Abstract
Background
The incidence of cervical cancer is increasing at an alarming rate in many countries and presently, it is the most common form of malignant cancer being reported among women in India. Development of novel approach for cervical cancer therapy, sparing healthy normal cells overcoming the limitations of prevailing therapies is of prime importance. Mangroves constitute a significant repository of medicinally important plants. Thus, in this study, we aimed to determine the anticancer activity of the mangrove Excoecaria agallocha L. leaf extracts on human cervical cancer (SiHa HPV 16+) cell line with subsequent characterization of the bioactive compounds conferring the anticancer activity and studying the probable underlying mechanism of action of the purified plant extract.
Results
The plant extract was subjected to silica gel column chromatography and the fractions obtained were analyzed for cytotoxic activity against SiHa cells by MTT assay. One out of the three eluted fractions exhibited selective toxicity against SiHa cells with an IC50 value of 15.538 ± 0.577 µg/mL, while it had no cytotoxic effect on normal healthy human peripheral blood mononuclear cells. High-resolution liquid chromatography mass spectroscopy, coupled to electron spray ionization and diode array detection analysis, led to the structure elucidation and identification of a few pharmacologically important compounds, with Bergenin being present in the highest abundance. Fluorescence microscopy results revealed that the plant extract fraction induced LC3 puncta formation, in EGFP- SiHa cells indicating the onset of autophagy, with simultaneous stimulation of mitophagy. The plant extract also inhibited proliferation of the SiHa-smac-mCherry cells by second mitochondria-derived activator of caspase (SMAC)—induced cytochrome c dependent apoptosis, that was further confirmed with Caspase-3 activation by colorimetric assay. The GFP-dgn in SiHa cells was remarkably protected from proteasomal degradation that might upregulate the survivability of the cells significantly. Flow cytometry followed by Western blot analysis further asserted the ability of the plant extract fraction to cause cell cycle arrest of SiHa cells in the G2/M phase by significantly reducing protein expression levels of cyclin B1 and D1, decreasing Cdc2 level and simultaneously increasing p21 and p53 levels.
Conclusion
It could be inferred that the aqueous extract of E. agallocha successfully decreased the proliferation of SiHa cervical cancer cells through induction of autophagy and apoptosis in a concerted manner, with simultaneous stimulation of mitophagy and G2/M phase cell cycle arrest, hinting at Bergenin being the major compound conferring the anti-cancer activity of the plant extract. Thus, isolation of the identified bioactive compounds from E. agallocha and their subsequent purification for drug development might serve as a novel medicinal approach for the treatment of cervical cancer in conjugation with existing therapeutic methods.
Similar content being viewed by others
Background
The Indian Sundarbans are one of the largest mangrove forests of the world, well known for the rich diversity of flora and fauna it harbors, along with the significant role it plays in maintaining the ecological balance of the environment [1]. Studies have revealed that mangrove plants possess a repertoire of phytochemicals that are of pharmacological and medicinal importance with potential prospect of application in modern chemotherapeutics [2,3,4]. For centuries, the tribal population of the Sundarbans employed mangrove plant extracts as their traditional medicine for healing health disorders [5, 6]. However, unlike other natural herbs or medicinal plants, the use of mangroves in chemotherapeutics has been comparatively less explored.
Excoecaria agallocha is one such mangrove plant that has been traditionally used in folklore medicine by the inhabitants of Sundarbans but limited research has been conducted to isolate and purify the bioactive compound from it that confers the biological activity exhibited. The genus Excoecaria belonging to the family Euphorbiaceae comprises of 37 tree species [7]. These trees are found in tropical areas of Asia, Africa, and Australia. However, only E. agallocha and E. indica grow in the mangroves. E. agallocha is widely found in the Indian Sundarbans [8, 9]. Studies have revealed its anti-diabetic, antimicrobial, anti-larvicidal, anti-nociceptive and anti-cancerous properties [10].
At a global level, cervical cancer is one of the most common malignant forms of cancers among women [11]. In recent years, resistant to chemotherapeutics and increasing toxicity of the drugs have been observed that have decreased the efficiency of the existing treatment strategies for cervical cancer [12,13,14]. Constant efforts are being put forward by scientists to develop effective chemotherapeutics. Novel metallodrugs and compounds such as 2-(4-N, N,-Dimethyl) pyridine containing oxadiazole moiety, Schiff base derivatives of 5-(2-phenoxypyridin-3-yl)-1,3,4-thiadiazol-2-amine, novel amine derivatives and aryl-substituted acetamide derivatives have been recently synthesized and have exhibited moderate to potent anticancer activity against a wide range of cancer cell lines such as HeLa, MCF7 and Caco-2 [15,16,17,18]. However, such compounds are challenging from the viewpoint of designing and development of such drugs, because of structural complexity. Not all countries have the amenities and infrastructure to bear the expenditures of developing such drugs on an industrial scale. Moreover, cases of induction of genotoxicity by metallodrugs have also been reported in a few cases; molecular basis of their activities are yet to be clearly understood and efficacy of such compounds is highly dependent on stages of the cell cycle and body clock that poses limitations to its clinical administration [19]. These factors demand the development of more potent chemotherapeutics, preferably from a natural source, that will cause minimum toxicity to the normal cells. Most drugs used generally work by inducing apoptosis of the cancerous cells. However, other forms of cell damage mechanisms that are non-apoptotic should be studied as well so that the co-existing mechanisms are well understood to culminate them into an efficient cancer treatment strategy [20]. To understand the correlation, real-time imaging analysis for detection of apoptosis and autophagy as well as other mechanisms that occur parallelly at single cell level is crucial [21].
Though the anti-cancer activity of E. agallocha extracts have been reported earlier, the specific bioactive compound responsible for conferring such properties and the underlying mechanism of action have not been clearly deciphered till date. Our study thus aims to determine the potent bioactive fraction purified from the extract of E. agallocha leaves, determine its anticancer activity against SiHa cervical cancer cell lines and monitor the associated mechanism of action through live cell imaging and fluorescence microscopy analysis, that would enable us to put forward the findings for the development of a novel chemotherapeutic strategy for the treatment of human cervical cancer.
Methods
Plant collection and preservation
Fresh leaf samples of Excoecaria agallocha were collected from Bali Island of the Indian Sundarbans (between 21° 013′ N and 22° 040′ N latitude and 88°003'E and 89°007'E longitude), with the assistance of traditional medical practitioners, a botanist and the local administration. The plant was identified at St. Xavier’s College (Autonomous), Kolkata, and voucher specimen were deposited for future reference (MCB2018/541/005). Collected plants were washed with distilled water stored at 4 °C after collection and utilized within 7 days for extract preparation [22].
Preparation of E. agallocha leaf extract
Fresh leaves of E. agallocha were washed under running tap water, dried in sunlight for about 3–4 days and then grounded to fine a powder and stored in airtight bottles. 25 g of the powdered plant material was added to 50 mL double distilled water and left on an orbital shaker for 48 h. Extracts was filtered with a membrane filter and dried using a rotary vacuum evaporator (RotaVap, India). The dried extract was collected and stored in aliquots at 4 °C for further experiments [23]. Crude aqueous extract of E. agallocha was subjected to column chromatography.
