Abstract
Background
The conventional oral dosage forms are not effective in dealing with chronopathological conditions, such as nocturnal asthma. Therefore, there is an unmet need to develop a delivery system that can deliver drug as per the chronopharmacology of the diseases. The purpose of the study is to use quality by design (QbD) technique and pulsatile principles for the development of Eudragit-coated dual release bilayer tablets. The dual layer consists of immediate release layer of fexofenadine HCl and sustained release layer of montelukast sodium.
Results
The quality target product profile of the formulation was developed, and the critical quality attributes were identified. Three-level, three-factor Box-Behnken design was used for the optimization of the bilayer tablets. Based on the design, a total of 13 formulation combinations (F1–F13 and M1–M13) were made having acceptable micromeritic properties. The developed immediate and sustained release layers were evaluated for physicochemical properties. Depending upon the value of the diffusion exponent, the Fickian diffusion mechanism is dominant among immediate and sustained release tablet layers. Response curve for immediate release layer showed that concentrations of sodium starch glycolate and sodium bicarbonate had a negative effect on disintegration time and a positive effect on drug release. For sustained release tablet layer, concentrations of HPMC E 5 LV and magnesium stearate had a significant effect on drug release. The ANOVA and diagnostic plots confirmed the significance and goodness of fit of the used model. Based on desirability plot values, optimized formulation was developed and coated with Eudragit coat. The coated bilayer tablet showed met the requirement of providing an immediate release during the first hour and a sustained release action for a period of more than 8 h after passing the gastric region.
Conclusions
The formulation can be fruitful in curbing the menace of nocturnal asthma and providing a high degree of patient compliance as the patient will not have to wake up at night to take the medication.
Similar content being viewed by others
Background
Nocturnal asthma can be defined as a drop in the forced expiratory volume in 1 s (FEV1) of at least 15% in between the bedtime and awakening in patients who suffered from asthma [1]. There is an increase in bronchial hyper-reactivity at night along with a decrease in lung function. This happens due to the circadian changes in the body’s cortisol levels during the night [2]. An examination was directed at 7729 patients with asthma keeping in mind the end goal to investigate the pervasiveness of nocturnal asthma symptoms. Results showed that 64% of the patients indicated nocturnal asthma symptoms no less than 3 times per week. About 26% of 3015 patients associated with mild asthmatic condition awakened at the night [3]. In another study, 3129 patients with nocturnal asthma were investigated and about 94% of them showed dyspneic episodes between 10 pm and 7 am [4]. Studies have shown that nocturnal asthma is associated with mortality and the majority of the sudden death occurs between midnight and 5 am [5, 6]. The conventional dosage forms were found less effective in the management of nocturnal asthmatic attacks. Therefore, there is a need for a modified system in order to overcome the present limitations.
The pulsatile systems have shown positive outcomes in the management of ailment with chronopathalogy, such as nocturnal asthma [7]. The chronotherapeutic pulsatile systems release the drug in a pulsatile manner at a predetermine off-release period (lag time) in a specific site in order to emulate the chronopathological symptoms [8]. The bi- or multilayer drug delivery frameworks are gaining more acceptance due to various benefits, such as two different or same therapeutic entity can be delivered with different release profile, and therapeutic efficacy can be increased due to the synergistic effect of two drugs and reduces the dosing unit burden thus improving patient compliance [9,10,11]. To date, different technologies have been used in the development of the modified tablets, such as OROS®, L-OROSTM, EN SO TROL, DUROS, and DUREDAS [9]. In the present investigation, Dual Release Drug Absorption System (DUREDAS) technology was used for the development of fexofenadine HCl- and montelukast sodium-loaded bilayer tablet.
In the development of any pharmaceutical product, the quality of the dosage form is of prime importance for regulatory bodies. Considering the specific end goal to maintain the quality within the product, the quality by design (QbD) approach has become mandatory by all regulatory bodies. The principle of QbD is described in the Q8 (R2) guideline of the International Conference on Harmonisation (ICH) as “a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management” [12]. Three ICH guidelines, ICH Q8 (pharmaceutical development), ICH Q9 (pharmaceutical risk management), and ICH Q10 (pharmaceutical quality systems), cover various aspects of QbD [13, 14]. The important elements of QbD include defining the objective, i.e., Quality Target Product Profile (QTPP), determination of critical quality attributes (CQAs), risk assessment, development of experimental design, implementation of the control strategy, and continuous improvement.
The present investigation is focused on the application of QbD for the development of chronomodulated dual release bilayer tablets. Bilayer tablets of fexofenadine HCl and montelukast sodium were developed. Montelukast sodium is a leukotriene receptor antagonist used for the treatment of asthma and seasonal allergies. Fexofenadine HCl is a second generation H-1 receptor antagonist or antihistamine used for the management of allergic rhinitis. In addition, fexofenadine HCl also has an anti-inflammatory effect due to mediators [15]. A combination therapy of antihistamines and antileukotrienes will show a synergistic effect for the better management of nocturnal asthma. The pulsatile principle was used for the time release of the drugs from each layer of the bilayer tablets. A three-factor, three-level Box-Behnken design was used for the design of experiment (DoE). The powder blends were assessed for their micromeritic properties and used for the development of bilayer tablets. The prepared two different core tablet layers (immediate and sustained layers) were evaluated for various parameters including drug release kinetics. The impact of formulation variables on disintegration time and drug release of two separate tablet layers was evaluated. The optimized layers were used to prepare bilayer tablet by direct compression. Finally, the bilayer tablet was coated with Eudragit S100 and evaluated for various parameters including in vitro drug release.
Methods
Materials
Fexofenadine HCl and montelukast sodium were purchased from Aspen Life Sciences, Amritsar, India. The polyvinylpyrrolidone K30 (PVP K30), microcrystalline cellulose, lactose monohydrate, magnesium stearate, and sodium starch glycolate were purchased from BB Chemicals, Aurangabad, India. Isopropyl alcohol, indigo carmine blue, starch, hydroxypropyl methylcellulose (HPMC E 5 LV), and phosphate buffer saline (PBS) were purchased from Loba Chemie Pvt. Ltd., Mumbai, India. All other chemicals are of analytical grade.
Drug-polymer compatibility studies
The drug-drug and drug-polymer compatibilities were investigated using FTIR spectroscopy. The FTIR spectra were recorded for the drugs alone and in combination with polymers using the potassium bromide (KBr) dispersion method. The drug and combination samples were added to KBr (1:1) and examined at the range of 400 to 4000 cm−1 with the resolution of 4 cm−1.
Initial risk assessment
The Quality Target Product Profile (QTPP) is an integral part of the quality by design (QbD) approach and has been described in ICH Q8 and Q9. In like manner, it is imperative to recognize the objective at a beginning period of the product development [13, 14]. The QTPP incorporates item product attributes to guarantee that the item meets the base wanted quality particulars. The QTPP and CQAs for the bilayer tablet were determined in light of the past investigations and literature reviews.
