Abstract
Background
This study aimed to determine the association of diametrical antral follicles with the ovarian response by Follicular Output Rate (FORT) ratio in 100 females undergoing in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI). This study selected 100 women undergoing controlled ovarian hyperstimulation (COH). The number of antral follicles (diameter of 3–10 mm) was calculated with two-dimensional vaginal sonography on days 1–3 of the cycle. Then, on a triggering day with human chorionic gonadotropin (HCG), the number of follicles (with a diameter of 16–22 mm) and the ratio of FORT were determined. The correlation among FORT with age, antral follicle count (AFC), AFC ≤ 5, AFC > 5, number of preovulatory follicles (16–20 mm), number of metaphase II (MII) oocytes, body mass index (BMI), infertility period, and anti-Mullerian hormone (AMH) was assessed.
Results
There was a significant correlation between FORT and total AFC, AFC > 5, number of preovulatory follicles (16–20 mm), and number of MII oocytes retrieved. There is no significant relationship between FORT and examined two variables (AMH and AFC ≤ 5). Multiple linear regression analysis showed no significant relationship between FORT and examined two variables (AMH and AFC > 5). There was a significant correlation between MII oocytes retrieved and age, total AFC, AFC ≤ 5, AFC > 5, number of preovulatory follicles (16–20 mm), and AMH. A significant positive relationship existed between MII oocytes retrieved and examined two variables (AFC ≤ 5 and AFC > 5).
Conclusion
There was not much difference in the correlation between the AFC ≤ 5 and AFC > 5, and both positively correlated with the number of MII oocytes retrieved.
Similar content being viewed by others
1 Background
Follicular harvesting and optimization of the response to controlled ovarian hyperstimulation (COH) with gonadotropins are essential in treating infertility with assisted reproductive techniques [1]. Prediction of this response, assessed by ovarian reserve, is critical for prognosis and patient-specific treatment. Specifying treatment requires the selection of the best gonadotropin-releasing hormone (GnRH) analog protocol and the best initial dose of gonadotropin to achieve the ideal ovarian response because it is known that both potentially low and excessive responses lead to cycle cancellation and increased costs [2]. While a low ovarian response may reduce the risk of pregnancy, an exaggerated response indicates the risk of ovarian hyperstimulation syndrome [3,4,5]. Ovarian storage has complex mechanisms of age, genetics, and environmental factors [6]. Several ovarian reserve markers have been studied in the last few decades, but no ideal marker remains. Prediction of response through ovarian reserve examination is superior only through calendar age. At present, the number of antral follicles (AFC) and anti-Mullerian hormone (AMH) have the best sensitivity and specificity for predicting ovarian response, despite the presence of a false positive rate of 10–20% [7]. Because ovarian response appears to be influenced by many factors, for determination of the individual starting dose of FSH in in vitro fertilization (IVF) cycles, nomograms are mainly based on age and AMH, AFC, and follicle-stimulating hormone (FSH) levels during the third menstrual period. This situation leads to a reduction in costs and an improvement in the possibility of pregnancy [8, 9].
The ovarian response is one of the most commonly studied parameters in clinical trials on IVF treatment [10]. Traditionally, the number of oocytes retrieved is the main result of the ovary responding to gonadotropin stimulation [5]. However, the number of obtained preovulatory follicles at the end of COH is not a reliable reflection of the sensitivity of the antral follicle to FSH. It correlates highly with the number of antral follicles before ovarian stimulation [11]. The diameter of antral follicles and the number of antral follicles may also be critical. The FORT evaluates the ratio of FSH-responsive follicles. These parameters can be calculated as below:
This ratio is associated with IVF outcomes, including pregnancy rates [12]. Differentiation of different types of size may be related because several studies show that the endocrine function of a follicle is related to its diameter [13, 14]. A previous study reported that pregnant patients had the most significant number of antral follicles between 5 and 10 mm. Another study showed that the number of follicles with a diameter of 2–6 mm decreases with age, but the number of follicles with a diameter of 7–10 mm is constant. This situation suggests that follicles ≤ 6 mm may show ovarian function capacity. Bestow et al. showed a negative correlation between FORT and AFC. This group showed an inverse correlation between FORT and AFC at a significant level [15]. Bayu et al. showed that AFC ≥ 5 had a higher significant effect on pregnancy rate than the AFC < 5 in IVF [16]. Due to the small number of available studies in this field and to confirm or reject the limited results obtained in studies such as Bessow et al. and Bayu et al. study, this study was designed to evaluate the correlation between antral follicle diameter and FORT in women under controlled ovarian hyperstimulation for assisted reproductive techniques.
