Abstract
Background
Colorectal cancer (CRC) is a common and lethal malignancies with poor prognosis. CRC cells release extracellular vesicles called exosomes to facilitate tumor progression by passing bioactive molecules such as proteins and nucleic acids between cells of the tumor and their microenvironment. Granulocyte colony stimulating factor (G-CSF) is a hematopoietic growth factor which mainly affects the lineage of neutrophil and exerts direct anti-tumor effects on various tumor types. The purpose of our study is to investigate the effect of G-CSF on CRC cells and to evaluate its capability to attenuate the potentiality of CRC cells derived exosomes to induce bone marrow-derived mesenchymal stem cells (BM-MSCs) malignant transformation into cancer stem cells (CSCs).
Results
The level of both lncRNA metastasis associated lung adenocarcinoma transcript 1 (MALAT-1) (p = 0.014) & β-catenin (p = 0.01) was significantly decreased, whereas programmed cell death 4 (PDCD4) (p = 0.018) was increased in CRC exosomes pre-treated with G-CSF compared to untreated CRC exosomes. Additionally, there was a significant decrease in the cell proliferation in CRC cells pre-treated with G-CSF compared to untreated CRC cells (p = 0.008). Flow cytometric analysis of BM-MSCs showed that G-CSF could attenuate their transformation into CSCs.
Conclusion
G-CSF can be a promising therapeutic agent for CRC treatment.
Similar content being viewed by others
1 Background
Colorectal cancer (CRC) is one of the main causes of cancer deaths as there is about 1.4 million new cases yearly and one million new deaths yearly worldwide [1]. The microenvironment of the tumor is made up of a variety of cells, such as cancer stem cells (CSCs). CSCs are a group of cells exhibiting a stemness trait, such as the ability to self-renew and differentiate into multiple cell types. These cells are considered to be the cause of recurrence of the tumor and the failure of traditional cancer therapy [2].
Cancer cells release exosomes which are microscopic vesicles that transport biologically active molecules such as deoxyribonucleic acids (DNAs), long non-coding RNAs (LncRNAs), micro RNAs (miRNAs), and proteins [3, 4]. According to research, these exosomes have role in apoptosis, cell cycle progression, cell signaling, as well as cancer initiation and progression [5, 6] and could play a crucial role in transforming BM-MSCs into a tumor-like cells [7].
Overexpression of the metastasis-associated lung adenocarcinoma transcript 1 (MALAT-1)-LncRNA in CRC cells and their derived exosomes was later linked to the cell proliferation of CRC cell, invasion, migration, and metastasis via activation of the beta catenin (β-catenin) gene [8, 9].
Programmed cell death 4 (PDCD4) is a tumor suppressor whose expression is reduced in a variety of malignancies, including CRC. PDCD4 has a role in the inhibition of the cell proliferation PDCD4 as its overexpression inhibits cell proliferation [10].
CRC is often diagnosed at late stage. Chemotherapy and other therapies provide a limited increase of the survival for these patients [11], so there is a big need for discovery of novel therapeutic strategies in order to increase the survival rate of CRC. Granulocyte colony-stimulating factor (G-CSF) can stimulate differentiation and proliferation of myeloid precursor cells into granulocytes so that, it was used to treat neutropenia following cancer chemotherapy [12]. Surprisingly, some studies demonstrated that G-CSF could inhibit the progression of cancer in various cancer types [13,14,15].
This work aims to investigate the effect of G-CSF on CRC cells and to evaluate its capability to prevent CSCs formation induced by CRC derived exosomes.
2 Methods
Our study was an observational study that aimed to investigate the effect of G-CSF on CRC cells and to evaluate its capability to prevent BM-MSCs malignant transformation into CSCs induced by CRC exosomes.
