Abstract
Background
Vernonia amygdalina (leaf), Garcinia kola (seed), and Leucaena leucocephala (seed) are three well-known tropical plants used in African ethnomedicine to reduce parasitic worm burdens and are potential sources of alternative solution for controlling parasitic helminths infection in grazing livestock. This study investigated extracts from these plants for anthelmintic activity against adult Haemonchus placei, an haematophagous nematode from cattle abomasa. Powdered plant materials were macerated in acetone and the crude acetone extracts evaluated for anthelmintic activity using H. placei adult worm motility assay. Afterwards, fresh sample of V. amygdalina was macerated successively in chloroform and acetone and the extracts evaluated for anthelmintic activity. The chloroform extract was subjected to phytochemical and FT-IR analyses and fractionated by vacuum liquid chromatography. Anthelmintic data were fitted to a nonlinear regression equation (Log [extract or fraction] vs. lethality; variable slope) to produce best-fit sigmoidal curves and LC50 values computed with associated uncertainty.
Results
Of the three tropical plants, only V. amygdalina was active against adult H. placei with best-fit LC50 of 6.51 mg/mL (95% CI: 5.32–7.75). Evaluation of the two extracts obtained by successive maceration showed that chloroform extract (LC50, 2.46 mg/mL, 95% CI: 1.87–3.28) was 11 times as potent as acetone extract (LC50, 27.01 mg/mL, 95% CI: 21.32–48.57) (α < 0.0001). Chromatographic fractionation of the chloroform extract yielded four fractions (FA-FD) with FB (LC50, 2.38 mg/mL, 95% CI: 1.76–3.28) 2.19 times as potent as FC (LC50, 5.21 mg/mL, 95% CI: 4.40–5.79) against H. placei, while FA and FD were inactive. Phytochemical evaluation of the chloroform extract revealed the presence of saponins, steroids, terpenoids, cardiac glycosides, and the absence of tannins, flavonoids, alkaloids, and anthraquinones. FT-IR structural analysis of chloroform extract indicated the presence of key functional groups which are chemical fragments/ structural motifs known to be present in the two major classes of bioactive compounds (sesquiterpene lactones and steroid glucosides) reportedly to be found in V. amygdalina.
Conclusions
The findings showed that chloroform extract of V. amygdalina leaf possessed relatively good anthelmintic activity against adult H. placei. This could be indicative of its potential usefulness as an anthelmintic phytomedicine to control gastrointestinal nematodes infection in cattle.
Key highlights
-
Extracts of three different plant materials (one leaf, two seeds) were tested against adult Haemonchus placei in vitro;
-
Chloroform extract of Vernonia amygdalina was 11 times as potent as acetone extract;
-
Fractionation of the chloroform extract yielded a bioactive fraction responsible for about 90% of the total lethal effect of the chloroform extract.
-
Bioprocessing of V. amygdalina leaf could produce phytomedicines for organic livestock farming.
Graphical abstract

Similar content being viewed by others
1 Background
Food products from farm animals are important contribution towards the realization of global food security [1, 2]. While many factors threaten this food supply source, gastrointestinal nematode infection (GIN) of farm animals represents a fundamental constraint [2, 3]. Gastrointestinal nematode infection occurs as a result of grazing of animals on pasture contaminated with infective larvae of nematodes. Infection generally results in weight loss, decreased reproductive performance, poor food conversion, and anaemia [4, 5]. The infection is dominant in areas where inadequate livestock extension services, poor environmental hygiene, and prevailing weather conditions, altogether favour the development and transmission of infective larvae. Even though grazing animals usually suffer from mixed infection of nematodes, adult stages of the abomasal nematode Haemonchus species are devastatingly pathogenic as they feed on blood, resulting in anaemia and related complications [2, 6, 7].
With the advent of chemotherapy, the control of GIN has been mainly by the use of modern anthelmintic drugs. The hefty reliance and widespread application of these drugs, however, has inevitably led to the emergence of anthelmintic resistance [3, 8, 9]. Furthermore, this deplorable state is aggravated by the presence of drug residues in animal products, prompting heightened health and environmental concerns [10]. Thus, there is a resurgence of interest in the potential usefulness of plant extractives for effective control of gastrointestinal nematodes.