2 Methods
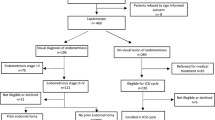
In this prospective longitudinal cross-sectional study, 100 women (with an age range of 20–40) referred to the infertility center of Isfahan University of medical sciences (between September 2021 and April 2022) who underwent COH were selected. Sampling was done by census method, and all patients who met the study criteria were included in the study in the mentioned period. Inclusion criteria were: age under 40 years, presence of both ovaries, absence of cyst and endometrium, no history of ovarian surgery, and performance of 2-dimensional transvaginal ultrasound with sufficient visualization. The exclusion criteria were: clinical signs of hyperandrogenism, polycystic ovary syndrome, hyperprolactinemia and hypothyroidism, and patients with CAH. Patients are admitted for initiation of the GnRH antagonist protocol for COH. Demographic variables (age, AMH, and BMI) are determined before the start of the cycle. The number of antral follicles (with a diameter of 2–10 mm) is calculated by two-dimensional vaginal sonography (supersonic imagine-aixplorer ultimate) on days 1–3 of the cycle. In this study, AFC count was divided into follicles with a diameter of 5 mm or less and those with a diameter greater than 5 mm. The follicles with a diameter of 5 mm or less are represented by AFC ≤ 5, and those with a diameter larger than 5 mm and up to 10 mm were represented by AFC > 5. For all patients, the GnRH antagonist protocol was used between days 1–3 of the menstrual cycle. The dose of Human Menopausal Gonadotropin (HMG) (Menotropin, Ferring) and recombinant FSH (Cinnal f, Follitropin alfa, Cinnagen, Tehran, Iran) was assessed based on patient weight, AMH, and previous response to ovarian stimulation. The dose range was between 150 and 300 IU. CETROTIDE 0.25 mg (GnRH antagonist, Serpero) was started when a follicle reached 14 mm. hCG 5000-10000 IU (human chorionic gonadotropin, Pregnyl, Organon, Netherlands) was injected after observation of three preovulatory follicles with a diameter of 16–22 mm. Oocytes were retrieved by transvaginal ultrasound-guided aspiration 36 h after hCG administration, and the embryologist determined the number of MII oocytes on the same day. On triggering day with HCG, the number of follicles 16–22 mm is determined, and the ratio of FORT was calculated. FORT calculation was done as the below formula:
It is noted that one specialist did vaginal sonography. After collecting the data, the information was entered into SPSS 25 (IBM, ARMONK NEW YORK, NY, USA). Mean and standard deviation were used to describe quantitative data, and frequency and percentage will be used for qualitative data. Pearson and Spearman’s tests were used to analyze the data in terms of data distribution.
2.1 Ethical consideration
This study was approved by the Ethics committee of Isfahan University of Medical Sciences (registration number: IR.MUI.MED.REC.1400.413).
