Abstract
Background
CD155 is an immune checkpoint protein that interacts with ligands on natural killer cells to regulate the tumor associated immunity. CD155 overexpression has been detected in many human cancer types. CD155 and its pathways are promising tumor immunotherapy targets. We aimed to evaluate the immunohistochemical expression of CD155 in invasive breast carcinomas and to correlate such expression with the pathological parameters of the tumors and also with natural killer - tumor infiltrating lymphocytes (NK-TILs) density in breast carcinomas tissue as highlighted by CD56 immunostaining. This study included 78 cases of breast carcinomas. Immunohistochemistry was performed using antibodies against CD155 which was detected on the tumor cells and CD56 as a marker for stromal NK cells.
Results
CD155 expression by the tumor cells was detected in 30.8% of the cases and correlated significantly with advanced prognostic stage, Estrogen receptor (ER) and Progesterone receptor (PR) negativity, high Ki-67 index and Human epidermal receptor 2 (HER2) enriched molecular subtype. High stromal TILs CD56 expression was detected in 28.2% of the cases and correlated significantly with high histologic grade, PR negativity, HER2 neu over-expression, high Ki-67 index, high stromal TILs and more aggressive molecular subtypes; triple negative breast cancer, HER2 enriched and Luminal B-HER2 positive. Finally, statistically significant direct correlation was detected between Tumor cells CD155 expression and high TILs CD56 expression.
Conclusions
Our results support tumor cell CD155 expression and TILs CD56 expression in breast cancers that are high grade, TILs rich and hormone receptors negative, highlighting those cases as possible candidates for CD155 targeted therapy.
Similar content being viewed by others
1 Background
Breast cancer (BC) is the most common female malignancy accounting for 37.7% of female cancers in Egypt [1]. It carries an unfavorable prognosis with 29% worldwide mortality [2].
Breast cancers show infiltration by a mixed immune cell population [3]. The importance of NK cells as tumor associated immune cells is increasingly appreciated in BC [4]. Mamessier et al. [5] reported that breast cancers are able to escape NK cell immune attack by modulating their surface receptors. A process that is associated with tumor progression.
CD155 is an immunoglobulin Type 1 transmembrane glycoprotein with a molecular weight of approximately 70 kDA [6]. It functions as an adhesion molecule that is involved in cell–cell contact. CD155 is normally located on the cell surface of dendritic cells, fibroblasts and endothelial cells [7].
CD155 has an immunoreceptor tyrosine based inhibition motif (ITIM), and functions as a ligand for DNAX-associated molecule 1 (DNAM- 1), T-cell immunoglobulin and ITIM domain (TIGIT), and CD96 receptors expressed on natural killer cells and T-cells. Interaction with DNAM-1 leads to activation of immune reactions, in contrast, interaction with CD96 and TIGIT, results in immunosuppression. It has been proposed that the functions of CD155 are modulated by the tumor microenvironment (TME), leading to tumor immune suppression; therefore, CD155 has been recognized as a potential anti-tumor target therapy [8].
Studies have shown that CD155 is overexpressed in a variety of malignant tumors, such as ovarian, colorectal, lung, and gastric carcinomas, as well as, neuroblastoma and melanoma [9]. The expression of CD155 has been also reported in BC [8,9,10].
Our study aimed to evaluate the immunohistochemical expression of CD155 in invasive BC, to detect the potentiality of targeting it as cancer immunotherapy. We tempted to correlate CD155 expression with the pathological parameters of the tumors including molecular subtypes. CD155 expression will also be correlated with presence and density of NK-TILs in BC tissue. The tumor infiltrating NK cells will be evaluated as CD56 positive lymphocytes, where, CD56 is the most significant biomarker for distinguishing NK cells from others lymphocyte populations [11].
2 Methods
This study is a retrospective observational cross-sectional one. Approval from research ethics committee (REC) at faculty of medicine, Cairo university (REC code: N-62-2021) was obtained before starting the study.
2.1 Cases collection
A total of 78 formalin fixed, paraffin embedded blocks of female breast carcinomas were collected from the archive of the Pathology Department at Kasr alainy hospital, faculty of medicine, Cairo university.
To preserve the patients’ privacy, the names of the cases were replaced by an ID number. Only this ID number was used afterwards on the glass slides, as well as, in the data sheet.
Inclusion criteria for our study included female patients undergoing conservative or radical mastectomy and not receiving any neoadjuvant therapy.