Vernonia amygdalina Del. is a tropical, edible vegetable whose leaves are used ethnomedicinally as remedies for gastrointestinal disorders, fever, general tonic, treatment of wound, venereal infections, as an anthelmintic and as a laxative [11]. This plant has been observed to be eaten by wild chimpanzees, possibly in a self-medicative behaviour against parasite-related illnesses [12]. Garcinia kola Heckel is a West African plant used as remedies for diarrhoea, worm infection, gonorrhea, stomach ache, jaundice, high fever, as chewing stick and to alleviate colic, chest colds and cough [13]. Leucaena leucocephala (Lam) De Wit leaf is commonly used as fodder for ruminants because of its high nutritive value. Its fresh seeds are used in Nigeria to deworm animals [14]. This paper reports the effect of extracts of these three tropical plants (V. amygdalina, G. kola and L. leucocephala) against adult H. placei, as none of these plants has ever been tested against this nematode which infect cattle primarily [6].
2 Methods
2.1 Plant materials
Vernonia amygdalina (leaves) and L. leucocephala (seeds) were obtained within the premises of University of Ibadan, Ibadan, Nigeria, while G. kola (seeds) were bought from Bodija Market, Ibadan, Nigeria. They were identified at Forestry Research Institute of Nigeria (FRIN), Ibadan. V. amygdalina leaves were air-dried under shade for four weeks, while G. kola seeds (chopped into smaller pieces) and L. leucocephala seeds were dried under shade for eight weeks. Afterwards, they were milled into coarse powder.
2.2 Source of adult nematodes
Actively moving adult H. placei nematodes were collected from the abomasal content of freshly slaughtered cattle at the Bodija Abattoir, Bodija Market, Ibadan, Nigeria, and maintained in normal saline solution. The identity of the worms was confirmed at the Parasitology Research Unit, by Prof I. O. Ademola, a parasitologist and head of the Department of Veterinary Parasitology and Entomology, University of Ibadan, Ibadan, Nigeria.
2.3 Chemicals and reagents
Acetone, n-hexane, chloroform, ethyl acetate, methanol, Tween 80, sodium chloride, vanillin, sulphuric acid (solvents and reagents were of analytical grades, Sigma-Aldrich, UK), silica gel 60 G (5–40 μm, Merck, Germany) and pre-coated TLC silica gel 60 (F254, aluminium sheets 10 × 20 cm, Merck, Germany).
2.4 Solvent extractions (crude and successive)
For the crude extraction, powdered materials (200 g each) of the three plants were extracted twice by maceration using acetone (1 L for 24 h in each instance). Afterwards, a fresh powdered material of V. amygdalina (300 g), after initial defatting using n-hexane (n-hex, 1L) for 24 h, was subjected to successive maceration in chloroform, and acetone (1L each, 2 times for 24 h), respectively. The various solvent extracts, filtered with the aid of filter paper into clean glass bottles, were concentrated into smaller volumes using rotary evaporator (40 °C), and subsequently evaporated to dryness under reduced pressure at 40 °C for 48 h. They were further dried in vacuum desiccator until constant mass.
2.5 Vacuum liquid chromatographic fractionation of V. amygdalina chloroform extract
Vernonia amygdalina chloroform extract (1 g, obtained by successive extraction) was dissolved using chloroform and then adsorbed on silica gel 60 G (5 g). The adsorbed sample, dried under reduced pressure at 40 °C, was packed onto sintered glass earlier prepacked with silica gel 60 G (25 g) with the aid of a vacuum pump and eluted using 200 mL each of varying solvent mixtures [(n-hexane → n-hexane/ethyl acetate (1:1) → ethyl acetate → ethyl acetate/methanol (1:1)]. The eluates were monitored by thin-layer chromatography analysis using ethyl acetate/methanol (90/10) as mobile phase, while the chromatogram was viewed under ultra violet light (356 nm), day light, and after spraying with vanillin-sulphuric acid reagent (0.1 g vanillin, 28 mL methanol, 1 mL sulphuric acid). The fractions, concentrated into smaller volumes under reduced pressure, were later dried in vacuo at 40 °C.