3 Results
This study was done on 100 women. The demographic and reproductive data are summarized in Table 1. Based on this table, the mean age of patients was 32.56 ± 4.7. The median for infertility period, BMI, and AMH was 6, 21, and 1.8, respectively. The mean of FORT (%), AFC ≤ 5, AFC > 5, and the number of MII oocytes was 71 ± 21.4, 4.28 ± 2.5, 5.14 ± 3.29, and 6.99 ± 4.8, respectively. The correlation between FORT and other related variables is shown in Table 2. Based on this table, there was a significant correlation between FORT and total AFC, AFC > 5, number of preovulatory follicles (16–20 mm), and number of MII oocytes retrieved (P < 0.05). There was no significant correlation between FORT with AFC > 5 and AFC ≤ 5 (P > 0.05). Other related variables (BMI, infertility period, AMH) also had no significant correlation with FORT (P > 0.05). For verification of the relationship between the AFC > 5 and FORT, multivariable linear regression was done. Acquired data are summarized in Table 3. This table indicated no significant relationship between FORT and examined two variables (AMH and AFC > 5). The correlation between the number of oocytes retrieved and other related variables is also shown in Table 4. Based on this table, there was a significant correlation between the number of MII oocytes retrieved and age, total AFC, AFC ≤ 5, AFC > 5, number of preovulatory follicles (16–20 mm), and AMH (P < 0.05). BMI and infertility period had no significant correlation with MII oocytes retrieved (P > 0.05). For verification of the relationship between the AFC and the number of MII oocytes retrieved, multivariable linear regression was done. Acquired data are summarized in Table 5. This table indicated a significant positive relationship between the number of MII oocytes retrieved and examined two variables (AFC ≤ 5 and AFC > 5). There was not much difference in the correlation between the AFC ≤ 5 and AFC > 5, and both positively correlated with the number of MII oocytes retrieved. The scatter chart between FORT with AFC ≤ 5 and AFC > 5 is shown in Fig. 1. This figure showed a direct relationship of FORT with AFC ≤ 5 and AFC > 5.
4 Discussion
This study evaluated the correlation between antral follicular count and diameter with FORT in women under controlled ovarian hyperstimulation for assisted reproductive technology (ART). Some studies considered the antral follicle count as a predictive marker in this field [17, 18]. According to previous studies, there is a positive relationship between FORT and outcomes of IVF. Thus, FORT can be considered a suitable marker for follicular responsiveness [19,20,21]. There are few studies about the correlation between FORT and demographic and related factors, as well as antral follicle size in ovarian responsiveness. Our study showed that there was no significant correlation between FORT and age. Grynberg et al. showed a similar result. This group also indicated that FORT was not significantly associated with age [22]. Similar to our study, Bessow et al. showed no correlation between FORT and age BMI [15]. There was a significant positive correlation between FORT and total AFC and AFC > 5 and the number of MII oocytes retrieved. However, no correlation was observed in regression analysis between AFC > 5 and FORT. According to Broer et al. [23] and Papathanasiou et al. study [24], AFC < 5, AFC > 5, and AMH are not sufficient for accurate prediction of ovarian response. We also showed that these variables had no significant correlation with ovarian response. Bestow et al. showed a negative correlation between the FORT and total AFC and the FORT and AFC < 6 and no correlation between FORT and AFC > 6 [15]. We considered number five as a cutoff for AFC. Unlike Bessow et al., we did not observe a negative correlation between FORT and AFC. In our study, a significant positive correlation was observed. Headsman et al. introduced follicular diameter as an effective parameter for the ovarian response. This group showed that AFC ≤ 6 correlated with ovarian responsiveness [14]. We used a better parameter, e.g., FORT. Grynberg et al. showed that FORT could be considered an efficient quantitative and qualitative marker of ovarian response during controlled ovarian stimulation (COS) [22]. These data are similar to our data. We also indicated that FORT positively correlates with AFC in women under controlled ovarian hyperstimulation for ART. Based on our acquired data, there is a significant correlation between the number of MII oocytes retrieved and age, total AFC, AFC ≤ 5, AFC > 5, number of preovulatory follicles (16–20 mm), and AMH. For verification of the relationship between the AFC and the number of MII oocytes retrieved, multivariable linear regression was done. The result showed a significant positive relationship between the number of MII oocytes retrieved and examined two variables (AFC ≤ 5 and AFC > 5). There was not much difference in the correlation between the AFC ≤ 5 and AFC > 5, and both had an almost equally positive correlation with the number of MII oocytes retrieved. An increase in age leads to a decrease in MII oocytes retrieved [25, 26]. Similarly, we showed a negative correlation between age and MII oocytes retrieved. Various studies indicated a positive correlation between MII oocytes retrieved and AFC, number of preovulatory follicles (16–20 mm), and AMH [27, 28]. In some studies, this correlation is significant. Some studies showed non-significant relationship [27,28,29,30,31,32]. Our study showed that all mentioned variables significantly positively correlated with MII oocytes retrieved. The ovarian response has an essential effect on the number of oocytes retrieved. This effect can be explained through Fort. AFC can be considered an independently associated factor in the oocyte retrieval cycle [20, 33, 34]. We showed a significant correlation between the number of MII oocytes retrieved and FORT. We also showed a significant correlation between the number of MII oocytes retrieved with total AFC, AFC ≤ 5, AFC > 5, and AMH.