Experimental design
In the present work, 13-run, 3 factor, 3-level Box-Behnken design (BBD) was used for creating second order polynomial models and analyzing quadratic response surfaces for optimizing the immediate and sustained release layers of core tablets using Design-Expert software (Trial version 11.0.5.0, Stat-Ease Inc., MN). The design was used for assessing the main, interaction, and quadratic effects of independent variables on dependent variables using the following quadratic model:
where Y is the dependent variable, β0–3 are the regression coefficients of independent variables and their mutual interactions, and A, B, and C are the independent variables [16]. The factors evaluated in the present work are concentrations of fexofenadine HCl (mg), sodium starch glycolate (mg), and sodium bicarbonate (mg) for the immediate release layer, and concentrations of montelukast sodium (mg), HPMC E 5 LV (mg), and magnesium stearate (mg) for the sustained release layer at low, medium, and high values. The dependent variables/responses were drug release (%) and disintegration time (seconds) for the immediate release layer and drug release (%) for the sustained release layer. The variables and their levels used in the development and optimization of immediate and sustained release tablet layers are illustrated in Table 1.
Micromeritic studies
In view of the design of experiment (DoE), diverse powder blends were prepared and set up for the development of immediate release (F1–F13) and sustained release (M1–M13) tablet layers. The powder blends were dried and mixed properly before evaluating their micromeritic properties. The composition of the powder blends used for the development of immediate and sustained release tablet layers is illustrated in Table 2.
Angle of repose (θ)
For the determination of the angle of repose, precisely measured powders (6 g) from each formulation (F1–F13 and M1–M13) were permitted to stream openly through a funnel fixed to a stand at a specific height (h). The height of the funnel was set approximately just above the height of the heap. The radius (r) and height (h) of the powder heap were determined to calculate the angle of repose. The following formula was used to measure the angle of repose:
where θ is the angle of repose, h is the height of heap, and r is the radius of the heap.
According to specifications of the monograph, the value of θ indicates the flowability of the powder blend: θ < 25°, excellent; 25–30°, good; 30–40°, fair; 40–45°, poor; and θ > 45°, very poor [17].
Bulk density and tapped density
Dry powder blends (6 g) from each formulation were gently shaken to break conceivable agglomerates present and then poured in a measuring cylinder (50 ml). The initial volume was noted, and the cylinder was held at a height of 2.5 cm and allowed to fall at an interval of 2 s. The tapping of the cylinder was continued until a constant volume was noticed. The process was repeated thrice (n = 3) for each formulation, and the average was taken. The following formula was used for the determination of bulk density (BD) and tapped density (TD):
Compressibility index (Carr’s index)
Compressibility index (Carr’s index) of the dry powder blends for immediate and sustained release tablet layers was calculated using the following formula:
Carr’s index also indicates the flowability of the powder blend: 5–15%, excellent; 12–16%, good; 18–21%, fair; 23–35%, poor; 33–38%, very poor; and > 40%, extremely poor [18].
Hausner’s ratio
Hausner’s ratio of dry powder blends for immediate and sustained release tablet layers was determined by the following formula:
The values lesser than 1.25 indicate good flow, whereas values greater than 1.25 indicate a poor flow. Values between 1.25 and 1.5 are considered as average flow, and generally, a glidant has to be added for improving the flow properties [19].
Immediate and sustained release tablet layers
Development of tablet layers
The powder blends with different combinations were used for the development of immediate and sustained release tablet layers. The ingredients were first passed through sieve number 44 to get a uniform particle size. All the ingredients except magnesium stearate were mixed in a mortar. Magnesium stearate was added at the last step and mixed well. The powder blends were then taken to the tablet punching machine. A three-punch hand-operated Trover Pharma Mach tablet punching machine was used for direct compression of the powder blends to get final tablet layer formulations.
Evaluation of tablet layers
A total of ten tablet layers (n = 10) from the batch of two different tablet layers (immediate and sustained) were randomly selected for the evaluation of various properties, such as hardness, percentage of friability, and weight variation. In addition, the tablet layers were also evaluated for the disintegration test for the immediate release layer, in vitro drug dissolution, and release kinetics. Disintegration test was performed using an altered disintegration method with six tablets (n = 6). The USP disintegration test apparatus was maintained at a temperature of 37 ± 0.5 °C in phosphate buffer saline (PBS, pH 6.8). The tablets were positioned in the baskets of the apparatus, and the time taken for complete tablet disintegration was noted [20]. The in vitro drug dissolution of the prepared tablet layer formulations was studied using eight-station USP type-II dissolution test apparatus maintained at a temperature of 37 ± 0.5 °C. The study was carried out in 900 ml of PBS (pH 6.8). The study was performed for 60 min for the immediate release tablet layer of fexofenadine HCl, while it was performed for 8 h for sustained release tablet layer of montelukast sodium [21]. Aliquots were withdrawn after a predetermined time interval, and an equal volume of fresh buffer was added into the basket after each withdrawal. The absorbance of the aliquots was taken at 259 nm for fexofenadine HCl and at 285 nm for montelukast sodium simultaneously using a validated UV spectrophotometer. For drug release kinetics studies, different models, zero order, first order, Higuchi, and Korsmeyer-Peppas models, were used.
Statistical optimization
The impact of factors on the tablet properties was assessed through response surface plot (3D) and contour plot (2D) and generated from Design-Expert software. Statistical model validation involved assessing statistical parameters of F value, correlation coefficient (R2), adjusted R-squared (R2Adj), predicted R-squared (R2Pred), predicted residual error sum of squares (PRESS), and adequate precision (AP) generated by ANOVA provision to ascertain model sufficiency and adequacy. An F value with p < 0.05 implied the significance of the model. A difference of less than 0.2 between R2Adj and R2Pred would prove that both values were in reasonable agreement with each other. Statistical validation of polynomial equations was done by assessing ANOVA specifications. Optimal values of the variables were then estimated using the numerical and graphical optimization tool by providing a set criterion of desirability to the software. A measure of fit was provided by PRESS statistics with a PRESS statistic of smaller value being preferred. Design-Expert software developed diagnostic plots like externally studentized residuals vs. predicted plot, predicted vs. actual plot, normal probability plot, and externally studentized residuals vs. run number plot which were analyzed to check if the points fell on the diagonal in normal probability plot and if they lay within the described limits or not.
Optimized bilayer tablet
Based on the optimized values of variables obtained, bilayer tablets of fexofenadine HCl and montelukast sodium were prepared by direct compression of tablet layers. Powder blends of both layers were prepared separately. The immediate release fexofenadine HCl powder blend was first poured into the die of the tablet punching machine. Tablets of the first layer were punched at a low compression force. Over this punched layer, sustained release montelukast sodium powder blend was poured. The compression force was increased, and both the layers were punched together to get bilayer tablets. The two layers could be clearly distinguished due to the addition of indigo carmine blue in the sustained release tablet layer. The developed bilayer tablet was evaluated for various tablet parameters.
