Abstract
Background
A specific, accurate, precise, robust, and cost-effective HPLC method was developed and validated for quantitative analysis of Fimasartan Potassium Trihydrate and Cilnidipine in fixed-dose combination. The isocratic elution was accomplished by Symmetry C18 column (150 mm × 4.6 mm, 5 µm) at 25 °C. Mobile phase composition is Methanol: Acetonitrile: Potassium Dihydrogen Phosphate buffer (pH 3) (60:05:35%v/v/v) at a flow rate of 1.0 mL/min, injection volume 20 µL with DAD detection at 240 nm.
Result
Fimasartan Potassium Trihydrate and Cilnidipine were eluted with retention time 2.65 min and 5.51 min respectively. This method was validated as per ICH guideline (Q2 R1). The calibration plots were over the concentration range of 15–90 μg/mL and 2.5–15 μg/mL for Fimasartan Potassium Trihydrate and Cilnidipine with correlation coefficient 0.9992 and 0.9989 respectively. Accuracy was obtained between 99.51–101.65% and 100.06–101.20% for Fimasartan Potassium Trihydrate and Cilnidipine respectively. LOD were found to be 0.97 μg/mL and 0.57 μg/mL and LOQ were found to be 2.95 μg/mL and 1.75 μg/mL for Fimasartan Potassium Trihydrate and Cilnidipine respectively.
Conclusion
The results showed that the developed method is reliable for the routine analysis for simultaneous determination of Fimasartan Potassium Trihydrate and Cilnidipine.
Similar content being viewed by others
Background
Hypertension is defined as a sustained increase in blood pressure ≥ 140/90 mm Hg, a criterion where the risk of hypertension-related cardiovascular disease is high enough to merit medical attention [1]. It is the most common cardiovascular disease. In India, 29.8% population is suffering from hypertension. A systolic pressure above 140 or a diastolic pressure above 90. Blood pressure is the lateral pressure exerted by blood on the wall of the blood vessel while flowing through it. It’s produced due to cardiac output and peripheral resistance [1].
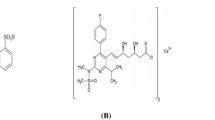
Fimasartan Potassium Trihydrate is chemically, potassium 5-[4ʹ-({2-butyl-5[(dimethylcarbamothioyl)methyl]-4-methyl-6-oxo1,6-dihydropyrimidin-1-yl}methyl)-[1,1ʹ-biphenyl]2-yl]-1H-1,2,3,4-tetrazole-1-ide Trihydrate, is a novel angiotensin II receptor blocker exhibiting potent and selective AT1 receptor blocking activity. The reported solubility found in methanol and Acetonitrile [2]. It is used for the treatment of mild to moderate essential hypertension. Fimasartan was rapidly absorbed following oral administration. Tmax ranged 0.5–3 h and t1/2 was 7–10 h [2]. The structure of the Fimasartan Potassium Trihydrate is given in Fig. 1.
Chemical Structure of Fimasartan Potassium Trihydrate [2]
Cilnidipine, a newer L/N-type Calcium channel blocker, is also an effective antihypertensive. It resolves the amlodipine induced pedel edema while maintaining adequate control of blood pressure [3]. It is chemically, 3-(2-methoxyethyl) 5-(2E)-3-phenylprop-2-en-1-yl 2, 6-dimethyl-4-(3-nitrophenyl)-1, 4-dihydropyridine-3, 5-dicarboxylate. The reported solubility found in Acetonitrile and methanol [4]. Cilnidipine is used along with proper diet and regular exercise to control high blood pressure. Cilnidipine presents a very rapid absorption with a maximum peaked concentration after 2 h. Its distribution tends to be higher in the liver as well as in kidneys, plasma and other tissues [5]. The structure of the Cilnidipine is given in Fig. 2.