In our study, four specific goals were pursued:
-
Determining the relationship between the number of antral follicles and the FORT ratio in women under COH showed a positive correlation (0.114) between FORT and AFC. Bestow et al. observed the negative correlation between FORT and AFC. This group showed an inverse correlation between FORT and AFC at a significant level [15].
-
Determining the relationship between antral follicle diameter and FORT ratio in women undergoing COH for IVF. The follicular size is considered an essential parameter for follicles [13, 14]. Our study divided the AFC count into AFC ≤ 5 and AFC > 5. Data analysis showed that FORT and AFC > 5 and FORT and AFC ≤ 5 were directly correlated at significant and non-significant levels, respectively. However, after regression, data showed no significant correlation between AFC > 5 and FORT.
-
Determining the relationship between age and FORT ratio in women undergoing COH for IVF: Different parameters can change ovarian reserve. One of these parameters is age [35]. However, there is no ideal parameter for ovarian reserve [36]. Grynberg et al. and Bessow et al. showed that FORT had not significantly associated with age [15, 22]. We acquired a similar result.
-
Determining the relationship between AMH levels and FORT ratios in women undergoing COH for IVF: AMH is a sensitive and specific variable for predicting ovarian response [37, 38].
In our study, the median AMH level was 1.8 ng/mL. We found a non-significant positive relationship between FORT and AMH. Based on Grynberg et al., FORT significantly decreased from the low, average, and high levels of AMH [22]. Genro et al. showed a negative association between AMH levels and FORT [13].
5 Conclusion
This study evaluated the correlation between FORT and total AFC and AFC diameter. Results showed a significant correlation between FORT and total AFC and AFC > 5. However, multiple linear regression analysis showed no significant relationship between FORT and AFC > 5. There is also a significant correlation between the number of MII oocytes retrieved and age, total AFC, AFC ≤ 5, AFC > 5, number of preovulatory follicles (16–20 mm), and AMH. Multiple linear regression analysis also showed that the AFC ≤ 5 and AFC > 5 had an almost equally positive correlation with the number of MII oocytes retrieved. Thus, based on the results, the effects of AFC ≤ 5 and AFC > 5 on follicular responsiveness are similar.
Availability of data and materials
Not applicable.
References
Lai Q, Chen C, Zhang Z, Zhang S, Yu Q, Yang P et al (2013) The significance of antral follicle size prior to stimulation in predicting ovarian response in a multiple dose GnRH antagonist protocol. Int J Clin Exp Pathol 6(2):258–266
La Marca A, Sunkara SK (2014) Individualization of controlled ovarian stimulation in IVF using ovarian reserve markers: from theory to practice. Hum Reprod Update 20(1):124–140
van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE et al (2006) Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online 13(4):476–480
Aboulghar MA, Mansour RT (2003) Ovarian hyperstimulation syndrome: classifications and critical analysis of preventive measures. Hum Reprod Update 9(3):275–289
Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A (2011) Association between the number of eggs and live birth in IVF treatment: an analysis of 400 135 treatment cycles. Hum Reprod 26(7):1768–1774
Tal R, Seifer DB (2013) Potential mechanisms for racial and ethnic differences in anti-Müllerian hormone and ovarian reserve. Int J Endocrinol 2013:818912
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB (2006) A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 12(6):685–718