2.6 Structural profile and phytochemical analysis of V. amygdalina chloroform extract
In other to obtain structural/ organic functional groups information on the V. amygdalina chloroform extract, it was subjected to Fourier transform infrared (FT-IR) spectroscopic characterization. About 1 mg of the extract was smeared on a KBr salt disc, placed in a disc holder and inserted into the sample beam of the FT-IR instrument, Perkin-Elmer® spectrum 2. The phytochemical analysis of the extract was done following standard procedures [15, 16].
2.7 Anthelmintic evaluation
Varying test concentrations of the crude acetone extracts (1–20 mg/mL; V. amygdalina, L. leucocephala, and G. kola), chloroform and acetone extracts (0.5–20 mg/mL) of V. amygdalina, and chromatographic fractions (0.5–15 mg/mL) of the chloroform extract were prepared in 20% Tween-80 in normal saline. Each concentration (0.5 mL, in duplicates) was dispensed into wells of 24-well standard plates (each well: diameter-14.00 mm, depth-16.10 mm, volume-2.39 mL), followed by placing ten adult H. placei nematodes into each well. The nematodes were exposed to these test concentrations for 3 h at ambient temperature (26‒30 °C). Afterwards, the nematodes were removed into Petri dishes containing distilled water, cleansed of the extracts and then exposed to warm (40 °C) normal saline for 10 min and observed for any revival of motility. At the end of the experiments, the nematodes were categorized as dead if there were no revival of mobility plus a complete lack of response to poking with a pick. The number of dead worms was recorded. Levamisole (as hydrochloride salt, from Reals Pharmaceutical Ltd, Nigeria) and 20% Tween-80 in normal saline were used as positive and negative controls, respectively. All experiments were replicated twice [17].
2.8 Statistical analysis
Statistical analysis was conducted using the GraphPad Prism Software 7 (GraphPad Software Inc., California, USA). Anthelmintic data were fitted to a nonlinear regression equation (Log [extract or fraction] vs. lethality; variable slope) to produce best-fit sigmoidal curves from which median lethal concentration (LC50) values were computed with associated uncertainty.
3 Results
3.1 Solvent extractions
The percentage yields of all the extracts—crude acetone extracts of the three screened plant species, and the chloroform and acetone extracts of V. amygdalina with (after initial defatting with n-hexane) are presented in Table 1.
3.2 Vacuum liquid chromatographic fractionation of V. amygdalina chloroform extract
After the anthelmintic evaluation of the two extracts (CE and AE) of V. amygdalina obtained by successive extraction, CE was selected and subjected to vacuum liquid chromatographic fractionation. This yielded four fractions, FA-FD (Table 2), with FB having the highest yield (52.7%). Chromatographic fractionation of CE resulted in good fractional separation of this bioactive extract as shown by the thin-layer chromatographic fingerprint of the fractions that revealed the various secondary metabolites in them (Fig. 1). Based on the solvent strength of the eluting solvents, the fingerprint revealed gradient elution as attested to by the relative positions of the metabolites on the plate (Fig. 1).
3.3 Structural and phytochemical analysis of V. amygdalina chloroform extract
Infrared spectroscopy detects the molecular vibrations (stretching and bending) of bonds within functional groups present in organic molecules. The FT-IR spectrum of V. amygdalina chloroform extract (Fig. 2) with its complexity of vibrational nodes represents a sum of the spectra of the individual constituent molecules present in the extract [18, 19]. The absorption peak frequency data are presented in Table 3.