Eudragit-coated bilayer tablet
The bilayer tablets were meant to release the active constituents in the intestine after a lag time of 2 h. To meet this objective, the prepared tablets were coated with a solution of Eudragit S100. Based on the literature survey and hit and trial method, a 5% w/v solution of Eudragit S100 solution was prepared using isopropyl alcohol and acetone (1:1) for coating the tablets. Dip coating technique was used for coating the bilayer tablet. The prepared tablets were first weighed and then transferred into a beaker containing 5% Eudragit S100 solution prepared in a mixture of isopropyl alcohol: acetone (1:1). Talc (5% w/w) was also added to the coating solution to prevent adhering of tablets. In addition, polyethylene glycol (PEG 400, 1.5% w/w) was added as a plasticizer [22]. The tablets were left in the beaker for 5 min and then taken out with the help of forceps. Following the dipping step, the tablets were placed in a glass plate (smeared with castor oil) for drying at a room temperature for 15 min. Finally, the tablets were dried at 60 °C in an oven for 30 min repeatedly, till the desired weight gain of the tablets was obtained. During drying step, the tablets were rotated occasionally. The dried coated tablets were again weighed to determine the weight of the coating layer.
Gradient pH dissolution method was used for the evaluation of drug release from the prepared coated bilayer tablets that were meant to release in the intestine [23]. For dissolution studies, USP dissolution apparatus II (paddle type) was used at 50 rpm and a temperature of 37 ± 0.5 °C. Eight-station LAB INDIA DS 8000 dissolution apparatus was used for the study. Six coated bilayer tablets, each containing fexofenadine HCl (60 mg) and montelukast sodium (6.35 mg), were placed in individual vessels of the dissolution apparatus and studied. For the first 2 h, the dissolution study was carried out in 900 ml of pH 1.2 HCl buffer at 50 rpm and a temperature of 37 ± 0.5 °C considering the average gastric emptying time is 2 h. Aliquots of 5 ml were withdrawn every 5 min, and an equal volume of fresh HCl buffer (pH 1.2) was added into the dissolution vessel. After 2 h, the volume of the dissolution medium was replaced with phosphate buffer (900 ml, pH 6.8). The study was continued in the medium for the next 8 h. Aliquots of 5 ml were withdrawn at predetermined time intervals, and an equal volume of fresh phosphate buffer (pH 6.8) was added into the dissolution vessel. The absorbance of each of the withdrawn aliquots of fexofenadine HCl and montelukast sodium was estimated simultaneously at two different wavelengths of 259 nm and 285 nm. The UV method was already validated for the estimation of these two drugs. However, the entire validation is not mentioned in this paper as the validation study is a part of our next manuscript.
Results
Compatibility studies
To date, studies have been shown that the possible drug-drug/drug-excipient interaction can affect the stability of the drug and its release from the developed formulation [24, 25]. The FTIR spectrum of the fexofenadine HCl showed various characteristic bands, such as −OH (alcohol) at 3298.3 cm−1, −CH stretching (alkane) at 2934.7 cm−1, −OH (carboxylic acid) at 2672.4 cm−1, CO stretching (carboxylic acid) at 1712.2 cm−1, C=C stretching (aromatic ring) at 1451.4 cm−1 and 1403.2 cm−1, CN stretching (amine) at 1279.8 cm−1, and CO stretching (carboxylic acid) at 1166.0 cm−1 (Fig. 1a). In addition, the FTIR spectrum of the montelukast sodium has also showed various characteristic bands, such as NH stretching (amine) at 3309.9 cm−1, C-H stretching (alkane) at 2979.1 cm−1, C=C stretching (alkene) 1603.1 cm−1, C=C stretching (aromatic ring) at 1491.0 cm−1, CN stretching (amine) at 1403.2 cm−1, and CO stretching (carboxylic acid) at 1137.0 cm−1 (Fig. 1b). The FTIR spectrum of the physical mixture of the drug (fexofenadine HCl and montelukast sodium) has shown all the characteristic bands of the individual drugs that indicate the compatibility between them (Fig. 1c).
The FTIR spectrum of the drug and polymer physical mixture was also recorded in order to evaluate any possible interaction among them. The FTIR spectrum of the fexofenadine HCl and sodium starch glycolate showed all the characteristic bands of the fexofenadine HCl (Fig. 2a). The FTIR spectrum of the montelukast and HPMC E 5 LV showed all the characteristic bands of the montelukast sodium (Fig. 2b). The FTIR spectrum of fexofenadine HCl and Eudragit S100 has shown the characteristic bands of the drug. The FTIR spectrum of montelukast sodium and Eudragit S100 showed characteristic bands of the drug (Fig. 2c, d). The FTIR studies has reviled that the drug and excipients were compatible with each other and suitable for the formulation development.
Initial risk assessment
This initial risk assessment was based on prior knowledge, experience, and published literature. The risk variables were indicated as high, medium, and low (Table 3). Based on currently available knowledge, medium risk variables can be considered acceptable. The Quality Target Product Profile (QTPP) and critical quality attributes (CQAs) for the bilayer tablet, such as dosage form, dosage design, route of administration, appearance, size, weight variation, hardness, friability, and dissolution, were considered. For this study, only dissolution rate and disintegration time were considered.
Experimental design
A 3-factor, 3-level BBD was used for the optimization of immediate release fexofenadine HCl and sustained release montelukast sodium tablet layers. The independent variables selected for immediate release layers were fexofenadine HCl (mg), sodium starch glycolate (mg), and sodium bicarbonate (mg). The independent variables selected for sustained release layers were montelukast sodium (mg), HPMC E 5 LV (mg), and magnesium stearate (mg). The effect of independent variables was investigated on the disintegration time and drug release of tablet layers (Table 1). A total of 13 different powder compositions (F1–F13 and M1–M13) were prepared for the development of immediate and sustained release tablet layer as per the BBD (Table 2). Before they used for the development of the immediate and sustained release tablet layers, these powder blends were first evaluated for their micromeritic properties. The properties studied were the angle of repose (θ), bulk density, tapped density, Carr’s index, and Hausner’s ratio (Fig. 3).
Evaluation of the tablet layers
The prepared powder blends were evaluated for micromeritic properties followed via development of immediate release tablet layers of fexofenadine HCl (F1–F13) and sustained release tablet layers of montelukast layers (M1–M13). The tablet layers were evaluated for various parameters, such as hardness, percentage of friability, weight variations, disintegration time of the immediate release layer, and percentage of drug release (Fig. 4). The developed immediate and sustained release tablet layers were also evaluated for diffusion release kinetics. Depending upon the value of the diffusion exponent (n), the Fickian diffusion mechanism is dominant among sustained release formulations.