Lensen SF, Wilkinson J, Leijdekkers JA, La Marca A, Mol BWJ, Marjoribanks J et al (2018) Individualised gonadotropin dose selection using markers of ovarian reserve for women undergoing in vitro fertilisation plus intracytoplasmic sperm injection (IVF/ICSI). Cochrane Database Syst Rev 2(2):Cd012693
Nyboe Andersen A, Nelson SM, Fauser BC, García-Velasco JA, Klein BM, Arce JC (2017) Individualized versus conventional ovarian stimulation for in vitro fertilization: a multicenter, randomized, controlled, assessor-blinded, phase 3 noninferiority trial. Fertil Steril 107(2):387–96.e4
Li HW, Lee VC, Ho PC, Ng EH (2014) Ovarian sensitivity index is a better measure of ovarian responsiveness to gonadotrophin stimulation than the number of oocytes during in-vitro fertilization treatment. J Assist Reprod Genet 31(2):199–203
Tomas C, Nuojua-Huttunen S, Martikainen H (1997) Pretreatment transvaginal ultrasound examination predicts ovarian responsiveness to gonadotrophins in in-vitro fertilization. Hum Reprod 12(2):220–223
Gallot V, Berwanger da Silva AL, Genro V, Grynberg M, Frydman N, Fanchin R (2012) Antral follicle responsiveness to follicle-stimulating hormone administration assessed by the Follicular Output RaTe (FORT) may predict in vitro fertilization-embryo transfer outcome. Hum Reprod 27(4):1066–1072
Genro VK, Grynberg M, Scheffer JB, Roux I, Frydman R, Fanchin R (2011) Serum anti-Müllerian hormone levels are negatively related to Follicular Output RaTe (FORT) in normo-cycling women undergoing controlled ovarian hyperstimulation. Hum Reprod 26(3):671–677
Haadsma ML, Bukman A, Groen H, Roeloffzen EM, Groenewoud ER, Heineman MJ et al (2007) The number of small antral follicles (2–6 mm) determines the outcome of endocrine ovarian reserve tests in a subfertile population. Hum Reprod 22(7):1925–1931
Bessow C, Donato R, de Souza T, Chapon R, Genro V, Cunha-Filho JS (2019) Antral follicle responsiveness assessed by follicular output RaTe(FORT) correlates with follicles diameter. J Ovarian Res 12(1):48
Bayu P, Syam HH (2021) P-604 Effectiveness comparison of antral follicular count (AFC), follicular-output-rate (FORT), follicle-to-oocyte-index (FOI), oocyte-sensitivity-index (OSI), and follicular-sensitivity-idex (FSI) for predicting clinical pregnancy rates in IVF. Hum Reprod 36(1):1306
Vrontikis A, Chang PL, Kovacs P, Lindheim SR (2010) Antral follice counts (AFC) predict ovarian response and pregnancy outcomes in oocyte donation cycles. J Assist Reprod Genet 27(7):383–389
Coelho Neto MA, Ludwin A, Borrell A, Benacerraf B, Dewailly D, da Silva CF et al (2018) Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound Obstet Gynecol 51(1):10–20
Solernou R, Peralta S, Casals G, Guimera M, Solsona M, Borras A et al (2021) The Follicular Output Rate (FORT) as a method to evaluate transdermal testosterone efficacy in poor responders. JBRA Assist Reprod 25(2):229–234
Li A, Zhang J, Kuang Y, Yu C (2020) Analysis of IVF/ICSI-FET outcomes in women with advanced endometriosis: influence on ovarian response and oocyte competence. Front Endocrinol 11:427
Zhang N, Hao CF, Zhuang LL, Liu XY, Gu HF, Liu S et al (2013) Prediction of IVF/ICSI outcome based on the follicular output rate. Reprod Biomed Online 27(2):147–153
Grynberg M, Labrosse J (2019) Understanding follicular output rate (FORT) and its implications for POSEIDON criteria. Front Endocrinol 10:246
Broer SL, van Disseldorp J, Broeze KA, Dolleman M, Opmeer BC, Bossuyt P et al (2013) Added value of ovarian reserve testing on patient characteristics in the prediction of ovarian response and ongoing pregnancy: an individual patient data approach. Hum Reprod Update 19(1):26–36
Papathanasiou A, Searle BJ, King NM, Bhattacharya S (2016) Trends in ‘poor responder’research: lessons learned from RCTs in assisted conception. Hum Reprod Update 22(3):306–319
Halvaei I, Ali Khalili M, Razi MH, Nottola SA (2012) The effect of immature oocytes quantity on the rates of oocytes maturity and morphology, fertilization, and embryo development in ICSI cycles. J Assist Reprod Genet 29(8):803–810