2.1 Colon adenocarcinoma cell culture
Human colorectal adenocarcinoma cell line (CACO-2) as colon cancer cell line was purchased from (Sigma-Aldrich Chemical Co., St. Louis, MO, USA, Cat. No MTOX1000P24). It was cultured in Park Memorial Institute (RPMI -1640) culture media with glutamine 2 mM (bio west, Nampa, cat n L0498-500), 10% Fetal Bovine Serum (FBS) (PAA, Pasching Austria, Cat nA11-151) and Penicillin with Streptomycin 1% (Lonza, Verviers, Belgium, CatN DE17-602E). CaCo-2 cells were grown in 5% CO2, 95% air at 37 °C (NEW BRUNSWICK SCIENTIFIC-Innova co-170). Cells were washed with cold phosphate buffer saline (PBS), trypsinized, harvested and centrifuged to form cell pellets. Then cells were divided into two groups; 1st group: untreated CRC cells, and the 2nd group: CRC cells treated with G-CSF (sigma-Aldrich Chemical Co., St. Louis, MO, USA, MDL number: MFCD00166475) at a single dose of 20 ng/ml at a dose of 20 ng/ml [16]. CRC cells from both groups were suspended for further MTT assay and exosomal extraction; then exosomes from both groups were harvested for molecular studies, and further application on BM-MSCs.
2.2 MTT cell proliferation assay
In order to investigate the anti-proliferative effect of G-CSF on CACO-2 cells (4,5-dimethylthiazol, 2,5-diphenyltetrazolium bromide (MTT) assay was used. We fixed CACO-2 cells (for 24 h) with the frequency of (1 × 106) cells into 96 well plates. The plate was divided equally into two groups as mentioned before. MTT reagent was added to the wells in line with the manufacturer’s instructions (Biospes, China, Cat n#BAR1005-1), detergent reagent was added (100 µl per well) when the purple precipitate was visible clearly, in order to make the formazan dye soluble. In the dim, plates were left with cover for 2 to 4 h. The cover of the plate was detached and we assessed the absorbance in each well at a range from 490 to 630 nm by using ELISA plate reader (Stat Fax 2200, Awareness Technologies, Florida, USA). Amount of absorbance was proportional to cell number.
2.3 Isolation, identification, and quantitation of exosomes derived from cultured cancer cells
Exosomes from both groups were isolated through differential centrifugation at 3000g to get rid of cells then 10,000g for 20 min to remove the debris, at 4° ultracentrifugation was done at 100,000g to cell-free supernatants (Beckman Coulter Optima L-90K ultracentrifuge) for 90 min, the supernatant was removed carefully, and crude exosome-containing pellets were re-suspended in 1 mL of ice-cold PBS and pooled. A second ultracentrifugation in the same conditions was preformed [17]. The exosomes’ protein content was quantified by Bradford method (BioRad, Hercules, CA, USA).
2.4 Extraction of exosomal RNAs from both studied CRC cells groups
A total RNA was extracted from exosomes derived from both groups by miRNeasy mini kit provided by Qiagen, Germany (Cat no: 217084). By using Beckman dual spectrophotometer (USA) at 260 nm, the total RNA obtained was determined.
2.5 Quantitative reverse transcriptase—polymerase chain reaction (qRT-PCR)
Reverse transcription and PCR amplication for mRNA expression was done using kit provided by Vivantis, Vi Prime PLUS One Step thermus aquaticus (Taq) RT-qPCR Green Master Mix I with ROX (SYBR Green Dye) (cat no #QLMM14-R) according to the manufacturer’s protocol. One Step Taq qRT-PCR Green Master Mix I with ROX Kit was compatible with three-step cycling, reverse transcription occurs for 10 min at 55 °C as one cycle, enzyme activation for 8 min at 95 °C as one cycle, denaturation occurs for 10 s at 95 °C and both annealing and extension for 60 s at 60 °C for forty cycles. The data were expressed in Cycle threshold (Ct) after the run of the RT-PCR. According to the calculation of delta-delta Ct (ΔΔCt), the quantification of the RQ of each target gene is done by normalization against house-keeping gene glyceraldehyde 3 phospate dehydrogenase for (mRNAs and LncRNA MALAT-1). Table 1 presented the sequence of the primer of all studied genes and LncRNA.
2.6 Preparation and isolation of human BM-MSCs
Under aseptic conditions, bone marrow (20 mL) was aspirated from the iliac crest and kept in heparinized tubes. A density gradient (Ficoll-Paque; GE HealthCare, Waukesha, WI) was used to separate nucleated cells and then re-suspended in culture medium (Delbecco’s Modified Eagle’s Medium) which was supplemented with 10% FBS and 1% penicillin–streptomycin (10,000 g/mL). Then the culture of the cells was done at 37 °C for 14 days in 5% CO2. The culture media was replaced every 2 to 3 days When the cells had reached 80–90%, the cultures were washed using PBS twice and trypsinized at 37 °C for 5 min with 0.25% Trypsin in 1 mM EDTA. Cells were re-suspended in media and subcultured for 10 days after centrifugation, so that an average count of 15 × 106 cells was obtained.