Exclusion criteria included male patients, cases with any missing data, cases subjected to incisional or excisional biopsies without axillary dissection or at least sentinel lymph node biopsy, cases of pure ductal carcinoma in situ (DCIS), patients who received neoadjuvant therapy and finally cases showing equivocal HER2 immunohistochemistry (IHC) results, with no available DISH (Dual in Situ Hybridization) report.
2.2 Data collection
The data collected from the pathology reports for each case included tumor size, and lymph node status, as well as, ER, PR, HER2 and Ki-67 results. A DISH report was obtained for equivocal HER2 cases (class 2 +) by IHC.
2.3 Histopathologic examination
The collected paraffin blocks were serially sectioned at 4 μm thickness and stained with Hematoxylin and Eosin (H&E) stains for histopathological examination. The tumors were histologically typed according to the World Health Organization (WHO) 2019 criteria. Histological grading was performed according to the Nottingham Grading System [12]. For further statistical evaluation, Grade one and two cases were grouped as low grade, while grade three cases were considered as high grade [13].
Lympho-vascular invasion (LVI) was defined by the presence of tumor cells within an endothelial lined space (lymphatic and/or blood vessel) [14].
2.4 Tumor infiltrating lymphocytes assessment
Tumor Infiltrating Lymphocytes were scored following the recommendations of the International TILs Working Group 2014. All mononuclear immune cells (lymphoplasma and histiocytes) in the stroma of the invasive tumor were evaluated and reported as a percentage of the stromal area (i.e. stromal area occupied by mononuclear cells) and not as a percentage of the stromal cells. TILs around DCIS and normal breast tissue or outside of the tumor border, as well as in areas of hyalinosis and necrosis were not included in the scoring. The working group recommended full assessment of average TILs in the tumor area rather than ‘hot spots’ [15].
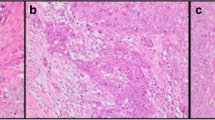
As the working group didn’t recommend a relevant threshold(s) for TILs assessment [15], we stratified our cases into Low (< 10%) and High (≥ 10%) TILs (Fig. 1) [16].
2.5 Staging and molecular subtyping
Tumors staging was performed using the TNM staging system. Anatomic and prognostic staging were performed according to the latest edition of the American Joint Committee on Cancer (AJCC) staging manual [17]. Concerning the prognostic staging, nine cases were excluded as their prognostic stages were missing in the AJCC classification. For statistical purposes, tumor stages (whether anatomic or prognostic) were classified into early; stages I and II and advanced; stages III and V [18].
Regarding the BC subtyping, we classified our cases following the St. Gallen International Expert Consensus 2013 recommendations: luminal A like; ER positive, PR positive, HER2 negative and low Ki-67, luminal B like-HER2 negative; ER positive, HER2 negative and either low PR or high Ki-67, Luminal B like-HER2 positive; ER positive, HER2 positive, any Ki-67 and any PR, HER2 positive–non luminal; HER2 positive and hormone receptor negative and Triple Negative; ER, PR and HER2 negative [19]. A cut point of 20% was used to stratify Ki-67 index into low and high. Luminal cases showing high histologic grade were considered as Luminal B rather than A according to St. Gallen International Expert Consensus 2017 recommendations [20].
2.6 Immunohistochemical staining
For immunostaining, two additional sections on positive charged slides were prepared from each paraffin block. Immunostaining was performed using a Ventana Benchmark Ultra immunostainer. For CD155 immunostaining, a CD155 (D8A5G) Rabbit monoclonal antibody (#81254: Cell Signaling Technology; Danvers, MA, USA) was used. NK cell assessment was performed using an anti- CD56 (MRQ-42) Rabbit monoclonal antibody (760-4596: Roche Diagnostics; USA).
2.7 Immunohistochemical evaluation
CD155 immunoreactivity was evaluated in the tumor cell membrane. We used an immunoreactivity (IR) score (Values 0–12) calculated as follows; IR = staining intensity × percentage of positive tumor cells, where intensities were scored as follows: 0; negative staining, one; weak staining, two; moderate staining and three; strong staining. The percentages of positive tumor cells were scored as follows: zero; no staining of cells, one; < 25%, two; 25–50%, three; 50–75% and four; > 75%. An IR score of two or above was considered positive (Fig. 2) [21].
CD56 immunoreactivity was evaluated only in stromal TILs. CD56 was examined in ten randomly selected areas. Density of NK cells was considered as high if there were more than five CD56 positive stromal TILs per ten high power fields (HPFs) (Fig. 3).