Grøndahl ML, Christiansen SL, Kesmodel US, Agerholm IE, Lemmen JG, Lundstrøm P et al (2017) Effect of women’s age on embryo morphology, cleavage rate and competence-A multicenter cohort study. PLoS ONE 12(4):e0172456
Poulain M, Younes R, Pirtea P, Trichereau J, Ziegler D, Benammar A et al (2021) Impact of ovarian yield—number of total and mature oocytes per antral follicular count—on live birth occurrence after IVF treatment. Front Med 8:1164
Moon KY, Kim H, Lee JY, Lee JR, Jee BC, Suh CS et al (2016) Nomogram to predict the number of oocytes retrieved in controlled ovarian stimulation. Clin Exp Reprod Med 43(2):112–118
Revelli A, Gennarelli G, Biasoni V, Chiadò A, Carosso A, Evangelista F et al (2020) The ovarian sensitivity index (OSI) significantly correlates with ovarian reserve biomarkers, is more predictive of clinical pregnancy than the total number of oocytes, and is consistent in consecutive IVF cycles. J Clin Med 9(6):1914
Guo Y, Jiang H, Hu S, Liu S, Li F, Jin L (2021) Efficacy of three COS protocols and predictability of AMH and AFC in women with discordant ovarian reserve markers: a retrospective study on 19,239 patients. J Ovarian Res 14(1):111
Danis RB, Sriprasert I, Ho JR, McGinnis LK, Kumar A, Stanczyk FZ (2021) Association of bioavailable inhibin B and oocyte yield in controlled ovarian stimulation. F&S Rep 2(2):189–194
Sonigo C, Simon C, Boubaya M, Benoit A, Sifer C, Sermondade N et al (2016) What threshold values of antral follicle count and serum AMH levels should be considered for oocyte cryopreservation after in vitro maturation? Hum Reprod 31(7):1493–1500
Bishop LA, Richter KS, Patounakis G, Andriani L, Moon K, Devine K (2017) Diminished ovarian reserve as measured by means of baseline follicle-stimulating hormone and antral follicle count is not associated with pregnancy loss in younger in vitro fertilization patients. Fertil Steril 108(6):980–987
Yakubu MT, Olutoye AF (2016) Aphrodisiac activity of aqueous extract of Anthonotha macrophylla P. Beauv. leaves in female Wistar rats. J Integrat Med 14(5):400–408
Scheffer GJ, Broekmans FJ, Dorland M, Habbema JD, Looman CW, te Velde ER (1999) Antral follicle counts by transvaginal ultrasonography are related to age in women with proven natural fertility. Fertil Steril 72(5):845–851
Tal R, Seifer DB (2013) Potential mechanisms for racial and ethnic differences in antimüllerian hormone and ovarian reserve. Int J Endocrinol. 2013:1–7
Moolhuijsen LME, Visser JA (2020) Anti-Müllerian hormone and ovarian reserve: update on assessing ovarian function. J Clin Endocrinol Metab 105(11):3361–3373
Umarsingh S, Adam JK, Krishna SBN (2020) The relationship between anti-Müllerian hormone (AMH) levels and pregnancy outcomes in patients undergoing assisted reproductive techniques (ART). PeerJ 8:e10390
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
In this study, all authors contributed to the design, writing, and review of the manuscript. FM carried out data collection. RDM contributed to data collection. HGT analyzed the acquired data. EN contributed to data collection and analysis. FM designed and managed the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics committee of Isfahan University of Medical Sciences (registration number: IR.MUI.MED.REC.1400.413).
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mardanian, F., Dehghani-Mohammadabadi, R., Tehrani, H.G. et al. Evaluation of correlation between antral follicle diameters with Follicular Output Rate (FORT) in women under controlled ovarian hyperstimulation for assisted reproductive techniques. Beni-Suef Univ J Basic Appl Sci 11, 145 (2022). https://doi.org/10.1186/s43088-022-00320-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00320-1