Chemical Structure of Cilnidipine [5]
A review of the literature reveals that various analytical methods are available for the estimation of individual drugs. It includes UV spectrophotometry [6,7,8], HPLC [9, 10], stability-indicating HPLC [11, 12], HPTLC [13], and ESI-LC–MS/MS [14,15,16,17,18,19,20]. Based on the literature survey it was found that there is no simple and RP-HPLC method available for simultaneous estimation of Fimasartan Potassium Trihydrate and Cilnidipine in fixed-dose combination.
Methods
Materials and instrumentation
Fimasartan Potassium Trihydrate was obtained from Ajanta Pharma Limited, Aurangabad. Cilnidipine was also provided by a reputed company. HPLC grades Methanol, Acetonitrile, and Milli Q water were used. All other chemicals were analytical reagent grade. Chromatographic analysis was carried out using an Agilent Technologies HPLC, DAD detector, and EZ Chrome software for data acquisition. A 0.45 μm Nylon filter was used for filtration.
Chromatographic condition
The Methanol, Acetonitrile, and Phosphate buffer pH 3.0 in the ratio of 60:05:35%v/v/v was used as a mobile phase. The flow rate was maintained at 1.0 mL/min. The injection volume is kept 20 μL and the detection was carried out at 240 nm.
Preparation of standard stock solution
A standard stock solution of 100 μg/mL for Fimasartan Potassium Trihydrate and Cilnidipine was prepared by using methanol as diluent.
Preparation of working solution of the mixture of Fimasartan Potassium Trihydrate and Cilnidipine
A working solution of 30 μg/mL for Fimasartan Potassium Trihydrate and 5 μg/mL for Cilnidipine were prepared by appropriate dilution.
Calibration standards
Six standard concentrations with 15, 30, 45, 60, 75 and 90 μg/mL were prepared from the stock solution of Fimasartan Potassium Trihydrate drug powder (pure form). Similarly, six standard strengths with 2.5, 5, 7.5, 10, 12.5 and 15 μg/mL were prepared from the stock solution of Cilnidipine drug powder (pure form).
Preparation of mobile phase
Prepare a mixture of Methanol, Acetonitrile and Potassium Dihydrogen Phosphate buffer in the volume ratio 60:05:35%v/v/v for method optimization. Mix well and sonicate to degas the mixture. Adjust pH 3.2 with Orthophosphoric acid.
Method validation
Analytical validation parameters for the analysis of the proposed method were determined according to ICH (Q2 R1) guideline. [21] The parameters such as linearity, range, accuracy, precision, specificity, robustness, LOD & LOQ were performed [21, 22].
System suitability
The typical values for evaluating system suitability of a chromatographic procedure include the RSD < 1%, Asymmetry factor < 2, and Theoretical plates > 2000. The determination of system suitability of the analytical method was accomplished by assaying three samples of the same strength as Fimasartan Potassium Trihydrate and Cilnidipine. The sample concentration of Fimasartan and Cilnidipine used in this analysis was 30 μg/mL and 5 μg/mL respectively. The retention time, peak area, theoretical plates, and asymmetry factor were evaluated for system suitability.
Linearity
The linearity response was determined by analysing 06 independent level of calibration curve in the range of 15–90 µg/mL (15, 30, 45, 60, 75 & 90 µg/mL) for FIK and 2.5–15 µg/mL (2.5, 5.0, 7.5, 10.0, 12.5 & 15.0 µg/mL) for CLN.
The plot of mean peak area against concentration was plotted. Correlation coefficient and regression line equations for FIK and CLN were calculated.
Accuracy and precision
The accuracy of the method was performed in triplicate at three different concentration levels of 50, 100 and 150% of the targeted concentration of drugs. The accuracy of the method was evaluated by calculating percentage recovery. Repeatability was performed under 6 replicates at the assay concentration of FIK and CLN. Intra-day and inter-day variations of FIK and CLN were performed in triplicate at three different concentration levels of 50, 100, and 150%. Results are expressed in the form of RSD.
Specificity
Specificity was performed by injecting diluent, placebo and sample solution to check the interference of excipients.