Evaluation of immediate release fexofenadine HCl tablet layer. a Hardness (kg/cm2). b Friability (%). c Disintegration time (seconds). d Drug release (Q+5%). Evaluation of sustained release montelukast sodium tablet layer. e Hardness (kg/cm2). f Friability (%). g Weight variation (mg). h Drug release (Q+5%) (Mean ± SD)
Optimization of immediate release tablet layer
Effect on disintegration time
The initial model without transformation did not fit into the model as it had a high percentage error. Hence, based on the suggestion of the software, log transformation was applied for disintegration time and further analysis and validation were done based on ln (disintegration time). The factors affecting disintegration time were concentrations of fexofenadine HCl (A), sodium starch glycolate (B), and sodium bicarbonate (C) (p = 0.0094) as depicted from the given equation:
The observed disintegration time of immediate release fexofenadine HCl layers (F1–F13) varied from 21 to 421 s (minimum disintegration time of 21 s). The predicted disintegration time values obtained by the model using the above equation were compared with observed values. A low percentage error of < 5% ascertained that the model has good predictability (Table 5). Based on the values and signs of the coefficients, it can be concluded that the concentration of fexofenadine HCl (A) had a negligibly small effect on disintegration time. However, the concentrations of sodium starch glycolate and sodium bicarbonate had a negative effect. It was observed that ln (disintegration time) increased rapidly with an increase in the concentration of sodium starch glycolate (B), which can be attributed to the improved moisture absorption by sodium starch glycolate that results in massive volumetric increase leading to faster disintegration (Fig. 5a–d) [26].
Response surface and contour plots illustrating a–d effect of fexofenadine HCl (FXD), sodium starch glycolate (SSG), and sodium bicarbonate (SB) on disintegration time (DT); e–h effect on drug release (DR); and i–l effect of montelukast sodium (MTK), hydroxypropyl methylcellulose (HPMC), and magnesium stearate (MS) on drug release (DR)
Effect on drug release
The factors affecting the drug release (%) were concentrations of fexofenadine HCl (A), sodium starch glycolate (B), and sodium bicarbonate (C) as depicted from the given equation:
The observed drug release in fexofenadine HCl layer varied from 50.5579 to 88.8778%. The predicted values of drug release (%) (Y1) obtained by the model using the above equation were compared with the observed values, and low percentage error proved that the designed model has good predictability (Table 5). The good fitting data was confirmed through the value of regression coefficient (r2) which was 0.9255. Based on the values and signs of the coefficients, it can be concluded that all the three factors had a positive effect on drug release (%). However, the concentration of API (A) did not have a significant effect on drug release. The concentrations of sodium starch glycolate (B) and sodium bicarbonate (C) had a significant positive effect on drug release (Y1) (Fig. 5e–h). This can be due to the improved moisture absorption by sodium starch glycolate that results in massive volumetric increase leading to faster disintegration and hence faster dissolution rate. Sodium bicarbonate, being an effervescence agent, generates carbon dioxide leading to pressure build-up within the formulation and thus causing the fast release of components. It acted synergistically by absorbing moisture and facilitating drug release. These findings are in agreement with previous reports [27].
Optimization of the sustained release tablet layer
Effect on drug release
The factors affecting the percentage of drug release were concentrations of montelukast sodium (A), HPMC E 5 LV (B), and magnesium stearate (C) as depicted from the given equation:
The observed drug release (%) in montelukast sodium tablet layers varied from 85.6923 to 99.5331%. The predicted values of drug release obtained by the model using the above equation were compared with the observed values, and low percentage error proved that the designed model has good predictability. The good fitting data was confirmed through the value of regression coefficient (r2) which was 0.9018. The concentrations of HPMC E 5 LV (B) and magnesium stearate (C) had a significant effect on drug release. As the concentration of HPMC E 5 LV was increased, there was a decrease in drug release (Fig. 5i–l). The mobility of the polymer chain gets enhanced in the presence of a solvent. As a result, a glassy matrix gradually transforms into a rubbery swollen gel. The higher concentrations of HPMC resulted in enhanced viscosity that retards the drug’s diffusion coefficient [28]. The magnesium stearate is hydrophobic in nature and forms a film at the surface of API and excipients that causes the dissolution medium to stay on the surface, thus decreasing wettability of the formulation and thereby decreasing the dissolution rate [29] (Fig. 5i–l).
Statistical assessment of formulation characteristics
Statistical analysis for immediate release layer
Model generated for ln (disintegration time) was found to be significant as suggested by the F value of 684.94 (p < 0.0001). There was a reasonable agreement between the predicted R2Pred value of 0.9906 and adjusted R2Adj value of 0.9942 as the difference between the two was less than 0.2. Precision was found to be 61.2807, which was greater than 4, thus suggesting an adequate signal and the model could be used for navigating the design space. PRESS value of 0.1433 indicated that the model is fit. The model generated for drug release was found to be significant as suggested by the F value of 37.28 (p < 0.0001). There was a reasonable agreement between the R2Pred value of 0.8457 and R2Adj value of 0.9007 as the difference between the two was less than 0.2. Adequate precision was found to be 14.6995, which was greater than 4, thus suggesting an adequate signal and the model could be used for navigating the design space. PRESS value of 411.80 indicated that the model is fit (Table 6).
Statistical analysis for sustained release layer
The model generated for the drug release (%) was found to be significant as suggested by the F value of 27.54 (p value < 0.0001). There was a reasonable agreement between the predicted R2 value of 0.7982 and adjusted R2 value of 0.8690 as the difference between the two was less than 0.2. The precision value was found to be 13.5002, which was greater than 4, thus suggesting an adequate signal and the model could be used for navigating the design space. PRESS value of 63.77 indicated that the model is fit (Table 6).
Diagnostic analysis
Diagnostic analysis of immediate release layer
The goodness of fit of the proposed model for disintegration time was investigated by plotting diagnostic plots (Fig. 6a–d) [30]. The normal probability plot of externally studentized residuals indicated that most of the colored points depicting values of disintegration time were located around the normal probability line, which proved the normality of residuals and suggested the relevant analysis of response data. The normality assumption for residuals was satisfied since the residuals are plotted around the straight line (Fig. 6a). The externally studentized residuals vs. predicted values plot revealed that the colored points of disintegration time lay within the set limits. The pattern of the plot showed the random distribution of studentized residuals in all the responses indicating that the assumption of constant variance was true (Fig. 6b). The residuals vs. run number plot explored the variables that may have influenced disintegration time during the experiment. All the points were lying within ± 4.01 which indicated that there was no outlying observation throughout and consistency was observed in all the runs (Fig. 6c). The predicted vs. actual values plot revealed that the experimentally observed values of disintegration time were in close agreement with the predicted values (Fig. 6d).
