZ) control group. ###P < 0.001 for the diabetic (STZ) control group compared to the normal non-diabetic group. MESI (100), MESI (200), and MESI (400): Methanolic extract of Sphaeranthus indicus Linn (MESI) 100, 200, and 400 mg/ kg, p.o. treated rats
Effect of MESIon diabetes-induced alterations in the superoxide dismutase (SOD) level. Data are expressed as mean ± SEM (n = 6) and analyzed by one-way ANOVA followed by a post hoc Tukey's multiple range test. *P < 0.05, **P < 0.01 for MESI, and ***P < 0.001 for metformin-treated groups compared to the diabetic (STZ) control group. ###P < 0.001 for the diabetic (STZ) control group compared to the normal non-diabetic group. MESI (100), MESI (200), and MESI (400): Methanolic extract of Sphaeranthus indicus Linn (MESI) 100, 200, and 400 mg/ kg, p.o. treated rats
Neural histoarchitectural findings
The architecture of the sciatic nerve appeared to be normal in normal non-diabetic (ND) rats without any neutrophilic and macrophagic infiltration, or neural necrosis and congestion (Fig. 8A). Four weeks after intraperitoneal treatment of STZ, diabetic (STZ) control rats had substantial nerve cell death as seen by the presence of neutrophils and macrophages, congestion, and edema in the nerve cells. Additionally, it was shown that vacuolization and necrosis in the nerve caused swelling of both myelinated and non-myelinated nerve fibers as well as a reduction in the number of myelinated fibers (Fig. 8B). Diabetic rats treated with STZ + MESI (100) showed neutrophil and macrophage infiltration, nerve cell edema, and degeneration of non-myelinated and myelinated nerve fibers (Fig. 8C). STZ + MESI (200) diabetic rats showed reduced infiltration of neutrophils as well as macrophages and degenerative changes of nerve fibers (Fig. 8D). STZ + MESI (400)-treated diabetic rats showed higher attenuation of the infiltration of neutrophils as well as macrophages along with neural necrosis and congestion with further improvement in the swelling of non-myelinated and myelinated nerve fibers (Fig. 8E). In diabetic rats treated with STZ + MET (500), regeneration of the sciatic nerve was observed, which was characterized by the absence of neutrophilic and macrophagic infiltration and necrosis, as well as nerve congestion (Fig. 8F).
A–F Effect of chronic treatment of MESI on histoarchitecture of sciatic nerve in STZ-induced diabetic neuropathy. Photomicrographs of sections of the sciatic nerve from rats stained with H & E. A Normal non-diabetic (ND), B Diabetic (STZ) control, C STZ + MESI (100), D STZ + MESI (200), E STZ + MESI (400), and F STZ + Metformin (500)-treated rats (microscopic examination under 400 × light microscopy). MESI (100), MESI (200), and MESI (400): Methanolic extract of Sphaeranthus indicus Linn (MESI) 100, 200, and 400 mg/ kg, p.o. treated rats
Discussion
Chronic hyperglycemia-assisted immunological damage, inadequacy of neural growth factors, and autonomic neural demyelination leading to peripheral nerve injury are most common in diabetic neuropathy patients [35, 36]. Damage to the capillary vasculature and peripheral nerves causes oxidative stress and hypoxia in the highly perfused organs via reactive oxygen species (ROS) [37,38,39].The proven determinants in diabetic neuropathy are polyol pathway activation, reactive oxygen species mediated oxidative stress, glycosylated hemoglobin accumulation, and collagen deposition [40].
Neuropathic pain in diabetes is characterized by symptoms such as allodynia and hyperalgesia, which are caused by an elevated nociceptive response, decreased motor nerve condition velocity, neuronal hypoxia, and decreased sensitivity to painful stimuli [41]. STZ-induced painful diabetic neuropathy in experimental animals has similar clinical manifestations [42]. Intraperitoneal administration of STZ in rats displays clinicopathological characteristics such as biochemical, oxidative, and metabolic alterations that are also present in humans [43].