2.8 Statistical analysis
All collected histopathological and immunohistochemical data were transferred to the Statistical Package of Social Science (SPSS) Software program, version 25 for statistical analysis. Comparison between groups was performed using Chi square test. A P value of ≤ 0.05 was considered statistically significant.
2.9 Slides screening and imaging
All slides were examined using an Olympus light microscope (model BX53F2). Images were obtained by digital Olympus high definition camera (model EP50) connected to the same microscope.
3 Results
This study included 78 cases of BC obtained from modified radical mastectomy and conservative breast surgery specimens. The pathological data of the cases are summarized in Table 1.
The pathologic characteristics of the studied cases stratified by CD155 and TILs- CD56 expression are summarized in Table 2. A statistically significant direct correlation was detected between Tumor cells CD155 expression and high TILs CD56 expression [P value = 0.004].
4 Discussion
CD155, an immune checkpoint transmembrane glycoprotein, is expressed on the tumor cells of many cancer types [6]. The receptors for CD155 are located on the NK cells, as well as, T lymphocytes and other cell types [8].
In this study, we investigated the immunohistochemical expression of CD155 and CD56 in BC tumor cells and BC TILs respectively. Such expressions were correlated to each other and also to the pathological parameters of the tumors.
CD155 expression was detected in 30.8% of our cases. This figure was close to the results of most of the studies targeting CD155 immunohistochemical expression. A study using the same CD155 clone as ours reported 38% CD155 positivity in BC 10. The reason for other studies reporting slightly higher rates of CD155 expression in BC may be related to different study populations; Yoshikawa et al. [8] reported 41% CD155 positivity in triple negative breast cancer cases or possibly to the used antibody clone; Yong et al. [9] reported 52.3% CD155 positivity in breast cancer cases using a polyclonal antibody.
In our study, CD155 showed an increased expression in high grade cases with a statistically significant correlation with high Ki-67 proliferation index. There was a wide agreement for such findings in the literature, suggesting a possible role for CD155 in increasing cell proliferation and loss of differentiation.
We reported statistically insignificant higher CD155 expression with advanced T stage, advanced anatomic stage and lymph node positive cases. Although many studies agreed with such finding [7, 9, 22]. One study reported statistically insignificant higher CD155 expression in T1 node negative cases [8].
Regarding the prognostic staging incorporating the ER, PR and HER2 status, as well as, the histologic grade in addition to the TNM, we reported statistically significant higher CD155 expression in advanced prognostic stage cases, however to our knowledge; no studies in the literature investigated such correlation.
In our study, CD155 expression showed slightly higher expression in LVI positive cases compared to LVI negative cases in keeping with our results of higher expression in lymph node positive cases. Some controversy existed in the literature regarding correlation of CD155 expression with LVI; while Yoshikawa et al. [8] reported higher CD155 expression in LVI negative cases, Trikia et al. [9] reported higher membranous CD155 expression in LVI negative cases and statistically significant higher cytoplasmic CD155 expression in LVI positive cases.
CD155 expression in our study showed statistically significant correlation with hormone receptor (ER and PR) negative cases and statistically insignificant correlation with HER2 overexpressing cases. This agreed with most of the studies in the literature [7, 9, 22].
On BC subtyping, we reported statistically significant highest CD155 expression in HER2 positive-non luminal cases followed by TNBC then luminal B and finally Luminal A cases. Trikia et al. [9] agreed with our results reporting highest rates of membranous CD155 expression in HER2 enriched cases, while others reported highest rates of CD155 expression in TNBC cases [10 and 22]. Notably, all studies agreed that the expression was higher in non-luminal than luminal cases highlighting such poor prognosis cases as possible candidates for CD155 targeting therapy.
In our study, CD155 expression was higher in cases with high stromal TILs compared to those with low stromal TILs. This was compatible with many others [7, 8, 22] keeping with the suggested immunologic role of CD155.
Regarding NK cell density among the stromal TILs, it was highlighted in our study using CD56 immunostaining. We detected high Stromal TILs CD56 expression in 28.2% of our cases. This figure was close to that reported by Trikia et al. [7] and Bouzidi et al. [23] who reported 23.8% and 25.2% cases with high CD56 TILs expression respectively. However, another study reported higher figures; 40% cases with CD56 positive stromal TILs and 45.5% cases with positive intra-tumoral TILs [11].
CD56 positivity in stromal TILs correlated significantly with the high histologic grade and high Ki-67 proliferation index in our study. This agreed with what was reported by many others [7, 11, 23, 25].
Although CD56 stromal TILs expression in our study was higher in more advanced T stage cases, it showed higher expression in lymph node negative, LVI negative and early anatomic stage cases, yet, all those relations were statistically insignificant. These findings agreed with what was reported by Bouzidi et al. [23].