3.4 Anthelmintic evaluation of extracts and fractions
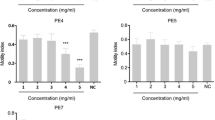
The results of anthelmintic evaluation of the extracts and fractions against H. placei are presented in Table 4. Of the three plants, only V. amygdalina was active with best-fit LC50 of 6.51 mg/mL (Fig. 3). The other two (L. leucocephala and G. kola) were inactive, even at the highest concentration (20 mg/mL). The best-fit LC50 values of the two extracts of V. amygdalina obtained by successive extraction were found to be significantly different (alpha < 0.0001): 2.46 mg/mL and 27.01 mg/mL for CE and AE, respectively (Fig. 4). For the four chromatographic fractions obtained after fractionation of CE, only FB and FC were active against H. placei (best-fit LC50 of 2.38 mg/mL and 5.21 mg/mL, respectively (Fig. 5). FA and FD were, however, not active. Levamisole, a reference anthelmintic, expectedly exhibited high potency with LC50 of 11.74 ng/mL. There was 100% survival of the worms in the 20% Tween 80 in normal saline used as the negative control.
Anthelmintic activities of FA-FD, obtained by vacuum liquid chromatographic fractionation of Vernonia amygdalina chloroform extract. Each value represents two replicates of two independent experiments after 3-h exposure to varying concentrations (0.5–15 mg/mL) at ambient temperature (26‒30 °C). *na indicates ‘not active’
4 Discussion
The three plants were selected for this study based on their ethnomedicinal uses and available documentation in the literature. The need to extract out much of their secondary metabolites content irrespective of their chemical classes informed the choice of acetone as solvent for the crude extraction. Acetone seems to have higher capacity to extract more bioactive secondary metabolites for screening purposes relative to other solvents [20, 21]. Acetone’s organic and polar character (methyl hydrocarbon skeletons interspersed with polarized carbonyl functional group) enables the extraction of both polar and non-polar secondary metabolites by solvation through dipole–dipole interactions with solute molecules [22, 23]. Based on the outcome of the anthelmintic evaluation of the three selected plants, fresh dried sample of V. amygdalina leaves was successively macerated using solvents of different polarities. This process afforded selective separation of the various secondary metabolites in V. amygdalina into two broad categories: less polar/intermediate-polar (Chloroform Extract, CE); and intermediate-polar/polar (Acetone Extract, AE). The two extraction solvents, chloroform and acetone, possess good selectivity property based on different dielectric constant (4.81 and 21.01, respectively), and relatively high solvent strength (4.1 and 5.1, respectively) [24, 25]. Accordingly, macerating first with chloroform facilitated the selective extraction of most non-polar compounds and some intermediate-polar compounds; macerating afterwards with acetone facilitated selective extraction of most intermediate-polar and polar compounds. Increasing the number of extraction solvents to three by macerating further with any of ethanol, methanol or water was considered, but dropped because these are ionic, amphiprotic solvents [18] with inherent capacity to extract out the more polar primary and secondary metabolites like sugars, amino acids, tannins and glycosides. The potential outcome will be increased extraction mass, and this often does not translate to higher bioactivity [20, 21, 26].
Analysis of the fundamental vibration frequencies that the spectrum revealed could yield structural information regarding the constituent’s mixture of phytochemicals in this bioactive extract. A number of fundamental frequency bands were identifiable in the spectrum and these include: 3442.93 cm−1, represents OH stretching vibration; 3009.50 cm−1, represents C–H stretching vibration coming from vinylic group or epoxy ring; 2918.81 and 2849.78 cm−1, represent C–H stretching vibrations from methylene group; 1731.30 and 1712.43 cm−1, are carbonyl stretching vibrations from ester group and lactone ring, respectively; 1649.50 and 1619.20 cm−1 can be assigned to C = C from unconjugated and conjugated alkene group, respectively; 1492.00, 1453.75 are frequency bands from C–H deformations; 1264.80 cm−1, representative of C–O stretching vibration from ester group and lactone ring; 1046.70 can be assigned to C–O stretching vibration from an alcohol, ester or lactone ring. All these functional groups are chemical fragments or structural motifs present in the two major classes of bioactive compounds (sesquiterpene lactones and steroid glucosides) reported to have been isolated from V. amygdalina [27, 28]. This was further affirmed by the result of the phytochemical analysis of the extract which revealed the presence of saponins, steroids, terpenoids and cardiac glycosides, as well as absence of tannins, flavonoids, alkaloids and anthraquinones.