The goodness of fit of the proposed model for the drug release was investigated by plotting diagnostic plots (Fig. 6e–h). The normal probability plot of externally studentized residuals indicated that most of the colored points depicting values of drug release (Q+5%) were located around the normal probability line, which proved the normality of residuals and suggested the relevant analysis of response data. The normality assumption for residuals was loosely satisfied in the linear model since the pattern of the residuals creates a little bit S-shaped curve (Fig. 6e). The externally studentized residuals vs. predicted values plot revealed that the colored points of Q+5% lay within the set limits. The pattern of the plot showed the random distribution of studentized residuals in all the responses indicating that the assumption of constant variance was true (Fig. 6f). The residuals vs. run number plot explored the variables that may have influenced Q+5% during the experiment. All the points were lying within ± 4.01 which indicated that there was no outlying observation throughout and consistency was observed in all the runs (Fig. 6g). The predicted vs. actual values plot revealed that the experimentally observed values of Q+5% were in close agreement with the predicted values (Fig. 6h).
Diagnostic analysis of sustained release layer
The goodness of fit of the proposed model for drug release from sustained release tablet layer was investigated by plotting diagnostic plots (Fig. 6i–l) [30]. The normal probability plot of externally studentized residuals indicated that most of the colored points depicting values of the percentage of drug release were located around the normal probability line. The normality assumption for residuals was loosely satisfied in the linear model (Fig. 6i). The externally studentized residuals vs. predicted values revealed that the colored points lay within the set limits (Fig. 6j). The predicted vs. actual values plot revealed that the experimentally observed values were in close agreement with the predicted values (Fig. 6k). Similar to the immediate release layer, that there was no outlying observation throughout and consistency was observed in all the runs (Fig. 6l).
Optimization and validation
Optimization and validation of immediate release layer
Design-Expert software explored the desirability function to obtain an optimized formulation, which was obtained using the set paradigm of maximum drug release and minimum disintegration time. Therefore, an additional batch of fexofenadine HCl immediate release layers was prepared for validation. The composition, point prediction, and confirmation of the experimental value lie within the limit. The response surface and contour plots for the immediate release fexofenadine HCl layer design suggested the values for fexofenadine HCl to be 60 mg, sodium bicarbonate to be 18.32 mg, and sodium starch glycolate to be 18.93 mg. The predicted value of the drug release according to the plot was 88.7258%, and for disintegration time, the predicted value was 29.3966 s with a desirability of 0.819301 (Fig. 7a–f). The mean values obtained for drug release and the disintegration time of the optimized immediate release fexofenadine HCl layer were found to lie within the set limits. The layer was thus confirmed to be optimized.
The optimized immediate release tablet layer represented via desirability plot (a, d), correlating response factor plots for drug release (%) (b, e) and disintegration time (seconds) (c, f). SSG, sodium starch glycolate; SB, sodium bicarbonate. The optimized sustained release layer tablet layer represented via desirability plot (g, h) and correlating response factor plots for drug release (%) (i, j). HPMC, hydroxypropyl methylcellulose; MS, magnesium stearate
Optimization and validation of sustained release layer
An additional batch of montelukast sodium-sustained tablet release layer was prepared for validation. For the set composition of the sustained release layer, the predicted drug release and desirability were found to be 98.542% and 0.6361 (Fig. 7g–j). The mean values obtained for drug release of the optimized sustained release montelukast sodium layer were found to lie within the set limits. The layer was thus confirmed to be optimized.
Evaluation of optimized bilayer tablet
The optimized immediate release tablet layer of fexofenadine HCl and sustained release tablet layers of montelukast sodium were used to develop bilayer tablet via direct compression (Fig. 8a). The bilayer tablets had a smooth surface. They were biconvex, elegant, and bicolored. The color distribution was uniform, and the two layers could be clearly distinguished based on the color difference. The immediate release fexofenadine HCl layer was blue in color, and the montelukast sodium sustained release layer was white in color (Fig. 8b). There were no chipping or mottling observed in any of the formulated tablets. The average thickness of the tablets was found to be 3.68 ± 0.0067 mm, and the average diameter was found to be 10.36 ± 0.0074 mm (Fig. 8c, d). The mean hardness of the prepared tablets was 6.77 ± 0.1033 kg/cm2, while the observed percentage of friability was 0.76, which was within the specified limit. The average weight of the tablets was 300 ± 0.032 mg, and the weight variation was within limits.
The in vitro drug release was first performed in pH 1.2 HCl buffer for 2 h and then in pH 6.8 phosphate buffer. According to the scope of the study, drug release had to be observed after an initial lag time of 2 h when the drug would be in the gastric fluid. To meet this requirement, a coating of 5% w/v Eudragit S100 in isopropyl alcohol was applied. The in vitro dissolution data suggests no drug release up to 80 min of run time (Fig. 8f). Drug concentration was observed in 85 min. However, the total amount of drug released till 120 min (2 h) was only 4.08% for fexofenadine HCl and 0.12% for montelukast sodium, which are within the acceptable limit. Post-2 h of dissolution, a burst release of fexofenadine HCl was observed with 97.65% drug release in 1 h (3 h from the beginning of the study). Only 4.47% montelukast sodium was released at the 3-h interval. The sustained release montelukast sodium layer continued to release drug steadily with 95.83% drug release at after 10 h of study. The formulation, thus, met the requirement of providing an immediate release during the first hour and a sustained release action for a period of 8 h after passing the gastric region.
Discussion
In the present investigation, we have developed Eudragit-coated chronomodulated time release bilayer tablet of fexofenadine HCl and montelukast sodium. Initially, the possible drug-drug and drug-excipient interactions were studies through IR spectroscopy. Results confirmed the drug-excipient compatibility and their suitability for the formulation development (Figs. 1 and 2). The initial risk assessment was performed through literature review. The QTPP and CQAs for bilayer tablet were established considering various parameters including dissolution rate and disintegration time (Table 3). In the fexofenadine HCl layer, starch and microcrystalline cellulose levels were found to have a high impact on friability, hardness, and dissolution. This is because of their binding properties. The microcrystalline cellulose is known to enhance tablet compressibility [31]. The sodium starch glycolate, being a super disintegrant, is a high-risk variable for disintegration followed by dissolution. In addition, sodium bicarbonate, which is an effervescence agent, is also a high-risk variable for disintegration and dissolution [32]. Other variables had medium to low risks on the CQAs. In the montelukast sodium layer, HPMC E 5 LV and polyvinylpyrrolidone (PVP K30) were found to be high-risk variables. The magnesium stearate, being hydrophobic in nature, may influence the dissolution of the formulation [29]. Other variables had medium to low risk on the CQAs of the tablets. Based on the initial risk assessment, fexofenadine HCl, sodium starch glycolate, and sodium bicarbonate levels in fexofenadine HCl layer, and montelukast sodium, HPMC E 5 LV, and magnesium stearate levels in the montelukast sodium layer were evaluated using a 3-level, 3-factor Box-Behnken design (BBD).
For the optimization of immediate release fexofenadine HCl and sustained release montelukast sodium tablet layers, a 3-factor, 3-level BBD was used. For immediate release layers, fexofenadine HCl (mg), sodium starch glycolate (mg), and sodium bicarbonate (mg) were taken as independent variable. In the similar manner, independent variables selected for sustained release layers were montelukast sodium (mg), HPMC E 5 LV (mg), and magnesium stearate (mg) (Table 1). Based on the design, a total of 13 formulation combinations (F1–F13 and M1–M13) were made and evaluated for micromeritic properties (angle of repose (θ), bulk density, tapped density, Carr’s index, and Hausner’s ratio) (Table 2).