2.7 Co-culture of BM-MSCS with CRC derived exosomes
BM-MSCS were divided as following into three groups: Group 1: untreated BM-MSCs (1 × 106) as a control group; Group 2: BM-MSCs (1 × 106) co-cultured with 100 µg/ml of exosomes derived from untreated CRC cells [18]; Group 3: BM- MSCs (1 × 106) co-cultured with 100 µg/ml of exosomes derived from CRC cells pre-treated with G-CSF [18]. BM-MSCs from the three studied groups were incubated for 24 h. BM-MSCs malignant transformation into CSCs was assessed by immunophenotyping analysis for CSCs markers (CD44 and 133) expression.
2.8 Statistical analysis
IBM® SPSS® Statistics Version 25 was used for statistical analysis. The mean and standard deviation of numerical data were presented. The paired sample t-test was used to compare G-CSF-treated and untreated CRC cells. One-way ANOVA was used to compare the three BM-MSC groups, followed by a Tukey post hoc test for multiple group comparisons [19]. The significance level was set at P ≤ 0.05 within all tests.
3 Results
3.1 Co-culture of CRC cells with G-CSF
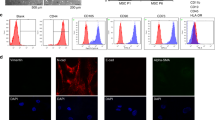
The morphological observations using inverted light microscopy in untreated CRC cells group revealed adherent growth with an increase in cell population (Fig. 1A), while there was a decrease in cell population, cell rounding and detachment in CRC cells pre-treated with G-CSF (Fig. 1B).
A untreated CRC cells; B CRC cells treated with G-CSF; showing decrease in the number of CRC cells, black arrows indicate cell rounding and detachment which are signs of apoptosis, C exosomes from untreated CRC cells, D exosomes from G-CSF treated CRC cells by TEM showing their cup shaped morphology and size (100 nm), scale bar (2 µm)
3.2 Identification of CRC derived exosomes from both studied groups
Exosomes from both untreated CRC cells and CRC cells pre-treated with G-CSF were identified by TEM according to their size (100 nm) and their morphology which is cup shaped as shown in (Fig. 1C, D).
3.3 G-CSF significantly inhibits CRC cell proliferation by MTT assay
The cell proliferation in CRC cells pre-treated with G-CSF was significantly decreased compared to untreated CRC cells (P = 0.008) (Fig. 2A).
3.4 G-CSF represses the expression of LncRNA MALAT-1 and β catenin and induces the expression of PDCD4
Concerning the expression levels of LncRNA MALAT-1 and β- catenin gene there were a significant decrease in their expression in the exosomes derived from CRC cells pre-treated with G-CSF compared to those derived from untreated CRC cells group (P = 0.014 and 0.01, respectively). (Fig. 2B, C).
Regarding PDCD4 gene expression there was a significant increase in its expression in exosomes derived from CRC cells pre-treated with G-CSF compared to exosomes derived from untreated CRC cells (P = 0.018) (Fig. 2C).
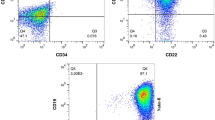
3.5 G-CSF could attenuate BM-MSCs transformation into CSCs
As shown by Flow cytometric analysis for CD markers of CSCs (CD44 and 133) in BM-MSCs studied groups, the CSCs markers CD44 and CD133 were significantly increased in BM-MSCs co-cultured with exosomes from untreated CRC cells group compared to other groups (P = 0.001, P = 0.001, respectively), whereas no significant difference was found between BM-MSCs co-cultured with exosomes from CRC cells pre-treated with G-CSF and BM-MSCs control group (P = 0.8, P = 0.99) (Fig. 3A, B).
3.6 The anti-proliferative effect of G-CSF is demonstrated by MTT cell proliferation in BM-MSCs
The cell proliferation in BM-MSCs co-cultured with exosomes from untreated CRC cells group was significantly increased compared to both control group of BM-MSCs and BM-MSCs co-cultured with exosomes from CRC cells pre-treated with G-CSF (P = 0.005; P = 0.002, respectively), whereas no significant difference was found in cell proliferation between BM-MSCs control group and BM-MSCs co-cultured with exosomes from CRC cells pre-treated with G-CSF (P = 0.85) (Fig. 3C).
