Abstract
Background
Schistosomiasis is a major, but generally overlooked, tropical disease carried by snails of the genus Biomphalaria, which have a large distribution in Egypt. Control of the intermediate host snail is critical in limiting schistosomiasis spread. On the topic of snails’ management, nanotechnology has gained more interest.
Results
Copper oxide nanoparticles, characterised by transmission electron microscopy and X-ray diffraction, showed a single crystal structure with an average crystallite size around 40 nm by X-ray diffraction and typical transmission electron microscopy (TEM) image. Also, the UV–VIS spectrophotometer displayed a sharp absorption band of CuO NPs. Molluscicidal activity of copper oxide nanoparticles against B. alexandrina snails was observed. Following exposure to CuO NPs (LC50 and LC90 was 40 and 64.3 mg/l, respectively), there was a reduction in the growth and reproductive rates of treated B. alexandrina at the sub-lethal concentrations, as well as, a drop in egg viability. Moreover, CuO NPs exhibited a toxic effect on miracidiae and cercariae of S. mansoni. Scanning electron microscopy (SEM) investigations of the head-foot and mantle of control and treated snails to the sub-lethal concentrations of CuO NPs (LC10 15.6 mg\l–LC25 27.18 mg\l) indicated morphological alterations in the ultrastructure.
Conclusions
CuO NPs caused a significant effect against the intermediate hosts of S. mansoni and provide a considerable scope in exploiting local indigenous resources as snail molluscicidal agents.
Similar content being viewed by others
1 Background
Schistosomiasis is the second neglected tropical parasitic infection after malaria [1, 2]. Human infection with Schistosoma mansoni is closely related to the existence of its intermediate host, Biomphalaria alexandrina [3]. These snails were found across Egypt, particularly in the Nile Delta and along the Nile's tributaries with a high prevalence [4]. Controlling these snails remains one of the most promising strategies for combating schistosomiasis [5,6,7]. Snail populations have been managed using a number of methods that interrupt their life cycle [8]. Although chemotherapy is one of the most effective techniques for schistosomiasis management, there is still a need for more selective and efficient molluscicides for snail vector control [9, 10]. Currently, nanoparticles are being used in a growing variety of products [11, 12] due to their unique properties compared with their larger counterparts, such as ultra-small size, large surface-area-to-mass ratio, and high reactivity [13] and have recently gained popularity in biomedical sciences as antibacterial [14], antiviral [15], antifungal [16], antiprotozoal [17, 18] and anthelmintic agents [19]. But only few studies have looked into NPs as cercaricides or molluscicides [20, 21]. Therefore, the goal of this study was to assess the effect of CuO NPs on B. alexandrina snails and how it is reflected on survival, growth by reproductive rates, egg hatchability, larvitoxicity and topographical architecture of these snails.
2 Methods
2.1 Snails
Adult B. alexandrina snails (8–10 mm) from Medical Malacology department, Theodor Bilharz Research Institute (TBRI), Giza, Egypt, were obtained. Ten snails were placed in each aquarium filled with one litre of dechlorinated water (pH 7–7.5) and covered with glass plates. Water temperature was adjusted to (25 ± 2 °C) and illumination was provided from 80 watts ceiling-level fluorescent lamps. Dead snails were collected every day and the water was changed twice weekly. Oven dried lettuce leaves, blue green algae (Nostoc muscorum) and dried flakes (TetraMin, Hanover, Germany) were used for feeding. Small pieces of polyethylene sheets were put into the aquaria to gather egg masses according to Pellegrino et al. [22] daily, then kept in tiny jars until they hatched according to El-Fiki and Mohamed [23] and Liang et al. [24].
2.2 Characterisation of CuO NPs
Copper oxide nanopowder (CuO NP) < 50 nm particle size was purchased from Sigma–Aldrich, St. Saint Louis, MO 63,103, USA. Structural studies of CuO NPs were done by high-resolution transmission electron microscope (FETEM, JEM-2100F, JEOL Inc., Japan) that used for the purpose of imaging and made by Nanotechnology and Advanced Material Central Lab (NAMCL), Agriculture Research Center (ARC). Two different modes of imaging were employed; the bright field at electron accelerating voltage 200 kV using lanthanum hexaboride (LaB6) electron source gun and the diffraction pattern imaging. The crystalline nature of CuO NPs was determined by observing the X-ray diffraction (XRD) pattern. The average hydrodynamic size and Zeta potential of CuO NPs also were determined by dynamic light scattering (DLS) (Nano-Zeta sizer-ZS, Malvern Instrument, UK). The optical absorption of the CuO NPs suspension was measured using a double beam UV–Vis-NIR spectrophotometer (Varian-Cary 5000) in the wavelength range of 200–800 nm at room temperature.