The observed activity of V. amygdalina extract in this study provided additional evidence justifying the ethnomedicinal use of the leaf in treatment of worm infection. The result suggests that the plant exhibited a fairly good potency against H. placei relative to acetone leaf extracts of Ocimum gratissimum and Cymbopogon citratus [29]. For the extracts obtained by successive extraction after the outcome of initial anthelmintic evaluation of the crude extracts, though CE and AE were both active, the result suggests that CE is 11 times more potent than AE against adult H. placei. In addition, CE’s potency was 2.65 times higher relative to the crude acetone extract. Thus, the successive extraction was value adding. It showed clearly that the activity of this plant against H. placei is largely contributed by the less polar/intermediate-polar components selectively extracted out by chloroform. For the two bioactive fractions, judging by their best-fit LC50 values only, FB was 2.19 times as potent as FC against H. placei. If, however, we factor in the mass obtained per fraction and calculate the lethal effect per fraction that kills 50% of worms, the result suggests that FB is responsible for almost 90% of the total activity of CE against H. placei [30]. In addition, this activity against adult H. placei is attributable to some of the phytochemicals present in FB and FC. Considering the mobile phases (n-hexane/ethyl acetate (1:1) and ethyl acetate (100%)) used for their elution, and the result of the structural analysis of CE, we submit that these phytochemicals would be of intermediate or medium polar compounds such as saponins (stigmastane-type and steroidal) and sesquiterpene lactones which have been reported in the literature to be present in the leaf [11, 31]. The structural motifs and moieties of these class of compounds with embedded polar fragments such as carbonyls, lactones, and hydroxyl groups made them to be easily dissolved or solvated by the ethyl acetate-based eluting solvents.
Overview of literature reports on the anthelmintic activity of V. amygdalina leaf revealed that aqueous/aqueous-organic solvents extracts were used, instead of varying solvents of different polarities as we have used in the current study. Nalule et al. [26] reported that aqueous ethanol extract (70%) with ED50 = 5.94 mg/mL, was twice as potent as the aqueous extract (ED50 = 13.70 mg/mL) against adult Ascaris suum at 48 h post-treatment [26]. Against earthworm (Lumbricus terrestris) at 50 mg/mL, [32] reported about a half time to death of 37.46 ± 13.55 min for aqueous ethanol extract (70%) relative to aqueous extract of 76.65 ± 12.73 min [32]. Also, against H. contortus eggs and larvae, aqueous acetone extract (70%) gave LC50 values of 957.00 and 508.20 µg/mL, respectively, at 48-h exposure [33]. Three other studies indicated varying activities against nematodes: aqueous ethanol extract (80%) gave 71.43% mortality at 24 h (500 mg/mL; dose too high) post-treatment against Heligmosomoides bakeri infective larvae [34]; aqueous ethanol extract (50%) gave 1.70% mortality at 72 h (1 mg/mL; longer duration of exposure) against Caenorhabditis elegans, a free-living nematode model [35]; while hot water extract exhibited poor inhibitory hatching effect against H. contortus egg [36]. The root extracts (methanol, water and acetone) were also reported to be active against adult H. contortus in vitro with mean mortality of 20‒33.3% at 6.25 mg/mL [37]. In addition, in vivo studies in goats showed the leaf aqueous extracts as efficacious against helminths [38, 39]. Overall, while all these studies used aqueous-based extracts, ours used primary extracts of different polarities and showed that the chloroform extract was more potent.
Garcinia kola seed did not show any activity in this study even though it was reported to possess anthelmintic activity (albeit weak) in some earlier studies using ethanol seed extracts. The seed exhibited 18.75% inhibition at 100 mg/mL and irreversibly paralyzed 76.52% at 50 mg/mL of H. bakeri egg and larvae, respectively [40]. Similarly, at 1.7 mg/ml, it was considered inactive against Haemonchus contortus larvae with less than 60% mortality [41]. Similarly, L. leucocephala seed was reported to exhibit good anthelmintic activity with the chloroform soluble alkaloidal seed extract furnishing an equivalent effect to mebendazole at 5 mg/mL using Ascaris suum [42]. Also, aqueous seed extract (LC50 = 0.586 mg/mL) and chromatographic fractions of the ethanol extract were active against H. contortus larvae [14, 43], while an investigation of seed protein extracts on H. contortus larvae gave a 50% hatching inhibition (0.48 mg/mL, cotyledon extract; 0.33 mg/mL, total seed extract) [44].