Isolation of compounds from plant extract by silica gel column chromatography
Crude aqueous extract of the leaf sample of E. agallocha was adsorbed onto silica gel (purchased from Sigma-Aldrich) by triturating in a mortar and left for about 10 h to dry [25]. Silica gel (100–200 mesh) was used as stationary phase, and a glass column was used that had dimensions of 25 cm × 2 cm (N.R. Scientific, Kolkata). Packing of the column with silica gel was done by wet slurry method. While packing, a padding of cotton was placed at the bottom of the column. Slurry was made with the required amount of stationary phase (silica gel) in the solvent of slowest polarity (n-hexane) and gradually poured into the column to form a bed of silica. The extract was mixed with the silica gel (1:3 ratio). It was then poured on to the top of the packed silica gel, a layer of cotton was covered again and the mixture was allowed to percolate. The column was then eluted with n-butanol: acetic acid: water (4: 1: 1), as this solvent system has been standardized for aqueous E. agallocha extracts in previous studies as well [24]. Twelve fractions were initially collected; each fraction was subjected to Thin Layer Chromatography (TLC) and their Retention factor (Rf) values noted. Silica gel 60 F254 plate (Merck) of uniform thickness of 0.2 mm was used a stationary phase [25]. 10 µl of the fraction was applied on the TLC plate and developed in the solvent system in a closed glass chamber to a height of about 8 cm. The plate was sprayed with Vanillin spray reagent (0.5 gm Vanillin in 100 ml ethanol and 1.5 ml of conc. Sulphuric acid) and the Rf values of each band was recorded according to the formula:
-
Retention factor (Rf) = Distance travelled by the fraction/Distance travelled by the solvent.
Maintenance of human cervical cancer cell lines
SiHa [cervical cancer cell-HPV 16+ (Human Papilloma Virus infected)] cells were maintained in RPMI-1640 medium (Invitrogen) containing 10% Fetal Bovine Serum (Sigma) and 1X antibiotic–antimycotic (Invitrogen) at 37 °C and pH 7.4 in a 5% CO2 humidified incubator. Peripheral blood mononuclear cells (PBMCs), used to study the selective cytotoxic potential of the plant extract, were maintained similarly in Roswell Park Memorial Institute-1640 medium (RPMI-1640) (HiMedia). SiHa LC3-EGFP (Light Chain III—enhanced green fluorescent protein), SiHaMitoKeima, SiHaSmac (second mitochondria-derived activator of caspases)-mCherry and SiHa expressing proteasome sensor [green fluorescent protein-degron (GFP-dgn)] stable cells stable cells were developed by Dr. T.R Santhosh Kumar’s Lab, Rajiv Gandhi Centre for Biotechnology (RGCB), Kerala.
Evaluation of cytotoxicity of isolated compounds by MTT assay
The evaluation of cytotoxicity of the 3 purified plant extract fractions of E. agallocha was done on cervical cancer cell line (SiHa HPV 16+) and healthy peripheral blood mononuclear cells (PBMC) by using 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium bromide (MTT) assay [26]. Briefly, the cells were cultured in 96-well plates at density of 2.5 × 104 cells per well in the presence of the plant extract. The concentrations of plant extract used were 1, 5, 10, 15, 20 and 30 µg/mL. After incubation for 48 h, 20 µl MTT dissolved in phosphate buffer saline (PBS, pH 7.4) was added to each well at a final concentration of 5 mg/mL and then incubated at 37 °C and 5% CO2 for 2 h. The water-insoluble dark blue formazan crystals that formed during MTT cleavage in actively metabolizing cells were dissolved in Dimethyl sulfoxide (DMSO, HiMedia). The optical density was read by a microplate reader at a wavelength of 570 nm. Results were expressed as the mean of three replicates as a percentage of control (taken as 100%). The extent of cytotoxicity was defined as the relative reduction of optical density (OD), which correlated to the number of viable cells in relation to cell control (100%). Cisplatin was used as reference standard drug. The cell viability was plotted in a graph and the IC50 was calculated accordingly to determine the optimum dosage of the purified extract for further studies. The fraction with potential anti-cancer activity was subjected to High Resolution Liquid Chromatography Mass Spectroscopy (HR LC–MS).
High resolution liquid chromatography-mass spectrometry (HR LC-MS) analysis of isolates
The bioactive fraction exhibiting potent anticancer activity against SiHa cells was subjected to High Resolution Liquid Chromatography and Mass Spectroscopy (HR-LCMS) with Diode Array Detection (DAD) for characterization and identification of the bioactive compound present in this fraction. DAD also enable us to know the wavelength at which the compound with highest abundance is present. HR-LCMS of the fraction was carried out in Sophisticated Analytical Instrument Facility (SAIF), Powai, IIT Bombay. [Instrument specification: Agilent Technologies, USA; Model: 1290 Infinity UHPLC system, 6550 iFunnel Q-TOFs; Mass range 50–3200 amu; Column details: Syncronis C18 100 × 2.1, particle size 1.7µ; acquisition time-30 min; flow rate 0.3 mL/min].
Effect on human cervical cell line by cellular imaging analysis—effect on autophagy, mitophagy, apoptosis and proteasome inhibition
The fraction containing the anti-cancer activity indicated from MTT assay was considered for these assays. For live cell imaging analysis, the fraction was preserved in lyophilized form before use. During experiment, it was dissolved in distilled water to obtain the aqueous extract with a stock concentration of 100 µg/mL. At RGCB, the methodologies have been standardized for all the assays. For the live cell imaging analysis, the SiHa cells were incubated with the purified plant extract in different concentrations 7.5, 15 and 30 µg/mL for 24 h in 5% CO2 at 37 °C. After 24-h incubations, cells were imaged viewed under Inverted fluorescent microscope EclipseTi2 (Nikon, Japan) equipped with Spectra X light source from Lumencore.
Autophagy study
SiHa LC3-Green fluorescent protein (GFP) cells were used. The GFP was excited using a 470/24 nm filter and Red fluorescent protein (RFP) at 575/35 nm filter. The emission of GFP 536/40 and RFP 593/40 was collected using specific filters placed on the external wheel. The images were captured with an electron multiplying charge coupled device (EMCCD) camera (AndoriXON 897) using NIS element software (Nikon). Cisplatin (at its IC50 dosage) was used as reference standard.
Mitophagy study
SiHa-MitoKeima (SiHa labeled with mtKeima) cells were imaged using excitation at 440 nm and emission range of 590 to 630 nm. Ratio of 561 nm/458 nm was calculated using NIS element software (Nikon).
Apoptosis study
SiHaSmac-mCherry cells were used. The fluorescent protein Smac was used to visualize the mitochondrial permeabilization event during apoptosis. Increase in red fluorescence was determined using fluorescence microscopy. The images were captured with an EMCCD camera (AndoriXON 897) using NIS element software (Nikon).
Study of proteasome inhibition
SiHa cells expressing proteasome sensor (GFP-dgn) stable cells were used. Proteasomal inhibition was monitored in cells treated with plant extracts along with untreated cells. The GFP was excited using a 470/24 nm filter and emission of GFP 510/20 was collected using specific filters placed on the external wheel. The images were captured with an EMCCD camera (AndoriXON 897) using NIS element software (Nikon).