2.3 Molluscicidal activity of CuO nanoparticles
A stock solution of 1000 mg/l was prepared and serial concentrations of CuO NPs (70, 60, 50, 40, 30, 20, and 10 mg/l) in glass beakers filled with 100 ml water were produced. For each concentration, three replicates of 10 adult snails were used. Each exposure lasted 24 h; at temperature 25 ± 2 °C and pH 7.4. Control snails were kept in dechlorinated water under the same experimental conditions. The snails were taken from each experimental test at the end of exposure period and washed thoroughly with dechlorinated water. Dead snails were documented as the average of the three replicates. The toxicity of CuO NPs has been expressed as LC50 and LC90 via probit analysis according to the procedure of Finney [25] using statistical program SPSS. The LC0 was estimated at 1/10 of the LC50 value according to El -Gindy et al. [26].
2.3.1 Effect of CuO NPs on survival and growth rates of juvenile snails
Four groups of juvenile B. alexandrina snails (2–3 mm) from the laboratory breeding colony each of 30 snails were used. A set of these groups was exposed to sub-lethal concentrations (LC0, LC10 and LC25) of Cu NPs for 24 h/ week followed by 6 days of recovery in clean dechlorinated water. This technique was repeated for four successive weeks. Another set of snails was maintained in clean dechlorinated tap water as control. Shell diameter was measured weekly under a dissecting microscope by a caliper according to Chernin, Michelson [27]. Dead snails were distinguished by immersion in a small amount of 15–20% sodium hydroxide solution, if bubbles and blood come out of snail, it is recorded as alive and if not, it is recorded as dead, then removed daily, and the survival rate was calculated according to Frank [28] by the following equation:
2.3.2 Effect of CuO NPs on egg laying capacity (M x) and net reproductive rate (Ro) of adult snails
Adult B. alexandrina snails were exposed for 24 h/week for four successive weeks to the tested concentrations of CuO NPs (LC0, LC10 and LC25). For each concentration, three replicates of ten adult snails (8–10 mm diameter) were used. Under the same experimental conditions, 30 snails were used as control group and kept in dechlorinated tap water. For egg deposition, polyethylene sheets were placed in the aquaria of treated and untreated snails, and egg masses were collected and counted weekly. The egg laying capacity is expressed in the form (Mx) and is calculated by dividing the total number of laid eggs in any given week by the total number of living snails at the start of the week as stated by El-Gindy and Radhawy [29] by the following equation.
(x)=Time of exposure in weeks
(Lx) is the survived snails at any given week as a fraction of the correct one (1.0=100%),
Fecundity (Mx) =the mean number of eggs/snail/week,
2.3.3 Larvicidal (miracidicidal and cercaricidal) activity
Five ml of water containing about 100 freshly hatched S. mansoni miracidia or cercariae were mixed with five ml of double concentration of the tested ones (LC0 4; LC10 15.6; LC25 27.18; LC50 40 and LC90 64.3 mg/l) of CuO NPs from each. As a control, 10 ml dechlorinated tap water with 100 newly hatched miracidia was used or cercariae according to Ritchie et al. [30]. The mortality rates of stationary one were reported at the end of the experiment since they were presumed to be dead as stated by WHO [31].
2.4 Scanning electron microscopy studies
Ten B. alexandrina snails were exposed for 24 h to each sublethal concentration of CuO NPs (LC10 15.6 mg/l and LC25 27.18 mg/l), then rinsed in dechlorinated water for 24 h for recovery. Ten snails were dipped in dechlorinated water as a control. Using a stereomicroscope, the soft parts were detached and washed twice in phosphate buffer saline (PBS) before fixed for 24 h in 2.5% glutaraldehyde and 0.2 Molar cacodylate buffer (pH 7.2). The specimens were rinsed in PBS, cold distilled water followed by dehydration with an ascending series of ethanol (70–100%). The dehydrated specimens were immersed in acetone and isoamyl acetate, and dried using a transitional medium of liquid carbon dioxide. Finally, the samples were coated with gold using an ion-sputter coater apparatus and photographed by a scanning electron microscope (JSM-5200 LA, JOEL Company, USA).