5 Conclusions
Results from this study suggest that chloroform leaf extract of V. amygdalina possesses anthelmintic activity against adult H. placei nematode. Bioprocessing of this extract could produce phytomedicines for organic livestock farming. Further studies to better characterize this extract are on-going in our laboratory.
Availability of data and material
Yes, in the main manuscript.
Abbreviations
- VA:
-
Vernonia amygdalina
- GK:
-
Garcinia kola
- LL:
-
Leucaena leucocephala
- CE:
-
Chloroform extract
- AE:
-
Acetone extract
- TLC:
-
Thin-layer chromatography
- FT-IR:
-
Fourier transform infrared
- FA:
-
Fraction A
- FB:
-
Fraction B
- FC:
-
Fraction C
- FD:
-
Fraction D
- FHI:
-
Forest Herbarium Ibadan
- LC:
-
Lethal concentration
- UV:
-
Ultra violet
- MeOH:
-
Methanol
- Hex:
-
Hexane
- EtOAc:
-
Ethyl acetate
- SV:
-
Strong vibration
- BV:
-
Bending vibration
References
Smith J, Sones K, Grace D, Macmillan S, Tarawali S, Herrero M (2013) Beyond milk, meat, and eggs: role of livestock in food and nutrition security. Anim Front 3(1):6–13
Fitzpatrick JL (2013) Global food security: the impact of veterinary parasites and parasitologists. Vet Parasitol 195(3–4):233–248. https://doi.org/10.1016/j.vetpar.2013.04.005
Vercruysse J, Charlier J, Van DJ, Morgan ER, Geary T, Von S-H et al (2018) Control of helminth ruminant infections by 2030. Parasitology. https://doi.org/10.1017/S003118201700227X
Krecek RC, Waller PJ (2006) Towards the implementation of the “basket of options” approach to helminth parasite control of livestock: emphasis on the tropics/subtropics. Vet Parasitol 139(4):270–282
Molento MB, Fortes FS, Pondelek DAS, de Borges FA, de Chagas ACS, de Torres-Acosta JFJ et al (2011) Challenges of nematode control in ruminants: focus on Latin America. Vet Parasitol 180(1–2):126–132
Giudici CJ, Cabaret J, Durette-Desset MC (1999) Description of Haemonchus placei (Place, 1893) (Nematoda, Trichostrongylidae, Haemonchinae), identification and intra-specific morphologic variability. Parasite 6(4):333–342
Emery DL, Hunt PW, Le Jambre LF (2016) Haemonchus contortus: the then and now, and where to from here? Int J Parasitol 46(12):755–769. https://doi.org/10.1016/j.ijpara.2016.07.001
Kaplan RM, Vidyashankar AN (2012) An inconvenient truth: global worming and anthelmintic resistance. Vet Parasitol 186(1–2):70–78. https://doi.org/10.1016/j.vetpar.2011.11.048
Ramos F, Portella LP, de Rodrigues FS, Reginato CZ, Pötter L, Cezar AS et al (2016) Anthelmintic resistance in gastrointestinal nematodes of beef cattle in the state of Rio Grande do Sul, Brazil. Int J Parasitol Drugs Drug Resist 6(1):93–101
Baynes RE, Dedonder K, Kissell L, Mzyk D, Smith G, Tell L et al (2016) Health concerns and management of select veterinary drug residues. Food Chem Toxicol 88:112–122. https://doi.org/10.1016/j.fct.2015.12.020
Oyeyemi IT, Akinlabi AA, Adewumi A, Aleshinloye AO, Oyeyemi OT (2017) Vernonia amygdalina: A folkloric herb with anthelminthic properties. Beni-Suef Univ J Basic Appl Sci. https://doi.org/10.1016/j.bjbas.2017.07.007
Huffman MA (2001) Self-medicative behavior in the African Great Apes: an evolutionary perspective into the origins of human traditional medicine. Bioscience 51(8):651–661
Iwu M (1993) Handbook of African medicinal plants. CRC Press, Boca Raton, pp 1–359