Caspase-3 activity by colorimetric assay
Caspase-3 Colorimetric assay kit (BioVision K106-25) was used and the protocol described in the kit. Briefly, Caspase-3/CPP32 Colorimetric Assay Kit provides a simple and convenient means for assaying the activity of caspases that recognize the specific sequence Asp-Glu-Val-Asp (DEVD). The assay is based on spectrophotometric detection of the chromophore p-nitroaniline (pNA) after cleavage from the labeled substrate DEVD-pNA. The pNA light emission can be quantified using a spectrophotometer or a microtiter plate reader at 400- or 405-nm. Comparison of the absorbance of pNA from an apoptotic sample, induced with the plant extract for 24 h, with an uninduced control allows determination of the fold increase in caspase activity (ID K106-25, BioVision Inc.; Mountain View, USA). Cisplatin (at IC50 dosage value) was used as reference standard.
Cell cycle analysis by flow cytometry and determination of protein expression analysis by Western Blotting
For cell cycle analysis, SiHa cells at 1 × 106 cells density was cultured in 6-well plates. After 16 h, the cells were treated with different concentrations of the plant extract for 48 h. After treatment, the cells were washed with PBS (phosphate buffer saline) and harvested by adding 300 µL of trypsin-ethylenediamine tetraacetic acid (EDTA). The harvested cells were washed in ice-cold PBS and centrifuged at 5000 rpm for 5 min. Further, the cells were resuspended and fixed with ice cold 70% ethanol for 2 h. After fixing, the cells were washed with ice cold PBS and centrifuged at 5000 rpm for 5 min. The pellets were resuspended in 250 µL PBS containing Propidium Iodide (PI) (10 µg/mL), RNase A (20 µg/mL) and incubated for further 30 min in the dark. Finally, the cells were analyzed by flow cytometry (BD FACSAria III, BD Biosciences, USA) and dot plot diagrams were generated using BD software (BD Biosciences, USA).
Western Blot analysis was done using a kit-based assay (Fast Western Blot Kit, Thermo Fisher Scientific). SiHa cells (1.0 × 106cells/60) were detached, washed in ice-cold cold PBS, and suspended in 100 1L of lysis buffer. The suspension was kept on ice for 20 min and then centrifuged at 5000 rpm for 20 min at 4 °C. The protein extracts were resuspended in sample buffer and this mixture was boiled for about 5 min. 50 µg of the proteins were loaded into each lane, resolved on a 10% Sodium dodecyl-sulfate (SDS) polyacrylamide gel (PAGE) and then transferred to nitrocellulose membrane [27] for western blot using the required antibodies against cyclin B1, cyclin D1, Cdc2, p21 and p53. Proteins of interest were visualized by using Trans-Blot Turbo Transfer system (Bio-Rad).
Statistical analysis
All experimental results are expressed as mean ± standard deviation from at least three independent experiments performed in triplicates. Statistical analysis was done using ANOVA test of Microsoft Excel (Version 2016) data analysis tool. When p < 0.05, the result was considered statistically significant.
Results
Isolation of bioactive fraction from E. agallocha extract by column chromatography and thin-layer chromatography
Column chromatography followed by thin-layer chromatography analysis is a widely used technique to purify a crude plant extract at a preliminary stage and simultaneous qualitative analysis by TLC indicates the plausible biochemical nature of the constituents present in the plant extract. Chromatographic techniques play a significant role in asserting the chemistry of natural products and contribute significantly in the discovery of compounds of pharmaceutical and biomedical importance. In our investigation, twelve fractions were eluted by column chromatography and when subjected to TLC, produced three fractions FI, FII and FIII with different (Table 1).The Rf values indicated the presence of diterpenoids, aromatic compounds and glycoside derivatives, which were further examined by HR-LCMS at a later stage. These three fractions were firstly assessed for cytotoxicity against SiHa cells using MTT assay.
Toxicity of the plant extract against healthy mammalian cell line and cancer cell line using MTT assay
MTT assay results exhibited a remarkable cytotoxic activity of FII against the SiHa cell line (Fig. 1a) while the other two fractions were not cytotoxic against SiHa cells within the range of concentration studied (Table 2). Cisplatin used as standard reference drug was observed to have an IC50 value of 5.122 ± 0.004 µg/mL (Fig. 1b).The results of the MTT assay indicated that the half-maximal inhibitory concentration (IC50) value of the FII plant extract was about 15.538 µg/mL ± 0.577 µg/mL (Fig. 2a). Moreover, the FII fraction induced a significant increase in cell proliferation when treated on healthy PBMC cells (Fig. 2b), which is indicative of its selective toxicity. Thus, this extract would produce no toxicity in normal healthy cells in the applied dosage concentration (15 µg/mL). This potent fraction possessing cytotoxic activity was thereafter subjected to LC–MS for identification of the bioactive compounds present.
Identification of the bioactive compound by HR-LCMS
The DAD (Diode array detector) analysis firstly confirmed that the fraction was devoid of any major impurities and, hence, was subjected to HR-LCMS for further analysis. HR-LCMS results of FII fraction indicated the presence of four major compounds at 280 nm scan (Fig. 3).The electron spray ionization (ESI) (positive and negative mode scan) analysis of the plant extract has been represented (in Fig. 4) where Bergenin and other pharmaceutically important compounds were detected in the positive ion mode. Khivorin, khayanthone, alpha-4-dihydroxytriazolam and chlorogenic acid were majorly detected in ESI negative ion mode while Bergenin, Chorismic acid, N-Acetyl ketoconazole and Nicotinamide mononucleotide were detected in the positive ion mode (Fig. 4a, b). Out of these compounds identified, Bergenin (328.08 g/mol) was present in the highest abundance (Table 2), hinting its contribution individually or in synergistic association with the other compounds, for the anti-cancer activity observed against SiHa cells. The detailed MS spectra (Fig. 5a) with the molecular formula determined (Table 3) further validated the DAD analysis results and the structure of the compound Bergenin was elucidated by HR LC–MS (Fig. 5b).
Induction of autophagy indicated by LC3 puncta formation in SiHa cells
During the process of autophagy, cytoplasmic LC3 (fluorescently labeled in our experiment) is processed and, thereby recruited to the autophagosomal membranes to form the autophagosome. It then fuses with the lysosome to form the autolysosome. Induction of autophagy is marked by puncta formation in cells. In our study, control cells (SiHa cells without plant extract treatment) showed absence of LC3 puncta formation while 24 h treatment of aqueous plant extract (7.5, 15, 30 µg/mL) showed increased LC3 Puncta formation in SiHa LC3-GFP cells in a dose-dependent manner (hole/puncta formation in cells, indicated by arrows in Fig. 6). Cisplatin also exhibits increased fluorescence but puncta formation is relatively lower.