It has been determined that cellular biosynthesis and metabolism are the primary causes of diabetes-induced polydipsia, polyphagia, polyuria, and body weight loss [44, 45]. Body weight reduction was significantly restored in MESI and metformin-treated rats in comparison with diabetic (STZ) control rats. Due to MESI and metformin therapy, typical signs of diabetes such as polyphagia, polydipsia, and polyuria were reduced, which in turn decreased excessive food and fluid consumption. MESI's effects on body weight, food and water ingestion, and urine production of diabetic rats are well matched with the past studies evaluating naringin [24], hesperidin [26], and chlorogenic acid [46].
During diabetes, the excess glucose present in the blood reacts with hemoglobin to form HbA1c, which is increased over a long period in diabetes mellitus [47]. There is evidence that glycation may itself induce the generation of oxygen-derived free radicals in a diabetic condition, which may be the leading cause of the development of diabetic neurological complications like neuropathic pain and depression [48,49,50]. An increase in HbA1c, as seen in poor diabetic control, has been linked to increased blood viscosity. Glycosylation of hemoglobin and increased glucose levels tend to affect RBC properties, decreasing RBC flexibility and increasing RBC aggregation, resulting in increased blood viscosity. Glycosylation of hemoglobin may also affect RBC membrane lipid protein interactions, altering internal viscosity, modifying the viscoelastic properties of erythrocyte membranes, and impairing RBC deformability. There is also evidence that glycosylation of hemoglobin impairs the relaxation of human mesenteric vessels caused by nitric oxide (NO). It has also been reported that hemoglobin glycosylation alters NO binding with thiols, resulting in decreased NO bioavailability and impaired vasodilation in rabbit aortic rings. Another way glycosylation of hemoglobin is thought to be vasoactive is through the formation of reactive oxygen species. Glycosylation of hemoglobin reduces its oxygen-carrying capacity, promoting hypoxia and the associated systemic vascular vasodilatory adaptations and responses. This leads to the formation of diabetic complications like atherosclerosis, retinopathy, neuropathy, and nephropathy in diabetes mellitus (DM) patients and animals [51].
In the current study, the observed increase in the level of glycosylated hemoglobin in diabetic (STZ) control rats might be due to the presence of excessive amounts of blood glucose. Furthermore, the level of glycosylated hemoglobin significantly decreased after chronic treatments with MESI and metformin, and this may be due to a decrease in the blood glucose level. These results are compatible with the previous studies carried out by Visnagri et al., 2014 [26]; Zhou et al., 2019 [52]; Solanki & Bhavsar, 2015 [53]; Tembhurne & Sakarkar, 2011 [54]; and Manoharan et al., 2011 [55].
The neural system's functioning encoding and processing of noxious stimuli is referred to as nociception. Behavioral responses to external stimuli are an ideal marker to indicate abnormal sensation and pain in diabetic neuropathy [56]. Hyperalgesia, or allodynia, is the form of nociceptive pain that manifests in inflamed tissue. According to reports, the validated techniques for assessing mechano-tactile allodynia, peripheral analgesia, and central pain in laboratory animals include von Frey hair, Randall–Selitto, and tail flick [57,58,59,60]. A transitory hyperalgesia phase and a subsequent hypoalgesia phase are the two stages of thermal pain perception observed after intraperitoneal injection of STZ [61]. According to numerous previous findings, hyperalgesia or allodynia appeared in the current study 4 weeks after STZ injection [62, 63]. Numerous processes, including the sensitization of peripheral receptors, ectopic activity in sprouting fibers, and changes in dorsal root ganglion cells, have been shown to be important in the development of nociception [64, 65]. A symmetric type of neuropathy which involves distal sensory and motor nerves is the classical feature of STZ-induced diabetic neuropathy (DN). With diabetes advancements, the sensation of distal extremities decreases, causing loss of pain sensation. Hence, an important parameter to assess the response of rats to thermal noxious stimuli is the tail withdrawal threshold. It has been reported that the STZ-induced diabetic rat exhibits an elevated tail-flick threshold response to noxious thermal stimuli [66].
In the current study, a significant reduction in mean paw withdrawal threshold, response latency (flickering and licking of hind paw), and tail withdrawal latency (tail-flick latency) was observed in the diabetic (STZ) control rats after 4 weeks of STZ injection as compared to normal non-diabetic rats. Chronic treatment with MESI and metformin showed significant and dose-dependent amelioration of the decrease in mean paw withdrawal threshold, response latency (flickering and licking of hind paw), and tail withdrawal latency (tail-flick latency) in diabetic rats.