Those findings can suggest a possible role for NK cells in preventing vascular dissemination of tumor cells and metastasis keeping with the previously reported association of high NK cell density with better outcome in some cancers [24]. Regarding Trikia et al. [7], they also reported highest CD56 TILs expression in LVI negative cases and in T3 cases, yet, they reported higher expression in N1 compared to N0 followed by N2 and N3 cases.
Although our cases showed higher stromal CD56 expression in early anatomic stage cases, they conversely showed higher expression in advanced prognostic stage cases owing to the higher positivity in high grade, ER negative, PR negative and HER2 positive cases.
In our study, Stromal CD56 expression was higher in ER negative cases and showed statistically significant association with PR negativity and HER2 overexpression. This was consistent with what was reported by Trikia et al. [7] and Bouzidi et al. [23]. Muntasell et al. [25] also reported high stromal NK cells in ER negative cases.
Regarding the BC subtypes, Stromal CD56 expression in our study showed statistically significant higher expression in more aggressive subtypes triple negative breast cancer (TNBC), HER2 positive-non luminal and Luminal B HER2 positive) compared to Luminal HER2 negative cases. Similarly, Trikia et al. [7] and Bouzidi et al. [23] reported highest Stromal CD56 positivity in HER2 enriched carcinomas and TNBC respectively.
In this study, high stromal CD56 correlated significantly with high density of stromal TILs, which agreed with Bouzidi et al. [23]. Finally, we detected a statistically significant correlation between CD155 expression by the tumor cells and CD56 expression by stromal TILs. Similarly Trikia et al. [7] reported statistically significant association of NK cell density with membranous but not cytoplasmic expression of CD155 by the tumor cells.
5 Conclusion
Our results supported CD155 expression by BC tumor cells and detected a strong correlation of such expression with the stromal NK density. We agreed with others that such expression is higher in a subset of tumors that are high grade, rich in stromal TILs and lacking hormone receptors, suggesting a possible role for CD155 targeted therapy in such cases. Possible limitation is the lack of correlation with the patient’s survival and cancer related morbidity.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on request.
Abbreviations
- BC:
-
Breast carcinomas
- NK - TILs:
-
Natural killer - tumor infiltrating lymphocytes
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER2:
-
Human epidermal receptor 2
- TNBC:
-
Triple negative breast cancer
- ITIM:
-
Immunoreceptor tyrosine based inhibition motif
- DNAM-1:
-
DNAX-associated molecule 1
- TIGIT:
-
T-cell immunoglobulin and ITIM domain
- TME:
-
Tumor microenvironment
- REC:
-
Research ethics committee
- DCIS:
-
Ductal carcinoma in situ
- DISH:
-
Dual in situ hybridization
- IHC:
-
Immunohistochemistry
- H&E:
-
Hematoxylin and eosin
- WHO:
-
World Health Organization
- LVI:
-
Lympho-vascular invasion
- AJCC:
-
American joint committee on cancer
- IR:
-
Immunoreactivity
- HPFs:
-
High power fields
- SPSS:
-
Statistical package of social science
- IDC-NST:
-
Invasive duct carcinoma of no special type
- ILC:
-
Invasive lobular carcinoma
References
Veruttipong D, Soliman AS, Gilbert SF, Blachley TS, Hablas A, Ramadan M et al (2012) Age distribution, polyps and rectal cancer in the Egyptian population-based cancer registry. World J Gastroenterol: WJG 18(30):3997
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127(12):2893–2917
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol 31(7):860–867
Jochems C, Schlom J (2011) Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med 236(5):567–579
Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R et al (2011) Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Investig 121(9):3609–3622
Ardolino M, Zingoni A, Cerboni C, Cecere F, Soriani A, Iannitto ML et al (2011) DNAM-1 ligand expression on Ag-stimulated T lymphocytes is mediated by ROS-dependent activation of DNA-damage response: relevance for NK–T cell interaction. Blood J Am Soc Hematol 117(18):4778–4786
Triki H, Charfi S, Bouzidi L, Kridis WB, Daoud J, Chaabane K et al (2019) CD155 expression in human breast cancer: clinical significance and relevance to natural killer cell infiltration. Life Sci 231:116543
Yoshikawa K, Ishida M, Yanai H, Tsuta K, Sekimoto M, Sugie T (2021) Immunohistochemical analysis of CD155 expression in triple-negative breast cancer patients. PLoS ONE 16(6):e0253176