Mitophagy induction detected by increased MitoKeima ratio in SiHa cells
MitoKeima is a pH-sensitive, dual-excitation ratiometric fluorescent probe that exhibits resistance to lysosomal proteases. At the physiological pH of the mitochondria (pH 8.0), the shorter-wavelength excitation (440 nM) predominates. Within the acidic lysosome (pH 4.5) after mitophagy, mt-Keima undergoes a gradual shift to longer-wavelength excitation (560 nm). The MitoKeima ratio (560 nm/440 nm) is observed for inferring the results. Low MitoKeima Ratio indicates absence of mitophagy while a higher ratio implies the occurrence of mitophagy. In our experiment, control cells showed low MitoKeima ratio. 24-h treatment with aqueous plant extract (7.5, 15, 30 µg/mL) showed increased MitoKeima ratio in Siha MitoKeima cells when compared to control cells, indicating the induction of mitophagy and permeabilization of the mitochondrial membrane in the SiHa cells (Fig. 7). The activity of cisplatin is relatively much higher as observed by the increased MitoKeima ratio that showed almost all the cells undergoing mitophagy, marked by red fluorescence.
a–e Mitophagy, indicated by MitoKeima ratio, was observed to increase in SiHa cells, treated with aqueous plant extract (7.5, 15, 30 µg/mL) for 24 h, in a dose-dependent manner whereas no puncta was observed in control (× 400 magnification). Cisplatin induced much higher stimulation of mitophagy as observed by the relatively higher intensity of red fluorescence detected
Smac-induced cytochrome c dependent activation of apoptosis
As mitochondrial membrane had been disrupted as observed from the Mitophagy screening assay, the next step was to assess the release of caspases from the mitochondria that might be triggered by the action of a mitochondrial protein. In our experiment, the Smac (Second Mitochondria-derived activator of caspases) protein had been fluorescently labeled in order to trace its release, which in turn indicates the activation of apoptosis. Control cell showed intact mitochondria without Smac release. 24-h treatment of plant extracts (7.5, 15, 30 µg/mL) showed mild increase in Smac release when compared to normal SiHaSmac-mCherry Cells (Fig. 8), indicating that autophagy and mitophagy consequently might lead to the apoptotic death of cells.
a–e Increased fluorescence in dose-dependent manner was observed due to Smac release and simultaneous apoptosis of SiHa-mCherry cells, treated with aqueous plant extract (7.5, 15, 30 µg/mL) for 24 h, while no Smac release was observed in control cells (400 × magnification). The increase in fluorescence in case of cisplatin treated cells was almost comparable to the effect observed in plant extract treated cells
Caspase-3 assay by colorimetric assay
This assay utilizes a synthetic tetrapeptide, Asp-Glu-Val-Asp (DEVD), labelled with a colorimetric molecule, p-nitroanilide (pNA) as substrates. The results indicate that the caspase-3 content increases in SiHa cells in a dose-dependent manner (Fig. 9), validating the imaging analysis observations. Induction of apoptosis by the plant extract indicates an increase in the DEVD-dependent protease activity that ultimately leads to apoptosis of the cancer cells, as activated caspase-3 in apoptotic cells cleave the substrate into free pNA which is determined spectrophotometrically at 405 nm.
Proteasome inhibition analysis
The GFP-dgn fusion protein contains a specific sequence which is targeted for proteasomal degradation, which corresponds to a decrease in GFP fluorescence signal. Treatment of SiHa cells with aqueous plant extract (7.5, 15, 30 µg/mL) for 24 h showed clear proteasomal inhibition of the protein in a dose-dependent manner (Fig. 10), indicated by the increased amount of fluorescence detected.
Cell cycle analysis
The deoxyribonucleotide (DNA) content profile of the plant extract treated SiHa cells (7.5–30 µg/mL) was observed using flow cytometry analysis (Fig. 11), that measured the fluorescence of DNA bound to PI. Our observations suggest that E. agallocha plant extract (FII) exposure to SiHa cells resulted in accumulation of cells in the G2/M phase in a dose-dependent manner. Results also indicated (Table 4) that increase in the percentage of cells in the G2/M phase was associated with simultaneous decrease in the G1 phase cells. Thus, it can be inferred that the E. agallocha aqueous extract caused inhibition of cell growth in cervical cancer cells (SiHa) by arresting cell growth at the G2/M phase. Cisplatin, on the other hand, arrested growth at G0/G1 phase. Hence, valinomycin (IC50 2.2 µg/mL against cervical cell line, reported by methods described by Kleuser et al. 1985), that induces arrest of cell growth at G2/M phase [28], was used as reference standard for further validation of the results. The IC50 value of valinomycin for SiHa cells was pre-determined and standardized in the lab (Table 5).
Effect of E. agallocha extract on the expression of cyclin A, cyclin B1, Cdc2, p21 and p53
In order to assess the underlying mode of action in the observed pattern of cell cycle, we analyzed the effect of the plant extract on a few cyclins and CDKs (cyclin-dependent kinases) that are associated in controlling cell cycle in SiHa cells. Our observations indicated that there was marked decrease in the expression of mitotic cyclins B1 and D1, mitotic-cyclin-dependent kinase Cdc2 with simultaneous increase in the expression of p21 and p53 in a dose-dependent manner (Fig. 12). Valinomycin also induced a similar change in protein expression of the treated SiHa cells, further validating the fact that the plant extract causes cell cycle arrest at G2/M phase.β-actin was used as loading control.
Discussion
In recent years, use of natural products from plants or herbal medicines in cancer treatment has received much attention due to the huge reservoir of phytochemicals that exhibit dynamic biological activity. The plant collected from the Sundarbans of West Bengal was analyzed for the presence of phytochemicals, antibacterial, antifungal and antioxidant activity that have been reported in our earlier investigations [29, 30]. Evaluation of the anticancer activity was our next objective that has been evaluated in this paper.
Anticancer activity of E. agallocha extract
Recent studies on anticancer activity of E. agallocha extracts of different plant parts indicate a few interesting findings. Recently conducted studies have reported that ethanolic stem extract of E. agallocha exhibits cytotoxic effect on various cell lines such as Miapaca-2, BxPC-3, PANC-1 and Capan-1, with IC50 values > 0.11 μg/mL. Researchers have concluded that the cytotoxic effect exhibited by the stem extracts might be due to the action of phytochemicals such as phenolic derivatives, glycosides and saponin present in the extracts of E. agallocha, as indicated by chromatographic results [31]. Konoshima et al. [32] have reported that E. agallocha wood contained biologically important compounds namely, diterpenoids that under in vitro condition exhibited potent inhibitory action against Epstein-Barr virus early antigen (EBV-EA). Further, a secolabdane-type diterpenoid was identified from the extract that had potent anti-tumour effect when analyzed by in vivo Two-Stage Mouse Skin Carcinogenesis Test with promoter (12-O-tetradecanoylphorbol acetate) and an initiator 7,12-dimethylbenz[a]anthracene. It is interesting to note that certain flavanol glycosides extracted from E. agallocha block the action of GLI-related protein (Glioma associated oncogene homolog). GLI is a transcriptional effector involved in tumour development; thus, blocking its action consequently leads to the inhibition of its translocation in to the nucleus. Hence, it acts as an effective inhibitor of the Hedgehog signaling pathway in cancer therapy [33, 34]. In 2012, anticancer activity on human lung cancer cell lines of the ethanol stem extract was reported by Patil et al. where the result showed potent cytotoxic activities in a dose-dependent manner [35]. However, as evident from literature studies and recent research findings, not much is known about the anticancer activity of its leaf extract with regard to human cervical cell lines, especially SiHa cells. Our study emphasizes on the aqueous extract so as to minimize toxicity factors and easy administration on cell lines because many a times, solvent system also culminates into the anticancer activity of a plant extract. The MTT assay allows to assess any intrinsic toxicity of the plant extract and helps to determine a safe dosage concentration administration. The results clearly indicate the anticancer activity of Fraction III against SiHa cells with an IC50 value of 15. 35 ± 0.577 µg/mL and no cytotoxic activity against normal healthy cells. Cisplatin has been used as reference standard drug since it is widely used clinically, for the treatment of lung, cervical and ovarian cancers. Hence, it is used for in vitro evaluation of cytotoxic potential of various potent chemotherapeutic drug candidates. The HR LC–MS indicates the presence of the compound Bergenin in the highest abundance in this fraction which is a novel finding since Bergenin has never been reported to be isolated from E. agallocha. Further, Bergenin is a trihydroxybenzoic acid glycoside and thus, this result is in accordance with the TLC observation, where the Rf value indicated the presence of acid glycoside or glycosidic derivatives.