2.5 Statistical analysis
The analyses included the calculation of the mean value, standard deviation, standard error and a "t" value at level p ≤ 0.05 according to Zar [32]. The median lethal concentration (LC50) value was determined by applying regression equation analysis to the probit transformed data of mortality as mentioned by Finney [25] using SPSS v. 17.0 for Windows (SPSS Inc. 2008).
3 Results
3.1 Properties of CuO NPs
Transmission electron microscopy shows the typical (TEM) image of CuO NPs, exhibits that the majority of the particles were polygonal in shape with smooth surfaces, and their average crystallite size was found to be around 40 nm (Fig. 1A). The structure of the CuO NPs was characterised by X-ray diffraction (Fig. 1B) confirmed the single crystal structure. No characteristic peaks of any impurities were detected, suggesting that high-quality CuO NPs were synthesized. The average hydrodynamic diameter and Zeta potential of CuO NPs were 503.6 nm and 23.6 mV, respectively (Fig. 1C, 1D). The UV–VIS spectrophotometer showed a sharp absorption band (Fig. 1E, F).
3.2 Molluscicidal activity of CuO NPs
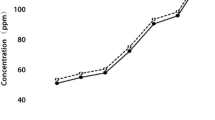
The present results showed that CuO NPs have a molluscicidal activity against B. alexandrina snails after 24 h exposure at LC50 40 mg/l (Table 1, Fig. 2).
3.2.1 Survival rate of B. alexandrina juveniles
The survival rate of B. alexandrina juvenile snails exposed to LC0 (4 mg/l) of CuO NPs for 24 h/ week decreased gradually during the 1st period of the experiment (Table 2, Fig. 3A). Increasing the concentration to LC10 (15.6 mg/l) and LC25 (27.18 mg/l) caused a sharp decrease in the survival rate of the treated snails, at the 4th week it was 35 and 5%, respectively, compared to 95% of the control one. At the 6th week, the survival rate was 25 and 5% for the groups exposed to LC0 and LC10, respectively, while no snails survived at the LC25 concentration at this week, compared to 90% for the control group.
3.2.2 Growth rate of B. alexandrina juveniles
The data presented in Table 2 and Fig. 3B showed that there was a highly significant reduction in the growth rates of the snail groups exposed to LC0 for 24 h/ week for 4 successive weeks of exposure (55%) compared to the control group (47.7%). The same trend was recorded for the treated snail group throughout the 2nd four weeks of the experiment (recovery period), as the growth rate was decreased by 87% compared with the control group. Also, the growth rate of snail group exposed to LC10 under these conditions was significantly decreased after the 4 weeks of exposure compared by the control group (86%). Thereafter, the growth rate of the treated snails was less than that of control snails after 2 weeks of the recovery period (at the 6th week), being 96% compared to control snails at this time. For LC25, the growth rate of this snail group was significantly lower than that of control group up to the 4th week of experiment (79.1%), as they died at 5th week.
3.2.3 Survival rate of adult B. alexandrina
The survival rate of adult B. alexandrina snails exposed to LC0 (4 mg/l) CuO NPs was slightly affected, being 0.8 at the 4th week of exposure. Thereafter, through the recovery period of four weeks, the snails survived till the end of the experiment, as their Lx values were 0.25, compared to 0.80 for the control group. Also, exposure of snails to LC10 (15.6 mg/l) considerably reduced their survival rate (Lx) to be 0.38 at the 4th week of exposure compared to 0.91 for the control group. This group died at 7th week of recovery. Rising the concentration to LC25 (27.18 mg/l), a quick and severe death of treated snails through the first 4 weeks of the experiment as their Lx was 0.25, then these survived snails could not tolerate treatment as they died by the 5th week of the experiment (Table 3, Fig. 4A).
3.2.4 Reproductive rate (RO) of B. alexandrina
Copper oxide NPs revealed that the reproductive rate of treated snails exposed to the tested concentrations was extremely highly suppressed (p < 0.001) in comparison to the control group. At LC0, the reproductive rate (Ro) was 4.507 with 88.8% reduction than control group (40.266). Also, the Ro values of snails treated with LC10 and LC25 were 0.645 and 0.198, respectively, compared to 40.266 for control one (Table 3, Fig. 4B).