LOD and LOQ
The limit of detection (LOD) and limit of quantification (LOQ) were calculated by formula as given in ICH (Q2R1).
Robustness
The robustness of the method was established by introducing small deliberate change in experimental conditions like flow rate, pH of Mobile phase and Mobile phase composition. The changes made in flow rate ± 0.1 mL/min, for pH of Mobile phase ± 0.2 and mobile phase composition ± 2%.
Assay
The combination of Fimasartan Potassium Trihydrate (60 mg) and Cilnidipine (10 mg) (Film Coated Tablet) is approved by CDSCO for clinical Trial Phase-III trial. So, there is no any single marketed pharmaceutical formulation is available.
The sample (synthetic mixture) was analyzed for assay containing 60 µg/mL of FIK and 10 µg/mL of CLN by optimized HPLC method. (conc. or dose ratio is suggested by CDSCO) [23].
Results
Optimized condition for method development
Various mobile phase compositions, different columns, pH, flow rate were investigated and the best result was achieved on Symmetry C18 (150 mm × 4.6 mm, 5 μm) column with the mobile phase composition was Methanol: Potassium Dihydrogen Phosphate buffer: Acetonitrile (60:35:05% v/v/v) pH 3 adjusted with OPA. The detection was carried out at 240 nm with 1.0 mL/min flow rate. The retention time of FIK and CLN is 2.65 and 5.51 min respectively with a total run time of 10 min. The optimized chromatogram and optimized conditions are mentioned in Fig. 3 and Table 1.
System suitability parameter
The optimized method is acceptable as system suitability parameters are valid as it passed the criteria of acceptability, as shown in Table 2.
Method validation
Linearity
The calibration curve obtained for FIK and CLN in the range of 15–90 μg/mL and 2.5–15 μg/mL and the correlation coefficient was found to be 0.9992 and 0.9989 respectively. Linearity graph of both the drugs is revealed in Additional file 1: Figs. S1 and S2 and Additional file 2: Table S1.
Accuracy
This method is accurate as % Recovery was found in the range of 99.51–101.65% for FIK, while for CLN it was found to be in the range of 100.06–101.20% are shown in Tables 3 and 4.
Precision
Repeatability and intermediate precision express in term of RSD. As RSD was found to be < 2 indicating the presented method is precise. The results are summarized in Additional file 2: Tables S2 and S3 respectively for repeatability study and intermediate precision.
Specificity
Excipients interference is not observed at the working wavelength of 240 nm, as % Interference was found less than 0.6% for both drugs. It is proven by comparing chromatogram of blank, Placebo and sample preparation solution; there was no interference of excipients with the peak of FIK and CLN. Thus, the method is Specific. The results are shown in Additional file 1: Figs. S3, S4 & S5 and Additional file 2: Tables S4 & S5.
LOD and LOQ
LOD and LOQ were found to be 0.97 μg/mL and 2.95 μg/mL for FIK and 0.57 μg/mL and 1.75 μg/mL for CLN respectively.
Robustness
Making a deliberate change in flow rate, pH of mobile phase and mobile phase composition were taken place and RSD was found to be less than 2, specify that the method is robust. Results, presented in Tables 5, 6, and 7 indicate that the selected factors remained unaffected by a small variation of these parameters.
Assay of synthetic mixture
A synthetic mixture of FIK & CLN containing 60 mg of FIK and 10 mg of CLN when analysed using the developed method, showed 100.31% assay for FIK and 100.01% assay for CLN.
The combination of Fimasartan Potassium Trihydrate and Cilnidipine (Film Coated Tablet) is approved by CDSCO for clinical Phase-III trial. So, there is no any single marketed pharmaceutical formulation is available. The results are tabulated in Table 8.