Numerous studies have found that hyperglycemia causes oxidative stress in STZ-induced diabetic rats, which is associated with the uncontrolled generation of free radicals [67, 68]. Non-enzymatic and enzymatic protein glycation pathways are involved in the generation of free radicals as a result of elevated glucose levels [69]. The increasing amount of oxidative stress may contribute to vascular dysfunction and neurological damage by the oxidation of cellular membrane lipoprotein, which would impair brain function, slow down nerve transmission, and make people more sensitive to unpleasant stimuli [70, 71]. Therefore, potent antioxidants with free radical scavenging potency like acetyl-L-carnitine, α-lipoic acid, methylcobalamin, benfotiamine, and topical capsaicin can be effective in the treatment of DN [72].
An essential antioxidant enzyme, SOD, affords protection against highly reactive superoxide anions (O2−) by converting them to hydrogen peroxide (H2O2) and thereby reducing oxidative stress [57, 73, 74]. SOD also maintains redox balance in neurons as well as vascular endothelium. The oxidation of nicotinamide adenine dinucleotide phosphate (NADP+/NADPH) caused by elevated glucose levels reduces SOD activity [75, 76], which activates aldose reductase (AR) and protein kinase C, resulting in pain perception. However, because of the antagonistic interaction between NADPH and glutathione disulfide (GSSG)—reductase, less GSH available to protect cells and the sulfhydryl group of cysteine in proteins [58, 77]. The activity of neural SOD and GSH was significantly reduced in diabetic animals in hyperglycemia [78]. Similar findings were observed in present study where the activity of SOD and GSH was significantly decreased after 8 weeks of STZ. Hence, diminished activity of SOD and GSH makes the sciatic nerve more prone to oxidative stress.
It has been reported that elevated MDA activity is responsible for cell membrane lysis and nerve damage by rearranging double bonds of unsaturated fatty acids in the lipid membrane [79, 80]. In diabetes, post-translational alterations of various antioxidants play a vital role. With the progression of diabetes, the activity of antioxidant enzymes like SOD and GSH is decreased, whereas the activity of MDA is increased in the sciatic nerve [81]. Similar findings were observed in the present study, where STZ control rats exhibit decreased SOD as well as GSH activity and elevated MDA activity in peripheral nerve tissue. Treatment with MESI and metformin significantly increased the decreased activity of SOD and GSH in the sciatic nerve of rats and reduced elevated MDA activity. The results of the present study are in accordance with the previous findings showing that MESI treatment raises levels of superoxide dismutase, catalase, and glutathione peroxides while lowering malondialdehyde levels in acetaminophen-induced hepatotoxicity in rats [82]. In 2006, Shirwaikar et al. reported the antioxidant potential of ethanolic extract of S. indicus in vitro [83]. Furthermore, previous studies evaluating the neuroprotective potentials of naringin [24] and hesperidin [26] support the present findings that MESI provides significant neuroprotection in STZ-sensitive diabetic rats.
In the current study, oral administration of S. indicus restored plasma glucose levels to near normal in rats with STZ-induced diabetes. Phytochemical studies carried out on the alcoholic extract of S. indicus revealed the presence of sterols, phenols, and flavonoids [84]. Shirwaikar et al. (2004) reported that flavonoids have a major role in reducing oxidative stress associated with diabetes, which in turn helps the regulation of plasma glucose concentration [85]. Antihyperglycemic action of flavonoids extracted from various sources has been reported [86].
In diabetic (STZ) control rats, examination of sciatic neural histoarchitecture showed neurocytic necrosis and congestion with massive neutrophilic and macrophagic infiltration with swelling of non-myelinated and myelinated nerve fibers and decreased mass of myelinated fibers due to neural necrosis and vacuolization. In contrast to this, the neuroprotective efficacy of MESI and metformin rats was characterized by regenerative changes in the architecture of the sciatic nerve with minimal signs of neural necrosis and congestion with further improvement in the non-myelinated and myelinated nerve edema.