3.2.5 Larvicidal activity of CuO NPs
a- Mortality rate of miracidiae: CuO NPs exhibited a larvicidal activity, where, after 20 min of exposure of miracidiae to CuO NPs LC10, moderate mortality rates of S. mansoni miracidia were observed (40%), while the miracidial mortality rate for LC25 was 75%, compared to 5% for the control group. Furthermore, prolonging miracidial exposure to LC10 and LC25 concentrations resulted in 100% mortality after 60 and 50 min, respectively, compared to 20 and 45% for the control group. Increasing the concentration to LC50 and LC90 induced severe and rapid mortality of treated miracidia during short exposure times, with a 100% death rate after 10 min at the LC90 concentration and 15 min at the LC50 concentration (Table 4, Fig. 5A).
b- Mortality rate of cercariae: The mortality rate of cercariae increased with increasing the concentration of CuO NPs and the time of exposure. After 30 min of exposure to LC90, and 50 min for LC25 and LC50, 100% of cercariae die, while after exposure to LC10 for 50 min, the death rate of cercariae was 45% compared to 10% of control group and 100% death was after 90 min of exposure compared to 75% of control group (Table 5, Fig. 5B).
3.3 Microscopic examination
Scanning Electron Microscopy (SEM) studies of the head-foot region of control B. alexandrina snails showed normal manner with a smooth tegmental surface and conspicuous microvilli. The tentacles have a flat surface with fine cilia, and the mantle has a smooth tegmental surface (Fig. 6A, B). After exposure of B. alexandrina snails to LC10 15.6 mg\l, the tentacles have rough folds with erosion at their apex. The tegmental surface of the mantle became turgid, rough, blebbing, peeling, and tortuosity (Fig. 6C, D). At LC25 (27.18 mg\l) mantle is ruptured, nipples appeared, and tegmental surface showed erosion and tortuosity. Also, tentacles are ruptured with rough folds and becoming more tortuose (Fig. 6E, F).
Scanning electron micrographs of B. alexandrina snails (soft parts) showing A Control snails displaying normal ultrastructure of head-foot region with smooth tegmental surface of mantle and conspicuous microvilli and the tentacle with fine cilia B Higher magnification of the tentacles with a smooth surface and fine spines in the tegmental surface of mantle (the arrow). C, D Snails exposed to LC10 (15.6 mg/l) of CuO NPs showing C Mantle became turgidity, rough, blebbing, peeling, and tortuosity. D Tentacles was rough with erosion at apex (the arrow). E, F Snails exposed to LC25 (27.18 mg/l) of CuO NPs showing E Ruptured and peeling mantle with rough tegmental surface F Tentacles became more rough with erosion of its folds, tortuosity (the arrow) and presence of nipples (N)
4 Discussion
Metal oxide nanoparticles, such as copper oxide nanoparticles (CuO NPs), have gained great interest among these nanomaterials due to their antibacterial, anticancer, antiprotozoal anthelmintic agents and antioxidant efficiency [33, 34]. The current study found that CuO NPs exhibit molluscicidal activity on adult Biomphalaria alexandrina snails with LC50 (40 mg/l). The calculation of LC50 value is critical because it aids in determining the safe amount or tolerance threshold of any contaminant [35]. These findings are consistent with those of Ganesan et al. [36], who discovered that CuO NPs was toxic to the freshwater crustacean Daphnia magna with LC50 values ranging from 0.06 to 9.80 mg/l. Also, Abd El-Atti et al. [37] validated the toxicity of CuO NPs on the crayfish Procambarus clarkii, finding that mortality rates were 0%, 6.7%, and 36.7% after exposure to 25, 125, and 250 mg/l of CuO NPs, respectively. Svobodová et al. [38] attributed these mortalities to the direct harmful effects of these nanoparticles on gill epithelium, which resulted in hypoxia and osmoregulatory stressors. The present study stated that exposing B. alexandrina juvenile snails to CuO NPs at concentrations of LC0, LC10 and LC25 is dramatically reduced their survival and growth rates compared to the control group, and this reduction was concentration dependent. These results are in accordance with Perreault et al. [39] who displayed that CuO NPs inhibited the development of Lemna gibba due to the release of copper ions from the NPs in the media. Also, Wu et al. [40] indicated that CuO NPs could considerably reduce the algal growth rate, Daphnia magna survival, and zebra fish hatching and attributed this toxicity to the combined actions of both soluble Cu ions and CuO NPs (Fig. 3).