Discussion
The present method was developed using Methanol: Acetonitrile: Potassium Dihydrogen Phosphate buffer at pH 3.0 adjusted with diluted OPA in the ratio of 60:05:35% v/v/v. The method was developed with the minimum or reduced amount of organic solvents as the mobile phase which results in a more sensitive and cost-effective method. In the present method, Fimasartan Potassium Trihydrate and Cilnidipine were eluted with retention time 2.65 min and 5.51 min respectively. On the basis of literature survey it was found that there is no method available for simultaneous estimation of Fimasartan Potassium Trihydrate and Cilnidipine in combination.
Conclusion
A simple, rapid HPLC method was developed for simultaneous determination of Fimasartan Potassium Trihydrate and Cilnidipine in the synthetic mixture, which provides sufficient resolution between both drugs. The developed method was validated by testing its linearity, specificity, accuracy, precision, robustness and results were found within the acceptance limit as per ICH (Q2 R1) guideline. Statistical analysis proves that the method is suitable for the simultaneous determination of both drugs in pharmaceutical analysis and routine quality control without any interference.
Abbreviations
- HPLC:
-
High-Performance Liquid Chromatography
- FIK:
-
Fimasartan Potassium Trihydrate
- CLN:
-
Cilnidipine
- ACN:
-
Acetonitrile
- OPA:
-
Ortho-phosphoric acid
- Conc:
-
Concentration
- RSD:
-
Relative Standard Deviation
- LOD:
-
Limit of Detection
- LOQ:
-
Limit of Quantification
- DAD:
-
Diode Array Detector
References
Tortora GJ, Grabowski SR (2003) Principles of anatomy and physiology, 10th edn. Wiley, New Jersey, pp 758–759
Kim JH, Lee JH, Paik SH, Kim JH, Chi YH (2012) Fimasartan, a novel angiotensin II receptor antagonist. J Arch Pharm Res 35(7):1123–1126
Prasad RS (2015) Replacement of Amlodipine with Cilnidipine and assessment of pedal edema along with blood pressure control. Sch J Appl Med Sci 3(4A):1680–1682
Merk NJ (2006) In the Merk Index, 14th edn. Royal Society of Chemistry, London, p 2326
Drug Profile and Information of Cilnidipine, https://pubchem.ncbi.nlm.nih.gov/compound/CILNIDIPINE. Accessed March 2020
Mohammad SS, Avutu SL, Marreddy JL (2019) Development and validated UV method for fimasartan in pure and dosage form. Int J Adv Res Innov Ideas Educ 5(4):1410–1417
Agrawal KS, Gnadhi LR (2019) UV spectrophotometric method of fimasartan drug and its tablet formularion. Asian Journal of Pharmaceutical Research and Development 7(5):26–30. https://doi.org/10.22270/ajprd.v7i5.576
Ravisankar P (2019) Novel UV and RP-HPLC method for cilnidipine in dosage form. Int J Pharma Sci Res 10(4):1886–1894. https://doi.org/10.13040/IJPSR.0975-8232.10(4).1886-94
Indian Pharmacopoeia (2018) Government of India, Ministry of Health & Family Welfare, 8th edn. Indian Pharmacopoeia Commission, Ghaziabad, pp 4616–4618
Japanese Pharmacopoeia (2017) The Ministry of Health, Labour and Welfare, pp 704–707
Pandya CP, Rajput SJ (2017) Validated stability indicating RP-HPLC method for the determination of Fimasartan in presence of degradation products. Indo Am J Pharm Res 7(11):929–946
Balakrishna T, Kulkarni A (2020) Development and validation of stability indicating RP-HPLC for estimation of Cilnidipine. J Drug Deliv Ther 10(1):97–100
Badade S, Dole M, Wagh V (2019) Development and validation of HPTLC method for fimasartan in bulk and dosage form. Int J Pharmacy Anal Res 8(2):179–184
Yoon SH, Oh S, Kim HS (2015) Validated LC–MS/MS Assay for the quantitative determination of Fimasartan in human plasma: application to pharmacokinetic studies. J Chromatog Sci 53(8):1250–1256
Kim TH, Shin S, Landersdorfer CB (2015) Population pharmacokinetic modeling of the enterohepatic recirculation of fimasartan in rats, dogs, and humans. AAPS J 17(5):1210–1223
Hyun JY, Kim HJ, Gwon MR (2015) Fully validated ultra-performance liquid chromatography-tandem mass spectrometry method for the determination of Fimasartan in human plasma. J Anal Sci 31(12):1335–1339