4 Discussion
Throughout the years, CRC has been considered the third most diagnosed cancer and the second cause of death related to cancer globally [20]. Despite the current therapy available for CRC, the cancer prognosis for patients has not been satisfying, especially for patients with metastatic tumors [21]. Thus, it is crucial to have new alternative effective treatment strategies for CRC patients.
Furthermore, CRC cells release high amounts of nanovesicles (exosomes), enclosing proteins, DNA, miRNA, and LncRNA which play an important role in regulating tumor growth, angiogenesis, and metastasis [22]. Besides CRC cells derived exosomes have been shown to enhance malignant transformation of colonic stromal MSCs into CSCs and thus, promoting tumor growth and progression [23].
G-CSF, which is a key regulator of neutrophil production and activity, is commonly used to prevent and treat febrile neutropenia following chemotherapy and radiotherapy in cancer patients [24]. Surprisingly, recent studies have revealed that G-CSF has valuable direct antitumor effects on various types of cancers, including mammary adenocarcinoma [14] and lung cancer [15]. Despite these interesting studies, we have a limited knowledge about the G-CSF’s effects on CRC.
Our study was conducted to investigate the G-CSF’s effect on CRC cells and to evaluate its capability to prevent BM-MSCs malignant transformation into CSCs induced by CRC exosomes.
LncRNAs are considered a type of ncRNA and their length is more than 200 nucleotides whose aberrant expression has been linked to cellular proliferation, differentiation, autophagy, and metastasis [25, 26]. LncRNA MALAT-1 was highly expressed in CRC [27] and promote both tumor growth and metastasis through triggering the expression of β-catenin. Wnt/β-catenin signaling was shown to promote the CSCs’ differentiation greatly [28], that can be progenitors of mature cancer cells, in addition to enhancing epithelial to mesenchymal transition (EMT) [29]. Therefore, targeting this pathway may attenuate the abnormal behavior of stem cell and EMT involved in carcinogenesis.
Interestingly, the results of our study showed a significant decrease in the expression levels of LncRNA MALAT-1 and β catenin in exosomes derived from CRC cells pre-treated with G-CSF in comparison with exosomes derived from untreated cells of CRC, which in turn suggest that G-CSF may inhibit tumor initiation and progression through downregulation of LncRNA MALAT-1/β-catenin axis.
The tumor suppressor, PDCD4 that slows down the benign and malignant tumors’ progression by inhibiting AP-1 transactivation and the translation machinery, in addition to induction of apoptosis [30]. According to recent studies, it has been reported that in CRC, the expression of PDCD4 is decreased or even completely absent [31]. Accordingly, upregulation of PDCD4 expression can represent a promising therapeutic strategy in CRC’ treatment.
Our present study’s results demonstrated that expression of PDCD4 was significantly increased in CRC exosomes pre-treated with G-CSF compared to the exosomes from untreated CRC cells suggesting that G-CSF may exhibit direct antitumor effect on CRC through upregulating PDCD4 expression. Besides, MTT cell proliferation assessment showed that cell proliferation in CRC cells pre-treated with G-CSF was significantly decreased in comparison with the untreated CRC cells group, thus, emphasizing the anti-tumoral activity of G-CSF on CRC.
MSCs are multipotent cells, replicating as undifferentiated cells, in addition to their ability to differentiate into mesenchymal tissue lineages such as; adipocytes, osteocytes, and chondrocytes [32]. MSCs are recognized as vital constituents of the tumor microenvironment, that can augment cell proliferation, invasion and metastasis of the cancer, and also enhancing angiogenesis, and suppressing anti-tumor immune responses [33]. These diverse effects are the result of mutual interactions between MSCs and cancer cells via cancer cells derived exosomes [34].
Accumulating evidence indicates that CD44, 133 are colorectal CSCs markers and are crucial factors in regulating the properties of CSCs, including self-renewal, metastasis, and chemo-radio-resistance [35, 36].