Conclusion
The outcomes of the current research concluded that methanolic extract of Sphaeranthus indicus Linn (MESI) have significant antinociceptive potential as characterized by dose-dependent attenuation of mechanical and thermal hyperalgesia and mechano-tactile allodynia and evidenced by increased mean paw withdrawal threshold, response latency (flickering and licking of the hind paw), and tail withdrawal latency (tail-flick latency). The antioxidant potential of MESI has been highlighted with a reduced index of neural LPO and restoration of GSH and SOD. Furthermore, the histoarchitectural study of the sciatic nerve supports the neuroprotective properties of MESI. Therefore, the current study summarizes the role of MESI in the treatment of STZ-induced painful diabetic neuropathy by virtue of its antidiabetic, antinociceptive, antioxidant, and neuroprotective potentials.
Availability of data and materials
The datasets generated and/ or analyzed during the current study are available from the corresponding author on request.
Abbreviations
- PKC:
-
Protein kinase C
- PARP:
-
Poly (ADPribose) polymerase
- AEG:
-
Advanced glycation end products
- NT:
-
Neurotransmitters
- STZ:
-
Streptozotocin
- SSRIs:
-
Selective serotonin reuptake inhibitors
- MESI:
-
Methanolic extract of Sphaeranthus indicus Linn
- DTNB:
-
5, 5’-Dithiobis-2-nitrobenzoic acid
- IAEC:
-
Institutional animal ethics committee
- OECD:
-
Organization for economic co-operation and development
- w/v:
-
Weight/volume
- CMC:
-
Carboxy methyl cellulose
- Glu:
-
Glucose
- GOD-POD:
-
Glucose oxidase–peroxidase
- ND:
-
Non-diabetic
- i.p.:
-
Intraperitoneal
- p.o.:
-
Per oral
- MDA:
-
Malondialdehyde
- TBARS:
-
Thiobarbiturate acid reacting substances
- LPO:
-
Lipid peroxidation
- GSH:
-
Reduced glutathione
- SOD:
-
Superoxide dismutase
- H & E:
-
Hematoxylin and eosin
- ANOVA:
-
Analysis of variance
- SEM:
-
Standard error of the mean
- HbA1c:
-
Glycosylated hemoglobin
- DM:
-
Diabetes mellitus
- DN:
-
Diabetic neuropathy
- ROS:
-
Reactive oxygen species
- AR:
-
Aldose reductase
- O2 − :
-
Superoxide anions
- H2O2 :
-
Hydrogen peroxide
- NADP+/NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- GSSG:
-
Glutathione disulfide
References
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5):1047–1053
Tavakoli M, Yavuz DG, Tahrani AA, Selvarajah D, Bowling FL, Fadavi H (2017) Diabetic neuropathy: current status and future prospects. J Diabetes Res 2017:555
Adeghate E, Schattner P, Dunn E (2006) An update on the etiology and epidemiology of diabetes mellitus. Ann N Y Acad Sci 1084(1):1–29
Saraswat N, Chandra P, Sachan N (2018) A deep insight on diabetic neuropathy: the silent complication of diabetes, with inputs on its causes, diagnosis, pathways, and treatments. Asian J Pharmaceut Clin Res 11(12):112–119
Ziegler D, Fonseca V (2015) From guideline to patient: a review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Complicat 29(1):146–156
Didangelos T, Doupis J, Veves A (2014) Painful diabetic neuropathy: clinical aspects. Handb Clin Neurol 126:53–61
Vincent AM, Russell JW, Low P, Feldman EL (2004) Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr Rev 25(4):612–628
Pacher P, Obrosova IG, Mabley JG, Szabó C (2005) Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications. Emerging new therapeutical strategies. Curr Med Chem 12(3):267–275
Singh R, Kishore L, Kaur N (2014) Diabetic peripheral neuropathy: current perspective and future directions. Pharmacol Res 80:21–35
Furman BL (2015) Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol 70(1):5–47
Morrow TJ (2004) Animal models of painful diabetic neuropathy: the STZ rat model. Curr Protoc Neurosci 29(1):9–18
Hoybergs YM, Biermans RL, Meert TF (2008) The impact of bodyweight and body condition on behavioral testing for painful diabetic neuropathy in the streptozotocin rat model. Neurosci Lett 436(1):13–18
Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M (2014) Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol 723:202–206
Callaghan BC, Cheng HT, Stables CL, Smith AL, Feldman EL (2012) Diabetic neuropathy: clinical manifestations and current treatments. Lancet Neurol 11(6):521–534
Duby JJ, Campbell RK, Setter SM, White JR, Rasmussen KA (2004) Diabetic neuropathy: an intensive review. Am J Health Syst Pharm 61(2):160–173
Gilron I, Flatters SJ (2006) Gabapentin and pregabalin for the treatment of neuropathic pain: a review of laboratory and clinical evidence. Pain Res Manag 11(Suppl A):16A-29A
Kirtikar KR, Basu BD (1935) Indian medicinal plants. Indian Medicinal Plants.