Kim TH, Kim MG, Shin S, Chi YH (2016) Placental transfer and mammary excretion of a novel angiotensin receptor blocker Fimasartan in rats. BMC Pharmacol Toxicol 17(1):35
Zhang X, Zhai S, Zhao R (2006) Determination of Cilnidipine, in human plasma using LC-MS/MS. Anal Chim Acta. https://doi.org/10.1016/j.aca.2006.11.072
Sawaikar L, Kapupara P (2020) Isolation and force degradation products of Ca channel blocker by LC-MS technique. Rasayan J Chem 13(3):1330–1334
Lee HW, Seo JH (2008) Development of a LC-ESI/MS method for determination of Cilnidipine in human plasma. J Chromatogr B. https://doi.org/10.1016/j.jchromb.2007.11.016
ICH – Harmonized Tripartite Guideline (2005) Validation of analytical procedures: text and methodology Q2 (R1). In: International conference on harmonization, IFPMA, Geneva, Switzerland
Snyder LR, Kirkland JJ, Glajch JL (1997) Practical HPLC method development, 2nd edn. Wiley, New Jersey
CDSCO - Drug combination approval (2020) http://www.cdsco.nic.in. Accessed Nov 2021
Acknowledgements
The authors are heartly thankful to B. K. Modi Government Pharmacy College, Rajkot, Gujarat, India for providing necessary facilities to carry out the research work. Special thanks to Ajanta Pharma LTD, Aurangabad for providing the drug samples.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The method was performed and validated by RS. Concept and guidance were from UC, and the final manuscript was prepared and checked by RS and UC respectively. We declare that all authors have read and approved the manuscript before submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The data for verification is provided with a Supplementary file and the rest of the data, if required, will be available upon request.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Figure S1. The calibration standard curve has been performed for Linearity determination of FIK (Pure Form) in the concentration range 15-90 μg/mL. Figure S2. The calibration standard curve has been performed for Linearity determination of CLN (Pure Form) in the concentration range 2.5-15 μg/mL. Figure S3. In the Specificity determination, the chromatogram of Blank is performed for the comparison with sample solution. Figure S4. The chromatogram of placebo is performed for the comparison with sample solution for determination of Excipients interference. Figure S5. Excipients interference is not observed at the working wavelength of 240 nm, as % Interference was found less than 0.6% for both drugs. It is proven by comparing chromatograms of blank, Placebo and sample preparation solution; there was no interference of excipients with the peak of FIK and CLN.
Additional file 2:
Table S1. The calibration curve obtained for FIK in the range of 15-90 μg/mL and the correlation coefficient was found to be 0.9992. Table S2. The calibration curve obtained for CLN in the range of 2.5- 160 15 μg/mL and the correlation coefficient was found to be 0.9989. Table S3. Repeatability study and Intermediate precision has been performed according to guidelines and RSD was found to be <2 indicating the presented method is precise. Table S4. Specificity for FIK has been performed with and without spiking of Placebo; there was no interference of excipients with the peak of FIK. Table S5. Specificity for CLN has been performed with and without spiking of Placebo; there was no interference of excipients with the peak of CLN. Thus, the method is Specific.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sojitra, R.G., Chotaliya, U.J. Analytical method development and validation for simultaneous estimation of Fimasartan Potassium Trihydrate and Cilnidipine in synthetic mixture by HPLC for the treatment of hypertension stage-II. Futur J Pharm Sci 7, 189 (2021). https://doi.org/10.1186/s43094-021-00336-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00336-x