The present results showed that the survival rate (Lx) of adult B. alexandrina snails was markedly reduced post their exposure to sublethal concentrations (LC0, LC10 or LC25) of CuO NPs compared to the control group. Likewise, Shin et al. [41] who stated that copper induces reduction in survival rate of the bivalve Tegillarca granosa. Similarly, Croteau et al. [42] demonstrated the toxicity of CuO NPs on Lymnaea stagnalis (Fig. 4).
Also, the reproductive rate (Ro) and fecundity (Mx) of adult B. alexandrina snails were significantly decreased. This agrees with findings of Ibrahim and Ghoname [43] who attributed this decline to severe histological alterations in the snail's hermaphrodite gland cells and their findings were supported by lower testosterone and estradiol concentrations in the snails' tissues. In a similar line, Pang et al. [44] showed that nano-CuO had a negative impact on the reproduction of the deposit-feeding snail, Potamopyrgus antipodarum. Similarly, Azzam et al. [45] revealed that both B. alexandrina and B. truncatus snails subjected to sub lethal doses of lupine extracts NPs and copper sulphate NPs did not lay any egg masses following treatments. Sakran and Bakry [46] also discovered that persistent exposure to Bayluscide and copper sulphate completely suppressed the fertility of B. alexandrina snails. The inhibition of egg laying production after exposure to some metals may result from the tested metals' actions on steroid hormones [47].
According to the current results, CuO NPs have miracidicidal and cercaricidal effects against S. mansoni larval stages and these activities were concentration and time dependent. Di Giulio and Hinton [48] stated that the concentration– response correlation may reflect the link between the quantity of chemical pollutant and the degree of organism response. Exposing S. mansoni larval stages to LC25 of CuO NPs resulted in 100% mortality after 50 min, compared to 20% and 45% for the control group. Increasing the concentration to LC50 and LC90 induced severe and rapid mortality of treated miracidia during short exposure times, with a 100% death rate after 10 min at the LC90 concentration and 15 min at the LC50 concentration. While the mortality rate of cercariae increased with increasing the time of exposure, after 30 min of exposure to LC90, and 50 min for LC50, 100% of cercariae die. This is in accordance with Kovrižnych et al. [49] who demonstrated that CuO NPs were acutely lethal to zebra fish embryos at LC50 value 960 mg/l and reasoned this toxicity to the released Cu ions from the CuO NPs which accumulated in the zebra fish embryos causing oxidative stress. Also, Sun et al. [50] revealed hepatotoxicity and neurotoxicity in zebra fish eggs and larvae after short-term exposure to CuO NPs at high concentrations. Furthermore, Ebodi and Ahmed [51] showed that cercariae were more resistant to Randia nilotica fruit extract as a molluscicidal agent than miracidia. El-Deeb et al. [52] further claimed that the difference in mortality rates between the two larval stages appears to be due to the chemical structure of the tested agents rather than the biological character of these larvae (Fig. 5).
Scanning electron microscopy is an effective method for assessing the effects of environmental stressors on the biological structures of aquatic species [53]. In the current study, the ultrastructure of the head-foot region of B. alexandrina snails examined by SEM, displayed normal topography such as a smooth tegmental surface, noticeable microvilli, and tentacles with a flat surface and fine cilia. In contrast, exposing B. alexandrina snails to sublethal doses of CuO NPs caused several morphological changes on the snails' outer surface. Tentacles exhibit rough folds with erosion at their apex, and the mantle's tegmental surface has turgidity, roughness, blebbing, peeling, and tortuosity. Furthermore, the mantle is burst and showed nipples emerging on its tegmental surface with erosion, tortuosity, and tentacles have ruptured with rough folds, becoming more tortuose as CuO NPs concentrations increase. Similarly, Moëzzi et al. [54] revealed ultra-morphological changes in the gills of the swan mussel Anodonta cygnea following CuO NPs exposure. Also, Heinlaan et al. [55] observed ultrastructural alterations in the midgut epithelium of Daphnia magna after exposure to CuO NPs. Finally, Attia et al. [56], Rasel et al. [57] and Ibrahim et al. [58] concluded that these nanomaterials modifications had an effect on the membrane structures and macromolecules of treated snails, resulting in their mortality (Fig. 6).