Galani VJ, Patel BG, Rana DG (2010) Sphaeranthus indicus Linn.: a phytopharmacological review. Int J Ayurveda Res 1(4):247–253
Ramachandran S (2013) Review on Sphaeranthus indicus Linn. (Koṭṭaikkarantai). Pharmacogn Rev 7(14):157–169
Indian Pharmacopoeia Committee, India. Ministry of Health, & Family Welfare (1985) Pharmacopoeia of India: The Indian Pharmacopoeia (Vol. 2) Controller of Publications
CPCSEA (2018) Compendium of CPCSEA 2018 Committee for the purpose of control and supervision of experiments on animals. Ministry of environment, Forest and Climate Change. Government of India, pp 1–213
Organization for Economic Co-operation and Development (OECD) Guidelines for testing of chemicals, acute oral toxicity-up and down procedure, vol 425 (2006)
Choudhary GP, Jain AP (2021) Evaluation of acute, subacute and LD50 values of methanolic extract of Sphaeranthus indicus leaves in Albino mice. Res J Pharm Technol 14(5):2487–2492
Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL (2012) Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 83(4):650–659
Navale AM, Paranjape A (2018) Antidiabetic and renoprotective effect of Anogeissus acuminata leaf extract on experimentally induced diabetic nephropathy. J Basic Clin Physiol Pharmacol 29(4):359–364
Visnagri A, Kandhare AD, Chakravarty S, Ghosh P, Bodhankar SL (2014) Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm Biol 52(7):814–828
Santos-Nogueira E, Redondo Castro E, Mancuso R, Navarro X (2012) Randall-Selitto test: a new approach for the detection of neuropathic pain after spinal cord injury. J Neurotrauma 29(5):898–904
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53(1):55–63
Kulkarni YA, Agarwal S, Garud MS (2015) Effect of Jyotishmati (Celastrus paniculatus) seeds in animal models of pain and inflammation. J Ayurveda Integr Med 6(2):82
Sugimoto K, Rashid IB, Shoji M, Suda T, Yasujima M (2008) Early changes in insulin receptor signaling and pain sensation in streptozotocin-induced diabetic neuropathy in rats. J Pain 9(3):237–245
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95(2):351–358
Slater TF, Sawyer BC (1971) The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J 123(5):805–814
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77
Addepalli V, Suryavanshi SV (2018) Catechin attenuates diabetic autonomic neuropathy in streptozotocin induced diabetic rats. Biomed Pharmacother 108:1517–1523
Vinik AI, Erbas T, Casellini CM (2013) Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Invest 4(1):4–18
Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Ziegler D (2005) Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28(4):956–962
Fang ZY, Prins JB, Marwick TH (2004) Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev 25(4):543–567
Pittenger G, Vinik A (2003) Nerve growth factor and diabetic neuropathy. Exp Diabesity Res 4(4):271–285
Suryavanshi SV, Kulkarni YA (2021) Attenuation of cardiac autonomic neuropathy by escin in diabetic rats. Pharmacology 106(3–4):211–217
Gul H, Yildiz O, Dogrul A, Yesilyurt O, Isimer A (2000) The interaction between IL-1β and morphine: possible mechanism of the deficiency of morphine-induced analgesia in diabetic mice. Pain 89(1):39–45
Gao F, Zheng ZM (2014) Animal models of diabetic neuropathic pain. Exp Clin Endocrinol Diabetes 122(02):100–106
Hasegawa Y, Kishimoto S, Nomura H, Yonezawa K, Inotsume N, Takeuchi Y, Fukushima S (2011) Effects of streptozotocin dosing on the disease state of streptozotocin-induced diabetic rats. J Drug Deliv Sci Technol 21(5):441–444
Uao OD, Udokang N, Udobang J, Ekpenyong C (2012) Oral administration of aqueous leaf extract of Ocimum gratissimum ameliorates polyphagia, polydipsia and weight loss in streptozotocin-induced diabetic rats. Am J Med Med Sci 2(3):45–49
Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi SH, Farhangi A, Verdi AA, Rad B (2007) Induction of diabetes by streptozotocin in rats. Indian J Clin Biochem 22(2):60–64
Saraswat N, Sachan N, Chandra P (2020) Anti-diabetic, diabetic neuropathy protective action and mechanism of action involving oxidative pathway of chlorogenic acid isolated from Selinum vaginatum roots in rats. Heliyon 6(10):e05137
Florkowski C (2013) HbA1c as a diagnostic test for diabetes mellitus–reviewing the evidence. Clin Biochem Rev 34(2):75
Vlassara H, Striker GE (2013) Advanced glycation end products in diabetes and diabetic complications. Endocrinol Metab Clin North Am 42(4):697–719
Nowotny K, Jung T, Höhn A, Weber D, Grune T (2015) Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5(1):194–222
Campbell JN, Meyer RA (2006) Mechanisms of neuropathic pain. Neuron 52(1):77–92
Sherwani SI, Khan HA, Ekhzaimy A, Masood A, Sakharkar MK (2016) Significance of HbA1c test in diagnosis and prognosis of diabetic patients. Biomark 11:666–8440
Zhou G, Yan M, Guo G, Tong N (2019) Ameliorative effect of berberine on neonatally induced type 2 diabetic neuropathy via modulation of BDNF, IGF-1, PPAR-γ, and AMPK expressions. Dose-Response 17(3):1559325819862449
Solanki ND, Bhavsar SK (2015) An evaluation of the protective role of Ficus racemosa Linn. in streptozotocin-induced diabetic neuropathy with neurodegeneration. Indian J Pharmacol 47(6):610
Tembhurne SV, Sakarkar DM (2011) Effect of fluoxetine on an experimental model of diabetes-induced neuropathic pain perception in the rat. Indian J Pharm Sci 73(6):621
Manoharan S, Umadevi S, Jayanthi S, Baskaran N (2011) Antihyperglycemic effect of Coscinium fenestratum and Catharanthus roseus in alloxan-induced diabetic rats. Int J Nutr Pharmacol Neurol Dis 1(2):189
Newby AC (2005) Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev 85:1–31
Kandhare A, Raygude K, Ghosh P, Bodhankar S (2011) The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Int J Green Pharm 5:236–243
Kandhare AD, Raygude KS, Ghosh P, Gosavi TP, Bodhankar SL (2011) Patentability of animal models: India and the globe. Int J Pharm Biol Arc 2(4):1024–1032
Raygude KS, Kandhare AD, Ghosh P, Ghule AE, Bodhankar SL (2012) Evaluation of ameliorative effect of quercetin in experimental model of alcoholic neuropathy in rats. Inflammopharmacology 20(6):331–341
Visnagri A, Kandhare AD, Kumar VS, Rajmane AR, Mohammad A, Ghosh P, Bodhankar SL (2012) Elucidation of ameliorative effect of Co-enzyme Q10 in streptozotocin-induced diabetic neuropathic perturbation by modulation of electrophysiological, biochemical and behavioral markers. Biomed Aging Pathol 2(4):157–172
Pabbidi RM, Yu SQ, Peng S, Khardori R, Pauza ME, Premkumar LS (2008) Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol Pain 4(1):1–17
Kapur D (2003) Neuropathic pain and diabetes. Diabetes/Metab Res Rev 19:S9-15
Wuarin-Bierman L, Zahnd GR, Kaufmann F, Burcklen L, Adler J (1987) Hyperalgesia in spontaneous and experimental animal models of diabetic neuropathy. Diabetologia 30(8):653–658
Jensen TS, Baron R (2003) Translation of symptoms and signs into mechanisms in neuropathic pain. Pain 102:1–8
Quattrini C, Tesfaye S (2003) Understanding the impact of painful diabetic neuropathy. Diabetes/Metab Res Rev 19:S2-8
Calcutt N, Freshwater J, Mizisin A (2004) Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia 47:718–724
Callaghan MJ, Ceradini DJ, Gurtner GC (2005) Hyperglycemia induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid Redox Signal 7:1476–1482
Niedowicz DM, Daleke DL (2005) The role of oxidative stress in diabetic complications. Cell Biochem Biophys 43:289–330
Greene DA, Sima AA, Stevens MJ, Feldman EL, Lattimer SA (1992) Complications: neuropathy, pathogenetic considerations. Diabetes Care 15(12):1902–1925