The powder blends for fexofenadine HCl immediate release layer (F1–F13) were evaluated for their micromeritic properties. It was observed that F1, F6, and F9 had excellent flow property, while F2, F4, F5, F8, F10, F11, F12, and F13 showed a good flow property (Fig. 3). However, F3 and F7 had the highest values for angle of repose, Carr’s index, and Hausner’s ratio and hence demonstrated a fair flow property (Fig. 3a, b). While there were variations in the bulk density of the formulations, the tapped density of all the formulations was found to be close to each other (Fig. 3b). It has been observed that as the concentration of lactose monohydrate increased, the flow property decreased. F1 had the best flow as it had the least amount of lactose monohydrate, while F3 showed a poor flow as compared to other formulations due to the presence of a high amount of lactose monohydrate. This is because lactose monohydrate has a poor flow property, and hence, it decreases the flow property of the formulation as its concentration in the formulation increases. Studies have shown that untreated lactose exhibited very poor powder flow. However, the flow properties of the lactose can be improved via intensive mechanical processing with magnesium stearate [33].
All the powder blends prepared for montelukast sodium-sustained release layer (M1–M13) showed good to fair flow property. Formulation M1 had the best flow property which can be confirmed through its observed angle of repose and Carr’s index values (Fig. 3c). The flow property improved with an increase in the amount of magnesium stearate. However, the flow properties became constant with further increase in magnesium stearate. A similar effect has been observed in the previous studies where magnesium stearate at low and medium concentration increases the flow properties [34]. The flow properties of M3, M6, M7, M8, and M9, each containing 3 mg of magnesium stearate, were superior to that of M2, M5, M10, and M11, having 2 mg of magnesium stearate (Fig. 3c, d). The flow properties of M1, M4, M12, and M13, each containing 4 mg magnesium stearate, did not differ much from M3, M6, M7, M8, and M9. The bulk density and tapped density values for all the formulations remained fairly constant (Fig. 3d). The powder blends were used for the development of immediate and sustained release tablet layers.
The powder blends were used for the development of immediate release tablet layers of fexofenadine HCl (F1–F13) and sustained release tablet layers of montelukast layers (M1–M13). The developed tablet layers were evaluated for various parameters, such as hardness, percentage of friability, weight variations, disintegration time of the immediate release layer, and percentage of drug release.
The hardness of immediate release tablet layers ranged from 4.20 to 4.73 kg/cm2. Maximum hardness was observed for F5, while F8 demonstrated the least hardness (Fig. 4a). All the tablet layers had acceptable hardness value. A direct relationship between percentage of friability and tablet hardness was observed. Tablets having high hardness value showed low percentage of friability and vice versa. The study has reported that there will be a decrease in the percentage of friability with an increase in the tablet hardness [35]. Thus, F5 had the least percentage of friability value of 0.65, while F8 had a maximum percentage of friability of 0.85 (Fig. 4b). All the formulations were within the compendial limit of < 1%. The harder the tablet, there will be fewer chances of chipping and breakage. Weights of immediate release tablet layers of all formulations were in close proximity to the actual value of 200 mg.
The disintegration time was found to decrease with an increase in the concentration of sodium starch glycolate and sodium bicarbonate (Fig. 4c). This is due to the swelling ability of sodium starch glycolate and the ability of sodium bicarbonate to liberate carbon dioxide upon coming in contact of water [36]. Disintegration time was also influenced by tablet hardness with tablets having low hardness showing low disintegration time [37]. Formulation F11 showed highest disintegration time. However, formulations F3, F5, and F10 have almost similar disintegration time (Fig. 4c). Formulation F13 had the least disintegration time of 21 s and the highest drug release (88.88%) at 15 min. On the other hand, formulation F11 had the maximum disintegration time of 421 s and a slow drug release of only 50.65% after 15 min (Fig. 4d). According to the USP, an uncoated immediate release tablet should disintegrate completely within 5 to 30 min. The immediate release compositions are selected in such a way they provide a disintegration time of less than about 30 min, preferably about 20 min or less, and more preferably about 18 min or less. This means that F3, F5, F10, and F11 failed to meet the criteria. In addition, the USP also states that an immediate release tablet should release not less than 80% of its drug within 15 min. Considering the disintegration paradigm, only formulations F1, F2, F8, and F9 meet the necessary requirements. The hardness of the sustained release montelukast sodium tablets layers (M1–M13) ranged from 4.22 to 4.57 kg/cm2 (Fig. 4e). Maximum hardness was observed for M4, while M11 demonstrated the least hardness. All the tablets had acceptable hardness value. A direct relationship between percentage of friability and tablet hardness was observed. Tablets having high hardness value showed low percentage of friability and vice versa. Thus, M4 had the least percentage of friability value of 0.60, while M11 had maximum percentage of friability of 0.83. All the formulations were within the compendial limit of < 1% (Fig. 4f).
Weights of tablet layers of all formulations (M1–M13) were in close proximity to the actual value of 100 mg (Fig. 4g). The concentration of HPMC E 5 LV and magnesium stearate influenced the percentage of drug release. It has been observed that the higher the concentration of HPMC E 5 LV, the slower was the drug release. The study has shown that HPMC can slow down the drug release from the tablet matrices. Similar observations were made in the case of varying concentrations of magnesium stearate. The HPMC forms a rubbery gel that retards drug dissolution, while the hydrophobic nature of magnesium stearate creates a barrier between the API and the outer environment [27]. M6, M7, M11, and M13 failed to sustain drug release for 8 h. Formulation M10 showed the maximum drug release of 97.68% after 8 h (Fig. 4h). The immediate release and sustained release tablet layers were also evaluated for diffusion release kinetics. Among the immediate release fexofenadine HCl tablet layers, formulations F3 and F6 followed the Hixson-Crowell model suggesting that the dissolution occurred in planes parallel to the tablet surface. Formulation F4 followed the Korsmeyer-Peppas model, which is similar to the release from a polymeric matrix. All the other formulations were found to follow first-order release kinetics suggesting that the release is concentration dependent. For immediate release fexofenadine HCl formulations, the value of the coefficient of correlation (r2) for the best fit model and diffusion exponent (n) ranges from 0.8107 to 0.9864 and 0.334 to 0.390, respectively. Depending upon the value of the diffusion exponent (n), the Fickian diffusion mechanism is dominant among immediate release formulations (Table 4) [28].