5 Conclusions
Copper oxide nanoparticles have the potential to be an effective molluscicide against B. alexandrina snails, the intermediate host of S. mansoni. As a result, more research is required to determine the best strategy for using such tested agents to reduce schistosomiasis while limiting water pollution and protect non-target species.
Availability of data and materials
Not applicable.
References
Kiros G, Erko B, Giday M, Mekonnen Y (2014) Laboratory assessment of molluscicidal and cercariacidal effects of Glinus lotoides fruits. BMC Res Notes 7:1–7
Rizk MZ, Aly HF (2015) Recent therapeutic approaches in control of parasitic diseases with special reference to schistosomiasis. - IJAR 1: 957–971.
Abou-El-Naga IF, Sadaka HA, Amer EI, Diab IH, Khedr SIA (2015) Impact of the age of Biomphalaria alexandrina snails on Schistosoma mansoni transmission: modulation of the genetic outcome and the internal defence system of the snail. Mem Inst Oswaldo Cruz 110:585–595
El-Sheikh YWA, Eltamny HM, Soliman HA, Farag AA, El Behary MHH (2012) Molluscicidal activity of eco-friendly natural compound (Rutin) gained from ethanolic flowers extract of Calendula officinalis on B. alexandrina B truncatus -. NY Sci J 5:19–27
Bakry FA, El-Hommossany K, Mossalem HS (2012) Immunological and physiological parameters of Biomphalaria alexandrina snails exposed to Azadirachta indica plant. Eur Rev Med Pharmacol Sci 16:133–143
Mohamed AM, El-Emam MA, Osman GY et al (2012) Effect of Basudin, Selecron and the Phytoalkaloid Colchicine (pesticides) on biological and molecular parameters of Biomphalaria alexandrina snails. Pestic Biochem Physiol 102:68–78
WHO (2014) Schistosomiasis. Fact sheet N°115
Abd El-Ghany AM, Abd El-Ghany NM (2017) Molluscicidal activity of Bacillus thuringiensis strains against Biomphalaria alexandrina snails. Beni-Suef Univ J Basic Appl Sci 6:391–393
WHO (2008) African Network for drug/diagnostics discovery and innovation (ANDI) "Creating a sustainable platform for R&D innovation in Africa". 1 meeting, Abuja, Nigeria
Singh SK, Yadav RP, Singh A (2010) Molluscicides from some common medicinal plants of eastern Uttar Pradesh. India J Appl Toxicol 30:1–7
Andualem WW, Sabir FK, Mohammed ET, Belay HH, Gonfa BA (2020) Synthesis of copper oxide nanoparticles using plant leaf extract of Catha edulis and its antibacterial activity. J Nanotechnol 2020:1–10
Akintelu SA, Folorunso AS, Folorunso FA, Oyebamiji AK (2020) Green synthesis of copper oxide nanoparticles for biomedical application and environmental remediation. Heliyon 6:e04508
Zhang XF, Choi YJ, Han JW (2015) Differential nanoreprotoxicity of silver nanoparticles in male somatic cells and spermatogonial stem cells. Int J Nanomedicine 10:1335–1357
Wang C, Liu L, Zhang A, Xie P et al (2011) Antibacterial effects of zinc oxide nanoparticles on Escherichia coli K88. Afr J Biotechnol 11(44):10248–10254
You J, Zhang Y, Hu Z (2011) Bacteria and bacteriophage inactivation by silver and zinc oxide nanoparticles. Coll Surf B Biointerfaces 85:161–167
Kim KT, Jang MH, Kim JY, Xing B (2012) Embryonic toxicity changes of organic nanomaterials in the presence of natural organic matter. Sci Total Environ 426:423–429
Baiocco P, Ilari A, Ceci P et al (2011) Inhibitory effect of silver nanoparticles on trypanothione reductase activity and Leishmania infantum proliferation. ACS Med Chem Lett 2:230–233
Delavari M, Dalimi A, Ghaffarifar F, Sadraei J (2014) In vitro study on cytotoxic effects of ZnO nanoparticles on promastigote and amastigote forms of Leishmania major (MRHO/IR/75/ER). Iran J Parasitol 9:6–13
Khan YA, Singh BR, Ullah R (2015) Anthelmintic effect of biocompatible zinc oxide nanoparticles (ZnO NPs) on Gigantocotyle explanatum, a neglected parasite of indian water buffalo. PLoS ONE. https://doi.org/10.1371/journal.pone.0133086