Bergenin—a promising candidate for designing effective chemotherapeutic drug from a novel natural source
Isolation of bioactive compounds using column chromatographic method is a widely adopted method and involves preparation of sample, packing of column, running the column with suitable stationary and mobile phase, elution of fractions and analysis of each eluted fraction using TLC [36]. The LC–MS results relate to the discovery of a new natural source of Bergenin, which is also known as Cuscutin, Ardisic acid B and Vakerin (IUPAC name is (2R,3S,4S,4aR,10bS)-3,4,8,10-tetrahydroxy-2-(hydroxymethyl)9-methoxy-3,4,4a,10b-tetrahydro-2H-pyrano[3,2-c] isochromen-6-one. Its chemical formula is C14H16O9 and has a molecular mass of 328.27 g/mol). Experimentally, Bergenin is known to have anti-inflammatory [37], antioxidant [38], antiulcerogenic, neuroprotective, immunomodulatory, antiarrhythmic [39], hepatoprotective [40] and antiretroviral [41] activities. However, anticancer activity on SiHa cells has not been reported earlier. In the known studies, there are also many other natural sources of Bergenin. Bergenin has been reported to be present in many plants—Aconophora compressa, Ardisia japonica, Astil bechinensis, Astil berivularis, Astil bethunbergii, Bergenia crassifolia, Caesalpinia digyna, Corylopsis spicata, Dryobalanops aromatic, Ficus racemosa, Flueggea leucopyrus, Flueggea virosa, Mallotus japonicus, Mallotus philippensis, Mallotus roxburghianus, Peltophorum africanum, Pentaclethra macrophylla, Sacoglottis gabonensis, Sacoglottis uchi, Saxifraga melanocentra, Shorea robusta, Teramnus labialis, Tridax procumbens etc. [42,43,44,45,46,47], but our finding suggesting that this chemical is present in significantly high amount in aqueous extract of E. agallocha, is a new finding and thus it appears to be an easy source of this chemical in West Bengal, India. Other compounds detected in the potent fraction may also contribute to the cytotoxic activity of the plant extract. Chorismic acid is present at a key branching point in aromatic acid biosynthesis. It is the precursor of tryptophan, tyrosine, and phenylalanine. It helps the biosynthesis of vitamin K and folate in plants and microorganisms. It can modulate t-RNA and is also a precursor of salicylic acid [48]. N-Deacetyl ketoconazole, a derivative of ketoconazole, is an antifungal compound used to treat and prevent fungal Infections. It works by inhibiting an enzyme required for the synthesis of ergosterol and ultimately altering the fungal cell membrane and is primarily fungistatic. It is lipophilic in nature, which leads to accumulation in fatty tissues, and is best adsorbed at the highly acidic level. In conventional treatment, ketoconazole is usually prescribed for infections such as ringworm, candidiasis, etc. The decrease in testosterone caused by the drug makes it useful for treating prostate cancer and for preventing postoperative erections following penile surgery [49]. On the other hand, Nicotinamide mononucleotide is a nucleotide derived from nicotinamide and ribose. It is a derivative of niacin, and in our body, it is converted to nicotinamide adenine dinucleotide (NAD) [50]. Khayanthone and khivorin are limonoids formed from apotirucallane after loss of four terminal carbons. Limonoids are also known as tetranortriterpenoids. Chlorogenic acid is a cinnamate ester and a tannin, having anti-inflammatory and anti-hypertensive properties. Alpha-4-dihydroxytriazolam is a benzodiazepine derivative and has anticonvulsant properties [51]. All the compounds detected are of pharmacological importance; however, only bergenin has been reported to have anti-cancer property. Though the other compounds do not have a direct role in inducing cytotoxicity in cancer cells, they might facilitate the overall survival of healthy normal cells while bergenin ceases the proliferation of cancer cells. All these compounds may be acting in a concerted manner, with Bergenin being the major contributor, for the observed selective cytotoxic potential of the plant extract against SiHa cells with minimal toxicity production in healthy PBMC cells.