Consistent with the previous findings, in our work the BM-MSCs’ isolation was successful, confirmed by their positivity for CD90 and CD105 surface markers, besides, FACS analysis of BM-MSCs co-cultured with exosomes derived from untreated CRC cells presented significant increase in CSCs markers’ expression; CD44 and CD133 compared to BM-MSCs control group and to BM-MSCs co-cultured with exosomes derived from cells of CRC pre-treated with G-CSF, thus emphasizing the capability of CRC derived exosomes to induce BM-MSCs malignant transformation into CSCs.
However, no significant difference was shown in the expression levels of CD44 and CD 133 between the BM-MSCs control group and BM-MSCs co-cultured with exosomes derived from cells of CRC pre-treated with G-CSF, indicating that the capability of CRC exosomes to induce BM-MSCs malignant transformation into CSCs could be attenuated by G-CSF.
Moreover, MTT cell proliferation assessment showed that the the cell proliferation in BM-MSCs co-cultured with exosomes derived from untreated cells of CRC group was significantly increased compared to BM-MSCs control group and to BM-MSCs co-cultured with exosomes from CRC cells pre-treated with G-CSF group, while there was no difference existed significantly in cell proliferation between BM-MSCs co-cultured with exosomes derived from CRC cells pre-treated with G-CSF group and BM-MSCs control group, thus confirming the capability of G-CSF to attenuate the ability of CRC cells derived exosomes to induce higher proliferation rate and malignant transformation of BM-MSCs into CSCs.
5 Conclusion
In conclusion, our present study clearly demonstrated that GCSF could exhibit direct antitumor effects on colorectal adenocarcinoma cell line CaCo-2; moreover, G-CSF could attenuate the potentiality of exosomes derived from CRC cells to induce malignant transformation of BM-MSCs into CSCs, thus, providing a new approach for the CRC’s treatment.
Availability of data and materials
Data generated or analyzed during this study are available from the corresponding author upon reasonable request.
Abbreviations
- CRC:
-
Colorectal cancer
- G-CSF:
-
Granulocyte colony stimulating factor
- BM-MSCs:
-
Bone marrow-derived mesenchymal stem cells
- CSCs:
-
Cancer stem cells
- PDCD4:
-
Programmed cell death 4
- MALAT-1:
-
Lung adenocarcinoma transcript 1
- CACO-2:
-
Human colorectal adenocarcinoma cell line
References
Rawla P, Sunkara T, Barsouk A (2019) Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol 14(2):89–103. https://doi.org/10.5114/pg.2018.81072
Xu J, Liao K, Zhou W (2018) Exosomes regulate the transformation of cancer cells in cancer stem cell homeostasis. Stem Cells Int 23(2018):4837370. https://doi.org/10.1155/2018/4837370
Demory Beckler M, Higginbotham J, Franklin J, Ham A, Halvey P, Imasuen I, Whitwell C, Li M, Liebler D, Coffey R (2013) Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics 12(2):343–355. https://doi.org/10.1074/mcp.M112.022806
Asghar S, Litherland G, Lockhart J, Goodyear C, Crilly A (2020) Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford) 59(1):57–68. https://doi.org/10.1093/rheumatology/kez462
Ren R, Sun H, Ma C, Liu J, Wang H (2019) Colon cancer cells secrete exosomes to promote self-proliferation by shortening mitosis duration and activation of STAT3 in a hypoxic environment. Cell Biosci 6(9):62. https://doi.org/10.1186/s13578-019-0325-8
Xiao Y, Zhong J, Zhong B, Huang J, Jiang L, Jiang Y, Yuan J, Sun J, Dai L, Yang C, Li Z, Wang J, Zhong T (2020) Exosomes as potential sources of biomarkers in colorectal cancer. Cancer Lett 476:13–22. https://doi.org/10.1016/j.canlet.2020.01.033
Lin L, Du L, Cao K, Huang Y, Yu P, Zhang L, Li F, Wang Y, Shi Y (2016) Tumour cell-derived exosomes endow mesenchymal stromal cells with tumour-promotion capabilities. Oncogene 35(46):6038–6042. https://doi.org/10.1038/onc.2016.131