For the optimization of immediate release tablet layer, the effect of variables on disintegration time and drug release was investigated through polynomial equation and response curves. The factors affecting disintegration time were concentrations of fexofenadine HCl (A), sodium starch glycolate (B), and sodium bicarbonate (C). The predicted disintegration time values obtained by the model using the above equation were compared with observed values. A low percentage error of < 5% ascertained that the model has good predictability (Table 5). The concentration of fexofenadine HCl (A) had a negligibly small effect on disintegration time. However, the concentrations of sodium starch glycolate and sodium bicarbonate had a negative effect. The disintegration of the tablet layer increased rapidly with an increase in the concentration of sodium starch glycolate (B). This could be due to the improved moisture absorption by sodium starch glycolate that results in massive volumetric increase leading to faster disintegration. Sodium bicarbonate, being an effervescence agent, generates carbon dioxide leading to the pressure build-up within the formulation and thus causing the fast release of components. It also led to a faster disintegration time but not as much as sodium starch glycolate.
The effect of variables on the drug release from the immediate release tablet layer showed that the concentration of the drug (A) did not have a significant effect on release. However, the concentrations of sodium starch glycolate (B) and sodium bicarbonate (C) had a significant positive effect on drug release (Y1) (Fig. 5e–h). Sodium bicarbonate, being an effervescence agent, generates carbon dioxide leading to pressure build-up within the formulation and thus causing the fast release of components. It acted synergistically by absorbing moisture and facilitating drug release.
For the optimization of sustained release tablet layer, the effect of variables on drug release was evaluated. Results showed that there was a decline in the drug release with increase in HPMC E 5 LV (Fig. 5i–l). The mobility of the polymer chain gets enhanced in the presence of a solvent. As a result, a glassy matrix gradually transforms into a rubbery swollen gel. The higher concentrations of HPMC resulted in enhanced viscosity that retards the drug’s diffusion coefficient. The magnesium stearate forms a film decreasing wettability of the formulation due to its hydrophobic nature and thereby decreasing the dissolution rate (Fig. 5i–l). The significance of the model used to evaluate the effect of variables on the performance of immediate and sustained release layers was evaluated through ANOVA analysis. The ANOVA and PRESS value suggested that the model generated was significant and fit for evaluation (Table 6).
The goodness of fit of the proposed model for immediate and sustained release tablet layer was investigated through diagnostic plots (Fig. 6a–d). The results through various diagnostic plots showed that the model used for the evaluation of variables on the properties of different tablet layers was fit for analysis. The optimized bilayer formulation with set paradigm of maximum drug release and minimum disintegration time was selected using desirability plots. The response surface and contour plots for the immediate release layer design suggested the values for fexofenadine HCl to be 60 mg, sodium bicarbonate to be 18.32 mg, and sodium starch glycolate to be 18.93 mg. The predicted value of the drug release according to the plot was 88.7258%, and for disintegration time, the predicted value was 29.3966 s with a desirability of 0.819301 (Fig. 7a–f). Similarly, sustained tablet release layer was prepared with set composition. The predicted drug release and desirability were found to be 98.542% and 0.6361 (Fig. 7g–j). Finally, the Eudragit-coated optimized bilayer tablet was developed and evaluated. The formulation, thus, met the requirement of providing an immediate release during the first hour and a sustained release action for a period of 8 h after passing the gastric region.
Conclusion
The present study demonstrates the successful preparation of the Eudragit-coated chronomodulated time release bilayer tablet of fexofenadine HCl and montelukast sodium. The aim of the study is to use a QbD approach to investigate the effect of variables on the CQAs of the bilayer tablet in an effective and economical manner with less number of experiments. The QTPP of the tablet was developed as per the previous studies, and CQAs were selected for the investigation. The fexofenadine HCl-loaded immediate and montelukast sodium-loaded sustained release layers were successfully optimized using a 3-factor, 3-level Box-Behnken design. The optimization model used in the study was successfully validated using ANOVA and diagnostic plots. The optimized formulation was confirmed as per the desirability plots. It was observed that the extended concentration of sodium starch glycolate prompted an increase in disintegration time and a relating increment in drug release. This was due to the swelling property of sodium starch glycolate that leads to a faster release of the tablet constituents. Sodium bicarbonate, being an effervescence agent, also contributed to the faster drug release. However, the effect of sodium starch glycolate was more pronounced than sodium bicarbonate. It was observed that with an increase in the concentration of HPMC, there was a decline in drug release. The HPMC frames a rubbery gel layer around the formulation, which leads to a slow drug release. Similar observations were made for magnesium stearate which is hydrophobic in nature that creates a barrier between the tablet components and the outer aqueous environment leading to a decrease in drug release. The design of the present bilayer tablet successfully provided the immediate release of fexofenadine HCl and a sustained drug release of montelukast sodium with an initial lag time of 2 h. Thus, the formulation can be fruitful in curbing the menace of nocturnal asthma and providing a high degree of patient compliance.
Availability of data and materials
All data provided in the manuscript is available upon request.
Abbreviations
- ANOVA:
-
Analysis of variance
- BBD:
-
Box-Behnken design
- BD:
-
Bulk density
- CQA:
-
Critical quality attributes
- DF:
-
Degree of freedom
- DoE:
-
Design of experiment
- DUREDAS:
-
Dual Release Drug Absorption System
- HPMC:
-
Hydroxypropyl methyl cellulose
- ICH:
-
International Conference on Harmonisation
- PBS:
-
Phosphate buffer saline
- PEG:
-
Polyethylene glycol
- PRESS:
-
Predicted residual error sum of squares
- PVP:
-
Polyvinylpyrrolidone
- QbD:
-
Quality by design
- QTPP:
-
Quality target product profile
- TD:
-
Tapped density
References
Xiongbin J (2016) Nocturnal asthma: a special type of asthma. Glob J Allergy 2:3–9
Silkoff PE, Martin RJ (1998) Pathophysiology of nocturnal asthma. Ann Allergy Asthma Immunol 81:378–384
Turner-Warwick M (1988) Epidemiology of nocturnal asthma. Am J Med 85:6–8
Dethlefsen U, Repgas R (1985) Ein neues therapieprinzip bei nachtlichen asthma. Klin Med 80:44–47
Hetzel MR, Clark TJ, Branthwaite MA (1977) Asthma: analysis of sudden deaths and ventilatory arrests in hospital. BMJ 1:808–811