E. agallocha extract harbors potential bioactivity to combat human cervical cancer, as revealed by live cell imaging analysis supported by Western blot analysis and cell cycle study
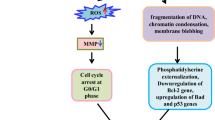
The mechanism of action of the bioactive fraction exhibiting anticancer activity against SiHa cells in our study has been assessed through live cell imaging analysis. We know loss of apoptosis and increased autophagy lead to cancer progression [52]. Apoptosis inducers and autophagy inhibitors are widely preferred as anticancer strategy. However, autophagy is often triggered along with apoptosis as a pro survival mechanism [53]. Few recently studied drugs such as chloroquine and hydroxychloroquine induce autophagic changes in treated cells before induction of apoptosis [54,55,56]. It might happen that when a cell is undergoing the death process, both apoptosis and autophagy may occur simultaneously because a certain level of cellular stress might initiate the autophagy process that leads to apoptosis. To have a better understanding of the associated events, real-time imaging methods to detect and monitor both the process of autophagy as well as apoptosis within the same cell is crucial. There are a number of standardized methods in this regard; however, the best real-time compatible method is LC3 puncta formation [57]. In order to track and follow the cellular event in vivo, labeling of phagophores and autosomes with simultaneous visualization is important. Only few proteins are uniquely associated with autophagic vesicles or precursors, out of which only LC3 labels autophagic structures both before and after fusion with lysosomes. Recruitment of LC3 to autophagosomes is observed as puncta formation where small pores are formed and mitochondrial permeabilization lately follows this process [58, 59]. The delivery of mitochondria to lysosomes has been traced by using mt-Keima (Keima with a mitochondria-targeting sequence). Mt-Keima has pH-dependent excitation profile and has resistance to lysosomal protease. When mitochondrial damage occurs, the membrane potential changes that leads to a change in its excitation level [60, 61]. Fluorescent fusion of intermembrane proteins such as Smac was used to screen the mitochondrial permeabilization process during apoptosis [62]. Further, caspase-3 activation was confirmed by colorimetric assay that showed an increase in the Caspase-3 activity in a dose-dependent manner. The method adopted for proteasomal inhibition study allows a simple, rapid and efficient way to study the ubiquitin–proteasome activity in living cells. A stable overexpression of the GFP reporter substrate has been designed to develop a GFP-dgn that rapidly undergoes proteasomal degradation in cancer cells, which can be observed with decreasing fluorescence [63]. The cell cycle in cancer is dysregulated or disrupted and hence, is a widely studied target for developing new and more potent anti-cancer drugs. Various independent research findings have reported that G2/M phase cell growth arrest is a plausible mode of action by which certain cytotoxic drugs work [64, 65]. In our investigation, flow cytometry analysis reveals that the E. agallocha extract exhibited a pronounced effect on the cell cycle regulation of SiHa cells, wherein accumulation of cells in the G2/M phase was observed. The cell growth arrest was accompanied by decrease in the expression of Cyclin A and B1, along with reduction in Cdc2 expression, with simultaneous increase in the expression of p21 and p53. Cdc2 kinases are mainly activated with activation of cyclin B1 during the G2/M phase [66, 67]. When the Tyr15 of Cdc2 is phosphorylated, the activity of Cdc2/cyclin A and B1 kinase complex is suppressed, thus, acting as a determining step for entry into mitotic phase. p53 detects DNA damage and stimulates DNA repair machinery or pro-apoptotic factors that leads to an increase in the susceptibility of the cells to undergo apoptosis. Further, p21 enable the arrest and maintenance of cells in the G2/M phase by inactivation of the cyclin B1/Cdc2 complex [68]. Our results indicate that reduction in expression of cyclin A, cyclin B1, Cdc2 and upregulation of p21 and p53 result in growth arrest in SiHa cells at G2/M phase, as has been reported by standard reference drugs such as Valinomycin, that induce cell cycle arrest at G2/M phase. Thus, analysis of our observations leads to the inference that the SiHa cell proliferation inhibition on treating with the E. agallocha plant extract may be due to the G2/M phase growth arrest. While the mode of action of the plant extract in case of inducing apoptosis, autophagy and mitophagy is comparable to that of cisplatin to a certain extent, the pattern of inducing cell cycle arrest and changes in protein expression (summarized in Fig. 13) are similar to the effects observed with treatment with valinomycin in vitro. Hence, a further intricate elucidation of signaling pathways and molecular biology analytical methods may be undertaken to precisely infer the mode of action of the purified plant extract fraction.
Conclusions
Based on the findings, it was observed that the aqueous plant extract of E. agallocha had potent anticancer activity against the human cervical cancer cell line (SiHa) and Bergenin was identified as the lead compound present in the extract. This is a unique finding because the specific anticancer activity on cervical cancer cells with minimal or no toxicity on normal healthy cells has not been reported on Excoecaria agallocha extract. Bergenin, the major bioactive compound along with other contents probably act in a synergistic manner or other ways that confer cytotoxic potential to the E. agallocha extract.
Availability of data and materials
All data generated and analyzed during the experiments that support the findings are included within the article.
Abbreviations
- CDK:
-
Cyclin-dependent kinase
- DAD:
-
Diode array detection
- DEVD:
-
Asp-Glu-Val-Asp
- DMSO:
-
Dimethyl sulfoxide
- DNA:
-
Deoxyribonucleotide
- EDTA:
-
Ethylenediamine tetra acetic acid
- EGFP:
-
Enhanced green fluorescent protein
- GFP:
-
Green fluorescent protein
- HR LC–MS:
-
High-resolution liquid chromatography-mass spectroscopy
- IC50 :
-
Half maximal inhibitory concentration
- LC3:
-
Light chain 3
- HPV:
-
Human papilloma virus
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OD:
-
Optical density
- p-NA:
-
P-Nitroanilide
- PBMC:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate buffer saline
- Rf:
-
Retention factor
- RFP:
-
Red fluorescent protein
- RPMI-1640:
-
Roswell Park Memorial Institute-1640 medium
- SMAC:
-
Second mitochondria-derived activator of caspase
- SDS-PAGE:
-
Sodium dodecyl-sulfate polyacrylamide gel electrophoresis
- TLC:
-
Thin layer chromatography
References
Sarker S, Reeve R, Thompson J, Paul N, Matthiopoulos, (2016) Are we failing to protect threatened mangroves in the Sundarbans world heritage ecosystem? Sci Rep 6:21234
Betoni JE, Mantovani RP, Barbosa LN, DiStasi LC, Fernandes Junior A (2006) Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. MemInst Oswaldo Cruz 101(4):387–390
Lewis K, Ausubel FM (2006) Prospects of plant derived antibacterials. Nat Biotechnol 24(12):1504–1507