Ji Q, Liu X, Fu X, Zhang L, Sui H, Zhou L, Sun J, Cai J, Qin J, Ren J, Li Q (2013) Resveratrol inhibits invasion and metastasis of colorectal cancer cells via MALAT1 mediated Wnt/β-catenin signal pathway. PLoS One 8(11):e78700. https://doi.org/10.1371/journal.pone.0078700
Xu J, Xiao Y, Liu B, Pan S, Liu Q, Shan Y, Li S, Qi Y, Huang Y, Jia L (2020) Exosomal MALAT1 sponges miR-26a/26b to promote the invasion and metastasis of colorectal cancer via FUT4 enhanced fucosylation and PI3K/Akt pathway. J Exp Clin Cancer Res 39(1):54. https://doi.org/10.1371/journal.pone.0078700
Wang Q, Zhu J, Wan Y et al (2017) Tumor suppressor Pdcd4 attenuates Sin1 translation to inhibit invasion in colon carcinoma. Oncogene 36:6225–6234. https://doi.org/10.1038/onc.2017.228
Van der Jeught K, Xu H, Li Y, Lu X, Ji G (2018) Drug resistance and new therapies in colorectal cancer. World J Gastroenterol 24(34):3834–3848. https://doi.org/10.3748/wjg.v24.i34.3834
Mehta H, Malandra M, Corey S (2015) G-CSF and GM-CSF in Neutropenia. J Immunol 195(4):1341–1349. https://doi.org/10.4049/jimmunol.1500861
Bottoni U, Trapasso F (2009) The role of G-CSF in the treatment of advanced tumors. Cancer Biol Ther 8(18):1744–1746. https://doi.org/10.4161/cbt.8.18.9453
Marino J, Furmento V, Zotta E, Roguin L (2009) Peritumoral administration of granulocyte colony-stimulating factor induces an apoptotic response on a murine mammary adenocarcinoma. Cancer Biol Ther 8(18):1737–1743. https://doi.org/10.4161/cbt.8.18.9210
Li Y, Ohno Y, Funaguchi N, Gomyo T, Sasaki Y, Toyoshi S, Kaito D, Yanase K, Endo J, Ito F, Kawasaki M, Minatoguchi S (2017) Granulocyte colony-stimulating factor enhances the anticancer effects of cisplatin against lung cancer by promoting angiogenesis. Adv Lung Cancer 6(1):1–11. https://doi.org/10.4236/alc.2017.61001
Liu L, Wu Y, Zhang C, Zhou C, Li Y, Zeng Y, Zhang C, Li R, Luo D, Wang L, Zhang L, Tu S, Deng H, Luo S, Chen Y, Xiong X, Yan X (2020) Cancer-associated adipocyte-derived G-CSF promotes breast cancer malignancy via Stat3 signaling. J Mol Cell Biol 12(9):723–737. https://doi.org/10.1093/jmcb/mjaa016
Lobb R, Becker M, Wen S, Wong C, Wiegmans A, Leimgruber A, Möller A (2015) Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles 4:27031. https://doi.org/10.3402/jev.v4.27031
Shahir M, Mahmoud Hashemi S, Asadirad A, Varahram M, Kazempour-Dizaji M, Folkerts G, Garssen J, Adcock I, Mortaz E (2020) Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J Cell Physiol 235(10):7043–7055. https://doi.org/10.1002/jcp.29601
Chan Y (2003) Biostatistics 102: quantitative data–parametric & non-parametric tests. Singapore Med J 44(8):391–396
Florescu-Ţenea R, Kamal A, Mitruţ P, Mitrut R, Ilie D, Nicolaesu A, Mogoanta L (2019) Colorectal cancer: an update on treatment options and future perspectives. Curr Health Sci J 45(2):134–141. https://doi.org/10.12865/CHSJ.45.02.02
Xie Y, Chen Y, Fang J (2020) Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct Target Ther 5(1):22. https://doi.org/10.1038/s41392-020-0116-z
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, Tao Y, He Z, Chen C, Jiang Y (2020) Exosomes: key players in cancer and potential therapeutic strategy. Sig Transduct Target Ther 5(1):145. https://doi.org/10.1038/s41392-020-00261-0
Lugini L, Valtieri M, Federici C, Cecchetti S, Meschini S, Condello M, Signore M, Fais S (2016) Exosomes from human colorectal cancer induce a tumor-like behavior in colonic mesenchymal stromal cells. Oncotarget 7(31):50086–50098. https://doi.org/10.18632/oncotarget.10574
Liu F, Du Y, Cai B, Yan M, Yang W, Wang Q (2017) A clinical study of polyethylene glycol recombinant human granulocyte colony-stimulating factor prevention neutropenia syndrome in patients with esophageal carcinoma and lung cancer after concurrent chemoradiotherapy. J Cancer Res Ther 13(5):790–795. https://doi.org/10.4103/jcrt.JCRT_320_17