Cochrane GM, Clark JH (1975) A survey of asthma mortality in patients between ages 35 and 64 in the Greater London hospitals in 1971. Thorax 30:300–305
Ali J, Saigal N, Qureshi MJ, Baboota S, Ahuja A (2010) Chronopharmaceutics: a promising drug delivery finding of the last two decades. Recent Pat Drug Deliv Formul 4:129–244
Mandal SA, Biswas N, Karim KM, Guha A, Chatterjee S, Behera M, Kuotsu K (2010) Drug delivery system based on chronobiology-a review. J Control Release 147:314–325
Abebe A, Akseli I, Sprockel O, Kottala N, Cuitino AM (2014) Review of bilayer tablet technology. Int J Pharm 461:549–558
Nirmal J, Saisivam S, Peddanna C, Muralidharan S, Nagrarajan M (2008) Bilayer tablets of atrovastatin calcium and nicotinic acid: formulation and evaluation. Chem Pharm Bull 56:1455–1458
Shiyani B, Gattani S, Surana S (2008) Formulation and evaluation of bi-layer tablet of metoclopramide hydrochloride and ibuprofen. AAPS PharmSciTech 9:818–827
ICHQ8(R2). The international conference on harmonization of technical requirements for registration of pharmaceuticals for human use, quality guideline Q8(R2) pharmaceutical development. 2009 http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf. (Accessed 2 Aug 2018)
ICH Q9. The international conference on harmonization of technical requirements for registration of pharmaceuticals for human use, ICH harmonised tripartite guideline Q9 Quality Risk Management. 2005 http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf. (Accessed 2 Aug 2018)
ICHQ10. The international conference on harmonization of technical requirements for registration of pharmaceuticals for human use, ICH harmonised tripartite guideline, Q 10 pharmaceutical quality system. 2008 https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf. (Accessed 2 Aug 2018)
Narang A, Nayak UY, Roy B, Narayan R (2016) Formulation design of bilayer dual-release tablet composition of fexofenadine HCl and montelukast sodium. Res J Pharm Tech 9:1410–1416
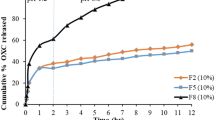
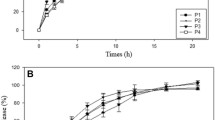
Tak W, Gupta B, Thapa RK, Woo KB, Kim SY, Go TG, Choi Y, Choi JY, Jeong JH, Choi HG, Yong CS, Kim JO (2016) Preparation and optimization of immediate release/sustained release bilayered tablets of loxoprofen using Box–Behnken design. AAPS PharmSciTech 18:1125–1134
Shah RB, Tawakkul MA, Khan MA (2008) Comparative evaluation of flow for pharmaceutical powders and granules. AAPS PharmSciTech 9:250–258
Govedarica B, Injac R, Dreu R, Srcic RS (2011) Formulation and evaluation of immediate release tablets with different types of paracetamol powders prepared by direct compression. Afr J Pharm Pharmacol 5:31–41
Hildebrandt C, Gopireddy SR, Fritsch AK, Profitlich T, Scherlie R, Urbanetz NA (2017) Evaluation and prediction of powder flowability in pharmaceutical tableting. Pharm Dev Technol 13:1–13
Shiyani BG, Dholakiya RB, Akbari BV, Lodhiya DJ, Ramani GK (2009) Development and evaluation of novel immediate release tablets of metoclopramide HCl by direct compression using treated gellan gum as a disintegration-accelerating agent. J Pharm Res 2:1460–1464
Bashar A, Alkhalidi A, Alkhatib HS, Khdair AA (2010) Comparative dissolution of diltiazem immediate and extended release products using conventional and innovative dissolution paddles. Open Drug Deliv J 4:48–54
Vemula SK (2015) Colon specific drug delivery: effect of eudragit enteric coating on hydroxypropyl methylcellulose matrix tablets of flurbiprofen. J Young Pharm 7:373–383
Roychowdhury S, Singh H, Deora G, Sharma S (2014) Formulation, evaluation and optimization of enteric coated tablets of erythromycin stearate by multivariate ANOVA method. AJADD 2:308–319
Huang RJ, Wigent JB (2008) Schwartz. Drug-polymer interaction and its significance on the physical stability of nifedipine amorphous dispersion in microparticles of an ammonio methacrylate copolymer and ethylcellulose binary blend. J Pharm Sci 97:251–262
Mitra J, Davoud H, Farhad K, Badir DL, Sara A (2011) Preparation and determination of drug-polymer interaction and in-vitro release of mefenamic acid microspheres made of cellulose acetate phthalate and/or ethylcellulose polymers. Iran J Pharm Res 10:457–467
Murtada AO, Abdelkarim MA, Huyam AM (2013) The effect of sodium starch glycolate concentration on physical effectiveness of chlorpheniramine tablets. J Pharm Edu Res 4:47–53
Ishikawa T, Watanabe Y, Takayama K, Endo H, Matsumoto M (2008) Effect of hydroxylpropylmethyl cellulose (HPMC) on the release profiles and bioavailability of a poorly water-soluble drug from tablets prepared using macrogol and HPMC. Int J Pharm 202:173–178
Arefin P, Hasan I, Reza MS (2016) Design, characterization and in vitro evaluation of HPMC K100 M CR loaded fexofenadine HCl microspheres. Springer Plus 5:1–11
Uzunovic A, Vranic E (2007) Effect of magnesium stearate concentration on dissolution properties of ranitidine hydrochloride coated tablets. Bosn J Basic Med Sci 7:279–283
Khan MA, Chaturvedi SC (2010) Formulation of sustained release zolpidem tartrate matrix tablets through optimization and their evaluation. Asian J Chem 22:4749–4762
Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B (2014) Microcrystalline cellulose, a direct compression binder in a quality by design environment-a review. Int J Pharm 473:64–72
Desai PM, Liew CV, Heng PWS (2016) Review of disintegrants and the disintegration phenomena. J Pharm Sci 105:2545–2555
Zhou Q, Armstrong B, Larson I, Stewart PJ, Morton DA (2010) Improving powder flow properties of a cohesive lactose monohydrate powder by intensive mechanical dry coating. J Pharm Sci 99:969–981
Yuksel N, Turkmen B, Kurdoglu AH, Basaran B, Erkin J, Baykara T (2007) Lubricant efficiency of magnesium stearate in direct compressible powder mixtures comprising Cellactose® 80 and pyridoxine hydrochloride. FABAD J Pharm Sci 32:173–183
Gordon MS (1994) Process considerations in reducing tablet friability and their effect on in vitro dissolution. Drug Dev Ind Pharm 20:0363–9045
Rahman MM, Roy S, Hasan S, Alam MA, Jha MK, Ahsan MQ, Ferdaus MJ (2011) Effect of mode of addition of disintegrants on dissolution of model drug from wet granulation tablets. Int J Pharm Sci Res 2:84–92
Kitazawa S, Johno I, Ito Y, Teramura S, Okado J (1975) Effects of hardness on the disintegration time and the dissolution rate of uncoated caffeine tablets. J Pharm Pharmacol 27:765–770
Acknowledgements
The authors would like to thank Dr. Rohit Bisht, Founder and Director, Scholars Paper Lab, Haldwani, Uttarakhand, India, for his guidance in making this manuscript.
Funding
No funding was availed for the present investigation. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript. BS contributed to the research guidance and analytical studies. GS contributed to the formulation evaluation and data analysis. MV contributed to the research guidance and data analysis. SV contributed to the formulation development and evaluation.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Non applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Singh, B., Saini, G., Vyas, M. et al. Optimized chronomodulated dual release bilayer tablets of fexofenadine and montelukast: quality by design, development, and in vitro evaluation. Futur J Pharm Sci 5, 5 (2019). https://doi.org/10.1186/s43094-019-0006-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-019-0006-9