Moustafa MA, Mossalem HS, Sarhan RM et al (2018) The potential effects of silver and gold nanoparticles as molluscicides and cercaricides on Schistosoma mansoni. Parasitol Res 117:3867–3880
Peng G, He Y, Zhao M et al (2018) Differential effects of metal oxide nanoparticles on zebrafish embryos and developing larvae. Environ Sci Nano 5:1200–1207
Pellegrino J, Goncalves M et al (1965) A simple method for collecting egg clutches of Biomphalaria glabrata (Australorbis glabratus) and for rearing newly hatched snails. J Parasitol 51(6):1014
El-Fiki SA, Mohamed AM (1978) Effect of some herbicides on the toxicity of certain molluscacides against Biomphalaria alexandrina snails Egypt. J Bilharz 5:91–100
Liang YS, Bruce JI, Body DA (1987) Laboratory cultivation of schistosome vector snails and maintenance of schistosome life cycle. In: Proceeding of the 1st Sino-American symposium (pp 34–48)
Finney DJ (1971) Probit analysis. Cambridge University Press, London
El-Gindy HI, Rawi SM, Abdel-Kader A, Ebeid FA (1991) Comparative effect of different pesticides on the transaminases activities in haemolymph of Biomphalaria alexandrina snails. J Egypt Ger Soc Zool 6:131–138
Chernin E, Michelson EH (1957) Studies on the biological control of schistosome-bearing snails: III. The effects of population density on growth and fecundity in Austbalobbis glabbatus. Am J Epidemiol 65:57–70
Frank GH (1963) Some factors affecting the fecundity of Biomphalaria pfeifferi (krauss). Bull World Health Organ 29:531–537
El-Gindy MS, Radhawy IA (1965) Effect of low concentrations of sodium pentachlorophenate on the fecundity and egg viability of Bulinus truncatus from Central Iraq. Bull Endem Dis (Baghdad) 7:44–54
Ritchie LS, Lopez V, Cora TM (1974) Prolonged application of an organtin against Biomphalaria glabrata and Schistosoma mansoni. In: Cheng TC (ed) Molluscicides in schistosomiasis control. Academic Press, New York, pp 77–88
WHO (1965) Molluscicide screening and evaluation. Bull W.H.O 33:567–581
Zar JH (1999) Biostatistical analysis. Pearson Education, India, pp 1–9
Navarro E, Baun A, Behra R et al (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Akintola AO, Kehinde BD, Ayoola PB et al (2020) Antioxidant properties of silver nanoparticles biosynthesized from methanolic leaf extract of Blighia sapida. IOP Conf Ser Mater Sci Eng 805(1):012004
Prenter J, MacNeil C, Dick JTA et al (2004) Lethal and sub lethal toxicity of ammonia to native, invasive, and parasitized freshwater amphipods. Water Res 38:2847–2850
Ganesan S, Anaimalai Thirumurthi N, Raghunath A, Vijayakumar S, Perumal E (2016) Acute and sub-lethal exposure to copper oxide nanoparticles causes oxidative stress and teratogenicity in zebrafish embryos. J Appl Toxicol 36:554–567
Abd El-Atti M, Desouky M, Mohamadien A, Said R (2019) Impact of copper oxide nanoparticles on freshwater crayfish, procambarus clarkia. A combined histopathological, biochemical and genotoxicological study. J Egypt Acad Soc Environ Dev D, Environ Stud 20:1–18
Svobodová J, Douda K, Fischer D et al (2017) Toxic and heavy metals as a cause of crayfish mass mortality from acidified headwater streams. Ecotoxicology 26:261–270
Perreault F, Samadani M, Dewez D (2014) Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicology 8:374–382
Wu F, Harper BJ, Crandon LE, Harper SL (2020) Assessment of Cu and CuO nanoparticle ecological responses using laboratory small-scale microcosms. Environ Sci Nano 7:105–115
Shin YK, Park JJ, Ju SM, Lee JS (2015) Copper Toxicity on survival, respiration and organ structure of Tegillarca granosa (Bivalvia: Arcidae) Korean. J Malacol 31(2):151–158
Croteau M, Misra SK, Luoma SN, Valsami-Jones E (2014) Bioaccumulation and toxicity of CuO nanoparticles by a freshwater invertebrate after waterborne and dietborne exposures environm. Sci Tech 48(18):10929–10937
Ibrahim AM, Ghoname SI (2018) Molluscicidal impacts of Anagallis arvensis aqueous extract on biological, hormonal, histological and molecular aspects of Biomphalaria alexandrina snails. Exp Parasitol 192:36–41