Ademola IO, Akanbi AI, Idowu SO (2005) Comparative nematocidal activity of chromatographic fractions of Leucaena leucocephala seed against gastrointestinal sheep nematodes. Pharm Biol 43(7):599–604
Evans WC (ed) (2009) Treas and evans pharmacognosy, 16th edn. Harcourt Pub. Ltd., Toronto
Sofowora A (1993) Medicinal plants and traditional medicine in Africa, 2nd edn. Spectrum Books Ltd., Ibadan
Aderibigbe SA, Idowu SO, Olaniyi AA, Wright CW, Fatokun AA (2021) Bioactivity and cytotoxicity profiling of strictosamide and vincosamide, anthelmintic epimers from Sarcocephalus latifolius (Smith) Bruce leaf. J Ethnopharmacol 265:1–10
Jeffery GH, Bassett J, Mendham J, Denney RC (eds) (1989) Vogel’s textbook of quantitative chemical analysis, 5th edn. Longman
Mayo DW, Miller FA, Hannah RW (2004) Course notes on the interpretation of infrared and raman spectra. John Wiley & Sons Inc, Hoboken, pp 1–462
Eloff JN, Angeh IE, Mcgaw LJ (2017) Solvent-solvent fractionation can increase the antifungal activity of a Melianthus comosus (Melianthaceae) acetone leaf extract to yield a potentially useful commercial antifungal product. Ind Crops Prod 110:103–112
Eloff JN (1998) Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol 60(1):1–8
Aulton M (2002) Dissolution and solubility. Aulton M (edS) Pharmaceutics: The Science of dosage form design, 2nd edn. Churchill Livingstone, London, pp 24–26
Clayden J, Greeves N, Warren S, Wothers P (2001) Organic Chemistry, 136th edn. Oxford University Press, Oxford, pp 20–21
Dean JA (ed) (1999) Lange’s Handbook of Chemistry, 15th edn. McGraw-Hill, New York, pp 477–481
Nyiredy S (2009) Thin layer chromatography: large-scale separations. In: Wilson ID, Poole CF (eds) Handbook of methods and instrumentation in separation science. Elsevier, UK, pp 778–780
Nalule AS, Mbaria JM, Kimenju JW (2013) In vitro anthelmintic potential of Vernonia amygdalina and Secamone africana on gastrointestinal nematodes. Agric Biol J North Am 4(1):54–66
Igile GO, Oleszek W, Jurzysta M, Burda S, Fafunso M, Fasanmade AA (1994) Flavonoids from Vernonia amygdalina and their antioxidant activities. J Agric Food Chem 42:2445–2448
Yeap SK, Ho WY, Beh BK, Liang WS, Ky H, Hadi A et al (2010) Vernonia amygdalina, an ethnoveterinary and ethnomedical used green vegetable with multiple bio- activities. J Med Plants Res 4(25):2787–2812
Aderibigbe SA, Idowu SO (2020) Anthelmintic activity of Ocimum gratissimum and Cymbopogon citratus leaf extracts against Haemonchus placei adult worm. J Pharm Bioresour 17(1):8–12
Eloff JN (2004) Quantification the bioactivity of plant extracts during screening and bioassay guided fractionation. Phytomedicine 11:370–371
Ohigashi H, Huffman MA, Koshimizu K, Kawanaka M, Sugiyama H, Kirby GC et al (1994) Toward the chemical ecology of medicinal plant use in chimpanzees: the case of Vernonia amygdalina, a plant used by wild chimpanzees possibly for parasite-related diseases. J Chem Ecol 20(3):541–553
Danquah CA, Koffuor GA, Annan K, Ketor EC (2012) The anthelmintic activity of Vernonia amygdalina (Asteraceae) and Alstonia boonei De Wild (Apocynaceae). J Med Biomed Sci 1:21–27
Ademola IO, Eloff JN (2011) Anthelminthic activity of acetone extract and fractions of Vernonia amygdalina against Haemonchus contortus eggs and larvae. Trop Anim Health Prod 43(2):521–527