Lee SB, Cha KH, Kim SN, Altantsetseg S, Shatar S, Sarangerel O, Nho CW (2007) The antimicrobial activity of essential oil from Dracocephalumfoetidum against pathogenic microorganisms. J Microbiol 45:53–57
Kokpal V, Miles DH, Payne AM, Chittawong V (1990) Chemical constituents and bioactive compounds from mangrove plants. Stud Nat Prod Chem 7:175–199
Premanathan M, Nakashima H, Kathiresan K, Rajendran N, Yamamoto N (1996) Invitro antihuman immunodeficiency virus activity of mangrove plants. Indian JMed Res 130:276–279
The Plant List Excoecaria (2013) Database http://www.theplantlist.org
Duke N (2006) Australia’s mangroves: the authoritative guide to Australia’s mangrove plants. University of Queensland, Brisbane
Yin B, Shen L, Zhang M, Zhao L, Wang Y, Huo C, Shi Q (2008) Chemical constituents of plants from the genus Excoecaria. Chem Biodivers 5:2356–2371
Kaliamurthi S, Selvaraj G (2016) Insights on Excoecariaagallocha: an overview. Nat Prod Chem Res 4:2–6
Liu Y, Fan P, Yang Y, Xu C, Hunag Y, Li D et al (2019) Human papillomavirus and human telomerase RNA component gene in cervical cancer progression. Sci Rep 9:15926
Falcetta FS, Medeiros LR, Edelweiss MI, Pohlmann PR, Stein AT, Rosa DD (2016) Adjuvant platinum-based chemotherapy for earlystage cervical cancer. Cochrane Database Syst Rev 11:005342
DattaNR SE, GomezS BS (2019) Efficacy and safety evaluation of the various therapeutic options in locally advanced cervix cancer: a systematic review and network meta-analysis of randomized clinical trials. Int J Radiat Oncol Biol Phys 103:411–437
Fu ZZ, Li K, Peng Y, Zheng Y, Cao LY, Zhang YJ et al (2017) Efficacy and toxicity of different concurrent chemoradiotherapy regimens in the treatment of advanced cervical cancer: a network meta-analysis. Medicine 96(2):e5853
Vinayak A, Sudha M, Adarsha HJ, Lalita KS, Kumar RP (2014) Design, synthesis and cytotoxic evaluation of novel 2-(4-N, N-Dimethyl) pyridine containing 1,3,4-oxadiazole moiety. Asian J Biomed Pharma Sci 4(37):1–5
Vinayak A, Sudha M, Adarsha HJ, Lalita KS, Kumar RP (2013) Synthesis, characterisation and anticancer activity of Schiff base derivatives of 5-(2-phenoxypyridin-3-yl)-1, 3, 4-thiadiazol-2-amine. Int Res J Pharm 4(12):62–66
Vinayak A, Sudha M, Adarsha HJ, Lalita KS, Kumar RP (2017) Design, synthesis and characterization of novel amine derivatives of 5-[5-(chloromethyl)-1, 3, 4-oxadiazol-2-yl]-2-(4-fluorophenyl)-pyridine as a new class of anticancer agents. Dhaka Univ J Pharm Sci 16(1):11–19
Vinayak A, Sudha M, Adarsha HJ, Lalita KS, Kumar RP (2014) Design, synthesis, characterization and cytotoxic evaluation of novel 2-Chloro-N-(aryl substituted) Acetamide derivatives of 5-[2-phenyl pyridin-3-yl]-1, 3, 4-oxadiazole-2-thiol. Int J Drug Dev Res 6(4):188–195
Anthony EJ, Bolitho EM, Bridgewater HE, Carter OWL, Donnelly JM, Imberti C et al (2020) Metallodrugs are unique: opportunities and challenges of discovery and development. Chem Sci 11:12888–12917. https://doi.org/10.1039/D0SC04082G
Datta NR, Stutz E, Liu M, Rogers S, Klingbiel D, Siebenhüner A et al (2017) Concurrent chemoradiotherapy vs radiotherapy alone in locally advanced cervix cancer: a systematic reviewand meta-analysis. Gynecol Oncol 145(2):374–385
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8(9):741–752
Sett S, Hazra J, Datta S, Mitra A, Mitra AK (2014) Screening the Indian Sundarban mangroves for antimicrobial activity. Int J Sci Inn Dis 4(1):17–25
Patra JK, Gouda S, Sahoo SK, Thatoi HN (2012) Chromatography separation 1H NMR analysis and bioautography screening of methanol extract of Excoecariaagallocha L. from Bhitarkanika. Orissa, India. Asian Pac J Trop Bio 2:S50–S56
Arockiamary N, Vijayalakshmi K (2014) Chromatographic separation of bioactive compounds from Ipomoea batatas lam (sweet potatoes) by column, high-performance thin layer chromatography, and gas chromatography-mass spectrum analysis techniques. Asian J Pharm Clin Res 7(5):4–8
Padmaja CK, Mundekkad D (2014) Preliminary phytochemical analysis and thin layer chromatography of the extracts of Excoecariaagallocha L. Int J Pharm Med 5(10):1000–1007
Hackenkamp J, Leszczynski D, Schiereck J, Kung J, La Muraglia GM (1999) Different effects of photodynamic therapy and gamma irradiation on vascular smooth muscle cells and matrix: implications for inhibiting restenosis. Arterioscler Thomb Vasc Biol 93:9821–9826
Saha S, Bhattacharjee P, Guha D, Kajal K, Khan P, Chakraborty S et al (2015) Republished: sulphur alters NKκB- p300 cross- talk in favour of p53–p300 to induce apoptosis in non-small cell lung carcinoma. Indian J Res Homeo 9(4):288–300
Iacobazzi RM, Annese C, Azzariti A, Accolti L, Franco M, Fusco C et al (2013) Antitumor potential of conjugable valinomycins bearing hydroxyl sites: in vitro studies. ACS Med Chem Lett 4(12):1189–1192
Sultana T, Mitra A, Das S (2019) A preliminary observation on an explicit antimicrobial action of mangrove plants on Pseudomonas aeruginosa. Asian J Pharm Clin Res 12(5):226–230
Sultana T, Mitra A, Das S (2021) Antimicrobial action of mangrove plant extracts against Salmonella typhi and Candida parapsilosis characterized by their antioxidant potentials and bioactive compounds. Int J Pharm Sci Res 12(9):1000–1016
Mathew R, Karantza-Wadsworth V, White E (2007) Role of autophagy in cancer. Nat Rev Cancer 7(12): 961–967
Patil RC, Manohar S, Upadhye M, Katchi VI, Rao A, Mule A et al (2012) Anti reversetranscriptase and anticancer activity of stem ethanol extracts of Excoecariaagallocha (Euphorbiaceae). Ceylon J Sci 40:147–155
Konoshima T, Konishi T, Takasaki M, Yamazoe K, Tokuda H (2001) Antitumor-promoting activity of the diterpene from Excoecariaagallocha. II Biol Pharm Bull 24(12):1440–1442
Kinzler KW, Vogelstein B (1990) The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Mol Cell Biol 10(2):634–642
Rifai Y, Arai MA, Sadhu SK, Ahmed F, Ishibashi M (2011) New Hedgehog/ GLI signaling inhibitors from Excoecariaagallocha. Bioorg Med Chem Lett 21:718–722
Bajpai VK, Majumder R, Park JG (2016) Isolation and purification of plant secondary metabolites using column chromatography technique. Bangladesh J Pharmacol 11:844–848
Patil RC, Manohar SM, Katchi VI, Rao AJ, Moghe A (2012) Ethanolic stem extract of Excoecariaagallocha induces G1 arrest or apoptosis in human lung cancer cells depending on their p53 status. Taiwania 57:89–98
GuoCai W, JiePing L, Ying Li W, Ye WenCai Q (2008) Chemical constituents from Flueggeavirosa. Chin J Nat Med 6(4):251–253
Pozharitskaya ON, Ivanova SA, Shikov AN, Makarov VG, Galambosi B (2007) Separation and evaluation of free radical-scavenging activity of phenol components of green, brown, and black leaves of Bergeniacrassifolia by using HPTLC-DPPH method. J Sep Sci 30(15):2447–2451
Sridhar C, Krishnaraju AV, Subbaraju GV (2006) Anti- inflammatory constituents of Teramnuslabialis. Indian J Pharm Sci 68(1):111–114
Kimura Y, Sumiyoshi M, Sakanaka M (2007) Effects of Astilbethunbergii rhizomes on wound healing, part 1: Isolation of promotional effectors from Astilbethunbergii rhizomes on burn wound healing. J Ethnopharmacol 109(1):72–77
Varshney VK, Dayal R (2006) Chemical constituents of Shorearobusta. Int J Chem Sci 4:298–304