Pang C, Selck H, Misra SK (2012) Effects of sediment-associated copper to the deposit-feeding snail, Potamopyrgus antipodarum: A comparison of Cu added in aqueous form or as nano- and micro-CuO particles. Aquat Toxicol 106–107:114–122
Azzam K, Abdel-Hady E, Khedr E (2019) Effect of copper coated lupine extract nanoparticles on some aquatic and terrestrial pest snails. Arab Univ J Agric Sci 27:2291–2302
Sakran AM, Bakry FA (2005) Biological and physiological studies on Biomphalaria alexandrina snails exposed to different plant molluscicides. J Egypt Ger Soc Zool 48(1):237
Rawi S, El-Gindy H, Haggag A, Hassan AE (1995) Few possible molluscicides from calendula Micrantha officinalis and Ammi majus plants. I. Physiological effect on B. alexandrina and B. truncatus. J Egypt Ger Soc Zool 16:69–75
Di Giulio RT, Hinton DE (2008) Liver toxicity. Toxicol Fish. https://doi.org/10.1201/9780203647295
Kovrižnych JA, Sotńikóva R, Zeljenková D (2013) Acute toxicity of 31 different nanoparticles to zebrafish (Danio rerio) tested in adulthood and in early life stages—comparative study. Interdiscip Toxicol 6:67–73
Sun Y, Zhang G, He Z et al (2016) Effects of copper oxide nanoparticles on developing zebrafish embryos and larvae. Int J Nanomedicine 11:905–918
Ebodi Ala YE, Ahmed MM (2017) Toxicity of Randia nilotica fruit extract on Schistosoma mansoni, Biomphalaria pfeifferi and Bulinus truncates. Cell Biol Dev 1:23–30
El-Deeb FAA, Marie MAS, Hasheesh WS, Sayed SSM (2016) Factors affecting the molluscicidal activity of Asparagus densiflorus and Oreopanax guatemalensis plants and Difenoconazole fungicide on Biomphalaria alexandrina snails. Comp Clin Path 25:775–783
Palaniappan PR, Sabhanayakam S, Krishnakumar N, Vadivelu M (2008) Morphological changes due to Lead exposure and the influence of DMSA on the gill tissues of the freshwater fish Catla catla. Food Chem Toxicol 46:2440–2444
Moëzzi F, Hedayati SA, Ghadermazi A (2018) Ecotoxicological impacts of exposure to copper oxide nanoparticles on the gill of the Swan mussel, Anodonta cygnea (Linnaeus, 1758). Molluscan Res 38:187–197
Heinlaan M, Kahru A, Kasemets K (2011) Changes in the Daphnia magna midgut upon ingestion of copper oxide nanoparticles: a transmission electron microscopy study. Water Res 45:179–190
Attia MM, Soliman SM, Khalf MA (2017) Hydrophilic nanosilica as a new larvicidal and molluscicidal agent for controlling of major infectious diseases in Egypt. Vet World 10:1046–1051
Rasel MAI, Singh S, Nguyen TD (2019) Impact of nanoparticle uptake on the biophysical properties of cell for biomedical engineering applications. Sci Rep. https://doi.org/10.1038/s41598-019-42225-7
Ibrahim AM, Mohamed F, Al-Quraishy S et al (2021) Green synthesis of Cerium oxide/Moringa oleifera seed extract nano-composite and its molluscicidsal activities against Biomophalaria alexanderina. J King Saud Univ Sci 33:101368
Acknowledgements
The authors gratefully thank the Medical Malacology Department, Theodor Bilharz Research Institute, Egypt for all of the support provide. Also, authors could thank the Parasitology Department of Faculty of Science, Cairo University for all the assistance they provide.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
FA, MF and AI have provided guidance during development of idea and MF, HH and AI prepared different figures required, MF and AI wrote and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have indicated that they have no conflict of interest regarding the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, A.M., Abdel-Ghaffar, F.A., Hassan, H.AM. et al. Assessment of molluscicidal and larvicidal activities of CuO nanoparticles on Biomphalaria alexandrina snails. Beni-Suef Univ J Basic Appl Sci 11, 84 (2022). https://doi.org/10.1186/s43088-022-00264-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00264-6