Schmitz S, Grote P, Herrmann B (2016) Mechanisms of long noncoding RNA function in development and disease. Cell Mol Life Sci 73:2491–2509. https://doi.org/10.1007/s00018-016-2174-5
He X, Yan Q, Kuang G, Wang Y, Cao P, Ou C (2018) Metastasis-associated lung adenocarcinoma transcript 1 regulates tumor progression: old wine in a new bottle. J Thorac Dis 10(9):S1088–S1091. https://doi.org/10.21037/jtd.2018.04.13
Zhang C, Yao K, Zhang J, Wang C, Wang C, Qin C (2020) Long noncoding RNA MALAT1 promotes colorectal cancer progression by acting as a ceRNA of miR-508-5p to regulate RAB14 expression. Biomed Res Int 2020:4157606. https://doi.org/10.1155/2020/4157606
Shang S, Hua F, Hu Z (2017) The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget 8(20):33972–33989. https://doi.org/10.18632/oncotarget.15687
Liang J, Liang L, Ouyang K, Li Z, Yi X (2017) MALAT1 induces tongue cancer cells’ EMT and inhibits apoptosis through Wnt/β-catenin signaling pathway. J Oral Pathol Med 46(2):98–105. https://doi.org/10.1111/jop.12466
Matsuhashi S, Manirujjaman M, Hamajima H, Ozaki I (2019) Control mechanisms of the tumor suppressor PDCD4: expression and functions. Int J Mol Sci 20(9):2304. https://doi.org/10.3390/ijms20092304
Long J, Yin Y, Guo H, Li S, Sun Y et al (2019) The mechanisms and clinical significance of PDCD4 in colorectal cancer. Gene 680:59–64. https://doi.org/10.1016/j.gene.2018.09.034
Ghaneialvar H, Soltani L, Rahmani H, Lotfi A, Soleimani M (2018) Characterization and classification of mesenchymal stem cells in several species using surface markers for cell therapy purposes. Indian J Clin Biochem 33(1):46–52. https://doi.org/10.1007/s12291-017-0641-x
Zhang T, Lee Y, Rui Y, Cheng T, Jiang X, Li G (2013) Bone marrow-derived mesenchymal stem cells promote growth and angiogenesis of breast and prostate tumors. Stem Cell Res Ther 4(3):70. https://doi.org/10.1186/scrt221
Lopatina T, Gai C, Deregibus M, Kholia S, Camussi G (2016) Cross talk between cancer and mesenchymal stem cells through extracellular vesicles carrying nucleic acids. Front Oncol 6:125. https://doi.org/10.3389/fonc.2016.00125
Yan Y, Zuo X, Wei D (2015) Concise review: emerging role of CD44 in cancer stem cells: a promising biomarker and therapeutic target. Stem Cells Transl Med 4(9):1033–1043. https://doi.org/10.5966/sctm.2015-0048
Chen S, Song X, Chen Z, Li X, Li M, Liu H, Li J (2013) CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLOS ONE 8(2):e56380. https://doi.org/10.1371/journal.pone.0056380
Acknowledgements
We would like to thank the research team of the Molecular Biology unit at the fourth floor, faculty of medicine, Cairo University.
Funding
This research was supported and funded by Kasr Alainy, Faculty of Medicine, Cairo University.
Author information
Authors and Affiliations
Contributions
Conceptualization: AM. Methodology and validation: AA, DS, MM and AM, Data curation: AM and MM. Writing—original draft preparation: MM, AA and AM Writing—review and editing: AA, and AM. Supervision, DS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study was ethically approved by the Ethical Committee of Faculty of Medicine, Cairo University, Egypt, on 21/1/2019. Consent to participate is not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, A.A., Monir, M., Sabry, D. et al. In vitro study to evaluate the effect of granulocyte colony stimulating factor on colorectal adenocarcinoma and on mesenchymal stem cells trans differentiation into cancer stem cells by cancer cells derived exosomes. Beni-Suef Univ J Basic Appl Sci 12, 31 (2023). https://doi.org/10.1186/s43088-023-00351-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00351-2