de Araujo EV, Soares CAG, Hagler AN, Mendonqa-Hagler LC (1995) Ascomycetous yeast communities of marine invertebrates in a Southeast Brazilian mangrove ecosystem. Antonie Van Leeuwenhoek 68:91–99
Chi Z-M, Liu T-T, Chi Z, Liu G-L, Wang Z-P (2012) Occurrence and diversity of yeasts in the mangrove ecosystems in Fujian Guangdong and Hainan Provinces of China. Indian J Microbiol 52(3):346–353
Das SK, Samantaray D, Mahapatra A, Pal N, Munda R, Thatoi H (2018) Pharmacological activities of leaf and bark extracts of a medicinal mangrove plant Avicenniaofficinalis L. Clin Phytosci 4:13
Patra JK, Panigrahi TK, Rath SK, Dhal NK, Thatoi H (2009) Phytochemical screening and antimicrobial assessment of leaf extracts of Exoecariaagallocha L.: a mangal species of Bhitarkanika, Orissa, India. AdvInd Nat Appl Sci 3:241–246
Sivaperumal P, Ramasamy P, Inbaneson SJ, Ravikumar S (2010) Screening of antibacterial activity of mangrove leaf bioactive compounds against antibiotic resistant clinical isolates. World J Fish Mar Sci 2:348–353
Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414(6863):562–565
Rodriguez RJ, Miranda CL (2000) Isoform specificity of N- deacetyl ketoconazole by human and rabbit flavincontaining monooxygenases. Drug Metab Dispos 28(9):1083–1086
Irie J, Inagaki E, Fujita M (2020) Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese. Endocr J 67(2): 153–160
Tajik N, Tajik M, Mack I, Enck P (2017) The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. European J Nutr 56(7):2215–2244
Bandaranayake WM (2002) Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wet Eco Man 10:421–452
Kondo Y, Kanzawa T, Sawaya R, Kondo S (2005) The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5:726–734
Shen S, Kepp O, Michaud M, Martins I, Minoux H, Metivier D et al (2011) Association and dissociation of autophagy, apoptosis and necrosis by systematic chemical study. Oncogene 30(45):4544–4556
Li J, Hou N, Faried A, Tsutsumi S, Kuwano H (2010) Inhibition of autophagy augments 5-fluorouracil chemotherapy in human colon cancer in vitro and in vivo model. Eur J Cancer 46(10):1900–1909
Levy JMM, Towers CG, Thorburn A (2017) Targeting autophagy in cancer. Nat Rev Cancer 17:528–542
Verbaanderd C, Maes H, Schaaf MB, Sukhatme VP, Pantziarka P, Sukhatme V et al (2017) Repurposing drugs in oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. ecancermedicalscience 23(11):781
Bampton ET, Goemans CG, Niranjan D, Mizushima N, Tolkovsky AM (2005) The dynamics of autophagy visualized in live cells: from autophagosome formation to fusion with endo/lysosomes. Autophagy 1(1): 23–36
Chen Y, Azad MB, Gibson SB (2010) Methods for detecting autophagy and determining autophagy-induced cell death. Can J Physiol Pharmacol 88(3):285–295
Lupitha SS, Chandrasekhar L, Varadarajan SN, Jayaprasad AG, Chandrasekharan A et al (2020) A reporter cell line for real-time imaging of autophagy and apoptosis. Toxicol Lett 326:23–30
Mizushima N, Murphy LO (2020) Autophagy assays for biological discovery and therapeutic development. Trends Biochem Sci 45(12):1080–1093
Munoz-Pinedo C, Guio-Carrion A, Goldstein JC, Fitzgerald P, Newmeyer DD, Green DR (2006) Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration. Proc Natl Acad Sci 103(31):11573–11578
Greussing R, Unterluggauer H, KozielR MAB, Dürr PJ (2012) Monitoring of ubiquitin-proteasome activity in living cells using a degron (dgn)-destabilized green fluorescent protein (GFP)-based reporter protein. J VisExp 10(69):3327
Carnero A (2002) Targetting the cell cycle for cancer therapy. Br J Cancer 87(2):129–133
Kaina B (2003) DNA damage- triggered apoptosis: critical role of DNA repair, double-stranded breaks, cell proliferation and signaling. Biochem Pharmacol 66(8):1547–1554
Wolgemuth DJ, Lele KM, Jobanputra V, Salazar G (2004) The A-type cyclins and the meiotic cell in mammalian male germ cells. Int J of Androl 27(4):192–199
Stark GR, Taylor WR (2004) Analyzing the G2/M checkpoint. Methods Mol Biol 280:51–82
Guillot C, Falette N, Paperin MP, Courtois S, Perret AG, Treilleux I et al (1997) p21WAF1/CIP response to genotoxic agents in wild type TP53 expression breast primary tumors. Oncogene 14:45–52
Acknowledgements
This research was supported by WB-DBT (Department of Biotechnology, Government of West Bengal) under the sanctioned project: Sanc. No. 541(Sanc)-BT/ST/P/S&T/2G-45/2017.
Plant authentication
Samples of the plant Excoecaria agallocha were collected, transported to St. Xavier’s College (Autonomous), Kolkata, where it was authenticated by Dr. Saurav Sett. Voucher specimen for the leaves of E. agallocha (MCB2018/541/005) was deposited for future reference. The leaves were collected with due permission and approval of the local administration and from the authorities of Department of Biotechnology, Government of West Bengal [Sanc. No. 541/BT/ST/P/S&T/2G-45].
Funding
This research was supported by WB-DBT (Department of Biotechnology, Government of West Bengal) under the sanctioned project: Sanc. No. 541(Sanc)-BT/ST/P/S&T/2G-45/2017. The authors sincerely thank Rev. Dr. Dominic Savio, S.J., Principal, St. Xavier’s College (Autonomous), Kolkata, for providing the laboratory facilities required for conducting the research. The authors acknowledge the support and cooperation from the Department of Microbiology, St. Xavier’s College (Autonomous), Kolkata, and Peerless Hospital & B.K. Roy Research Centre, Kolkata, for providing infrastructure and lab facilities to execute the antimicrobial assays. We wish to thank Dr. Saurav Sett, Department of Marine Science, University of Calcutta, for helping us in the procurement and identification of the plant samples. Our sincere thanks to the team at Sophisticated Analytical Instrument Facility (SAIF), IIT-Bombay, for providing the HR LC–MS facility. We convey our sincere gratitude to Dr. T.R. Santhosh Kumar and his team at Rajiv Gandhi Centre for Biotechnology, Kerala, for providing the cellular imaging analysis facility. We also acknowledge the support gained from CCRH, Kolkata, for conducting the MTT assay and protein expression studies.
Author information
Authors and Affiliations
Contributions
Conceptualization: SD, AKM. Methodology: SD, AKM. Validation: TS. Formal analysis: TS. Investigation: TS. Resources: SD, AKM. Data curation: SD, AKM. Writing – Original Draft: TS. Writing—Review & Editing: TS, SD, AKM. Visualization: TS. Supervision: SD, AKM. Project administration: SD, AKM. Funding acquisition: SD, AKM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The guidelines followed for blood isolation (PBMC used in MTT assay) from healthy volunteer donors have been approved by Department of Microbiology and the Institutional Ethical Committee, St. Xavier’s College, Kolkata. Consent to participate is not applicable in our investigation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sultana, T., Mitra, A.K. & Das, S. Evaluation of anti-cancer potential of Excoecaria agallocha (L.) leaf extract on human cervical cancer (SiHa) cell line and assessing the underlying mechanism of action. Futur J Pharm Sci 8, 3 (2022). https://doi.org/10.1186/s43094-021-00389-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00389-y