Yong H, Cheng R, Li X, Gao G, Jiang X, Cheng H et al (2019) CD155 expression and its prognostic value in postoperative patients with breast cancer. Biomed Pharmacother 115:108884
Wang RB, Li YC, Zhou Q, Lv SZ, Yuan KY, Wu JP et al (2020) Overexpression of CD155 is associated with PD-1 and PD-L1 expression on immune cells, rather than tumor cells in the breast cancer microenvironment. World J Clin Cases 8(23):5935
Rathore AS, Goel MM, Makker A, Kumar S, Srivastava AN (2014) Is the tumor infiltrating natural killer cell (NK-TILs) count in infiltrating ductal carcinoma of breast prognostically significant? Asian Pac J Cancer Prev 15(8):3757–3761
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Wang M, Liang L, Lei X, Multani A, Meric-Bernstam F, Tripathy D et al (2018) Evaluation of cMET aberration by immunohistochemistry and fluorescence in situ hybridization (FISH) in triple negative breast cancers. Ann Diagn Pathol 35:69–76
Gujam FJ, Going JJ, Edwards J, Mohammed ZM, McMillan DC (2014) The role of lymphatic and blood vessel invasion in predicting survival and methods of detection in patients with primary operable breast cancer. Crit Rev Oncol Hematol 89(2):231–241
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271
Kim A, Lee SJ, Kim YK, Park WY, Park DY, Kim JY et al (2017) Programmed death-ligand 1 (PD-L1) expression in tumour cell and tumour infiltrating lymphocytes of HER2-positive breast cancer and its prognostic value. Sci Rep 7(1):1–10
Hortobagyi GN, Connolly JL, D’Orsi CJ, Edge SB, Mittendorf EA, Rugo HS et al (2017) Breast. In: Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (eds) AJCC (American Joint Committee on Cancer) Cancer Staging Manual, 8th edn. Springer-Verlag, New York, pp 570–610
Zhou SJ, Bi TQ, Qin CX, Yang XQ, Pang K (2018) Expression of NAMPT is associated with breast invasive ductal carcinoma development and prognosis. Oncol Lett 15(5):6648–6654
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B et al (2013) Personalizing the treatment of women with early breast cancer: highlights of the St. Gallen International Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol 24(9):2206–2223. https://doi.org/10.1093/annonc/mdt303
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S et al (2017) De-escalating and escalating treatments for early-stage breast cancer the St. Gallen international expert consensus conference on the primary therapy of early breast cancer. Ann Oncol 28(8):1700–1712
Zheng Q, Gao J, Yin P, Wang W, Wang B, Li Y et al (2020) CD155 contributes to the mesenchymal phenotype of triple-negative breast cancer. Cancer Sci 111(2):383–394. https://doi.org/10.1111/cas.14276
Yu-Chen LI, Quan Z, Qing-Kun S, Rui-Bin W, Shuzhen L, Xiudong G et al (2020) Overexpression of an immune checkpoint (CD155) in breast cancer associated with prognostic significance and exhausted tumor-infiltrating lymphocytes: a cohort study. J Immunol Res 2020:1
Bouzidi L, Triki H, Charfi S, Kridis WB, Derbel M, Ayadi L et al (2021) Prognostic value of natural killer cells besides tumor-infiltrating lymphocytes in breast cancer tissues. Clin Breast Cancer 21(6):e738–e747
Huntington ND, Cursons J, Rautela J (2020) The cancer–natural killer cell immunity cycle. Nature Rev Cancer 20(8):437–454
Muntasell A, Rojo F, Servitja S, Rubio-Perez C, Cabo M, Tamborero D et al (2019) NK cell infiltrates and HLA class I expression in primary HER2+ breast cancer predict and uncouple pathological response and disease-free SurvivalNK cells and HLA class I as biomarkers in HER2 breast cancer. Clin Cancer Res 25(5):1535–1545
Acknowledgements
Not applicable
Funding
We declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [PEES]. The first draft of the manuscript was written by [AAE] and all authors commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to particpate
Approval from research ethics committee (REC) at faculty of medicine, Cairo university (REC code: N-62-2021) was obtained before starting the study. We enclosed a copy of the ethical approval.
Consent for publication
Not applicable.
Competing interests
We have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shibel, P.E.E., Abd Elmaogod, E.A. Immunohistochemical expression of CD155 in invasive female breast carcinoma and its correlation with tumor infiltrating natural killer cells. Beni-Suef Univ J Basic Appl Sci 12, 32 (2023). https://doi.org/10.1186/s43088-023-00370-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00370-z