Baydas G, Sonkaya E, Tuzcu M, Yasar A, Donder E (2005) Novel role for gabapentin in neuroprotection of central nervous system in streptozotocine-induced diabetic rats. Acta Pharmacol Sin 26(4):417–422
Serpell M (2006) Anatomy, physiology and pharmacology of pain. Surg Infect (Larchmt) 24:350–353
Head KA (2006) Peripheral neuropathy: pathogenic mechanisms and alternative therapies. Alternat Med Rev 11:294–329
Gosavi TP, Kandhare AD, Ghosh P, Bodhankar SL (2012) Anticonvulsant activity of Argentum metallicum, a homeopathic preparation. Der Pharmacia Lett 4:626–637
Halliwell B (1991) Drug antioxidant effects. A basis for drug selection? Drugs 42:569–605
Low PA, Nickander KK (1991) Oxygen free radical effects in sciatic nerve in experimental diabetes. Diabetes 40:873–877
Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, Low PA (1995) Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care 18(8):1160–1167
Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Gosavi TP, Badole SL, Bodhankar SL (2012) Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in rats. Asian Pacific J Trop Biomed 2(5):337–344
Arai K, Maguchi S, Fujii S, Ishibashi H, Oikawa K, Taniguchi N (1987) Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vitro glycated sites. J Biol Chem 262(35):16969–16972
Patil MVK, Kandhare AD, Bhise SD (2012) Anti-arthritic and antiinflammatory activity of Xanthium strumarium L. ethanolic extract in Freund’s complete adjuvant induced arthritis. Biomed Aging Pathol 2:6–15
Patil MVK, Kandhare AD, Bhise SD (2012) Effect of aqueous extract of Cucumis sativus Linn. fruit in ulcerative colitis in laboratory animals. Asian Pac J Trop Biomed 2:S962–S969
Kishi Y, Nickander KK, Schmelzer JD, Low PA (2000) Gene expression of antioxidant enzymes in experimental diabetic neuropathy. J Peripher Nerv Syst 5:11–18
Tiwari BK, Khosa RL (2009) Hepatoprotective and antioxidant effect of Sphaeranthus indicus against acetaminophen-induced hepatotoxicity in rats. J Pharm Sci Res 1(2):26–30
Shirwaikar A, Prabhu KS, Punitha IS (2006) In vitro antioxidant studies of Sphaeranthus indicus (Linn). Indian J Exp Biol 44:993–996
Shirwaikar A, Prabhu K, Kumar CD, Rajendran K, Lobo R (2006) Pharmacognostical evaluation of Sphaeranthus indicus (Linn). Nat Prod Sci 12:85–88
Shirwaikar A, Rajendran K, Kumar CD (2004) Oral antidiabetic activity of Annona squamosa leaf alcohol extract in NIDDM rats. Pharm Biol 42:30–35
Vertommen J, Van den Enden M, Simoens L, De Leeuw I (1994) Flavonoid treatment reduces glycation and lipid peroxidation in experimental diabetic rats. Phytother Res 8:430–432
Acknowledgements
The authors are thankful to the Department of Pharmacology, B. N. College of Pharmacy, B. N. University, Udaipur-313001, Rajasthan (India) for the infrastructure facilities to carry out this work.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
The entire theme and idea of the work were given by VBJ and JSV. Experimental studies were conducted and compiled by VBJ. Further, the overall results of the study were analyzed by both VBJ and JSV. Manuscript drafting was done by VBJ under the supervision of JSV. Both authors have carefully read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The protocol of the experiment was approved by the Institutional Animal Ethics Committee (IAEC) of Bhupal Nobles' College of Pharmacy, Udaipur-313001, Rajasthan (India) (870/PO/Re/S/05/CPCSEA).
Consent for publication
Not applicable.
Competing interests
This paper is jointly prepared by both authors and there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jadhav, V.B., Vaghela, J.S. Sphaeranthus indicus Linn ameliorates streptozotocin-induced experimental diabetic neuropathy by targeting oxidative stress-mediated alterations. Futur J Pharm Sci 8, 55 (2022). https://doi.org/10.1186/s43094-022-00444-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-022-00444-2