Nweze NE, Ogidi A, Ngongeh LA (2013) Anthelmintic potential of three plants used in Nigerian ethnoveterinary medicine. Pharm Biol 51(3):311–315
Agyare C, Spiegler V, Sarkodie H, Asase A, Liebau E, Hensel A (2014) An ethnopharmacological survey and in vitro confirmation of the ethnopharmacological use of medicinal plants as anthelmintic remedies in the Ashanti region, in the central part of Ghana. J Ethnopharmacol 158:255–263. https://doi.org/10.1016/j.jep.2014.10.029
Alawa CBI, Adamu AM, Gefu JO, Ajanusi OJ, Abdu PA, Chiezey NP et al (2003) In vitro screening of two Nigerian medicinal plants (Vernonia amygdalina and Annona senegalensis) for anthelmintic activity. Vet Parasitol 113:73–81
Sirama V, Kokwaro J, Owuor B, Yusuf A, Kodhiambo M (2015) In-vitro anthelmintic activity of Vernonia amygdalina Del. (Asteraceae) roots using adult Haemonchus contortus worms. Int J Pharmacol Res 5(1):1–7
Nalule AS, Karue CN, Katunguka-Rwakishaya E (2011) Anthelmintic activity of Phytolacca dodecandra and Vernonia amygdalina leaf extracts in naturally infected small East African goats. Livest Res Rural Dev 23(12):1–10
Adediran OA, Uwalaka EC (2015) Effectiveness evaluation of levamisole, albendazole, ivermectin, and Vernonia amygdalina in West African Dwarf Goats. J Parasitol Res. https://doi.org/10.1155/2015/706824
Onyenwe I, Ngongeh L, Udekwu C, Ezeugwu G (2010) Preliminary studies on the anthelmintic effects of ethanolic extract of Garcinia kola (Heckel) seed and methanolic extract of sacoglottis gabonensis (baillon) stem bark on Heligmosomoides bakeri larvae in Nsukka Nigeria. Glob J Pure Appl Sci. https://doi.org/10.4314/gjpas.v16i1.62827
Diehl MS, Atindehou KK, Téré H, Betschart B (2004) Prospect for anthelminthic plants in the Ivory coast using ethnobotanical criteria. J Ethnopharmacol 95(2–3):277–284
Villasenor IM, Gajo RMT, Gonda RC (1997) Bioactivity studies on the alkaloid extracts from seeds of Leucaena leucocephala. Phyther Res 11(8):615–617
Ademola IO, Idowu SO (2006) Anthelmintic activity of Leucaena leucocephala seed extract on Haemonchus contortus infective larvae. Vet Rec 158:485–486
dos Soares AMS, de Araújo SA, Lopes SG, Costa Junior LM (2015) Anthelmintic activity of Leucaena leucocephala protein extracts on Haemonchus contortus. Rev Bras Parasitol Veterinária 24(4):396–401
Sigma-Aldrich: IR Spectrum Table and Chart (2021) https://www.sigmaaldrich.com/NG/en/technical-documents/technical-article/analytical-chemistry/photometry-and-reflectometry/ir-spectrum-table. Accessed 22 Dec 2021
Acknowledgements
The support provided by Prof I. O. Ademola, and Dr I. M. Akanbi (Principal Veterinary Medical Officer, Department of Veterinary Services, Ministry of Agriculture, Natural Resources and Rural Developments, Ibadan, Oyo state, Nigeria) is gratefully acknowledged.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SAA and SOI designed the experiment; and all authors carried out the experiment. All authors analysed the results and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aderibigbe, S.A., Opayemi, O.S., Bolaji, S.A. et al. In vitro effect of three tropical plants on adult Haemonchus placei, an haematophagous nematode from cattle. Beni-Suef Univ J Basic Appl Sci 11, 72 (2022). https://doi.org/10.1186/s43088-022-00255-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00255-7