Abstract
Background
Breast cancer is the most common cancer among women, and melanoma incidence increases worldwide. The emergence of drug resistance and side effects of chemotherapy drugs has led to a great deal of attention being paid to the development of natural medicines, especially using essential oil. The preparation of essential oil-based nanoformulation has thus recently received more attention.
Results
In this study, chitosan nanoparticles (ChiNPs) containing Zataria multiflora essential oil with a particle size of 177 ± 10 nm, a narrow particle size distribution (SPAN 0.96), and a cubic-like shape were first prepared. IC50 values of the prepared nanoformulation against human melanoma (A-375) and breast cancer cell lines (MCF-7 and MDA-MB-468) were obtained as 32 (12–84), 46 (32–67), and 105 (85–131) µg/mL. Besides, an electrospun polycaprolactone–polyethylene oxide scaffold was prepared as a dressing after treatment with the nanoformulation. Fourier transform infrared analysis confirmed the scaffold's preparation as well as successful loading of the essential oil in chitosan nanoparticles. Furthermore, the scaffold did not show a cytotoxic effect on A-375, MCF-7, and MDA-MB-468, and its surface was hydrophobic as the water contact angle with the surface was 136.5°.
Conclusions
The prepared prototype with natural ingredients and high efficacy could be considered for further consideration in vivo study or complementary medicine.
Graphical abstract

Similar content being viewed by others
1 Background
After cardiovascular disease, cancers with approximately 17% of the global deaths are major health challenges worldwide. In addition, cancer imposes many onerous burdens, including emotional, physical, and financial encumbrance, on humankind societies [1]. Although the cancer rate was decreased by 3.1% in men yearly, it has a steady state in women (from 2009 to 2012) [2]. Melanoma (cancer of melanocytes) and breast cancer are two of the most dreadful cancers in the world. Breast cancer is the most predominant cancer in Asian nations [3]. As the incidence rate of melanoma has recently increased worldwide, an urgent consideration is required to reduce its morbidity and mortality [4, 5].
The side effects of synthetic or semisynthetic anticancer drugs, including a remarkable decrease in white blood cell count, loss of immunity, bone marrow depression, severe physical weakness, and alopecia, were given serious concern [6]. Therefore, natural products, especially essential oils (EOs), have received special attention in developing new anticancer drugs with less harmful effects [7]. However, since EOs are hydrophobic, to enhance their performance in laboratory and animal research, the preparation of EO-loaded nanostructures (e.g., nanofibers, nanoparticles, and lipid nanocarriers) has received more attention [8, 9]. For instance, as a natural biocompatible and biodegradable polymer, ChiNPs have been widely employed in drug delivery research. For example, ChiNPs containing Torreya grandis EO with a particle size of 349.6 nm offered a more potent antibacterial agent than non-formulated EO [10]. In another research, ChiNPs (30–80 nm) containing Carum copticum EO showed a better antioxidant effect than the bulk EO [11].
Our previous studies investigated the cytotoxicity of some EOs against A-375 melanoma cells and MCF-7 and MDA-MB-468 human breast cancer cells. For instance, the IC50 value of Myrtus communis EO against A-375 was 580.8 µg/mL [12]. Besides, Mentha spicata and Tanacetum balsamita EOs IC50 values’ against A-375 were 1136 and 1312 µg/mL. On the other hand, their efficacy against MDA-MB-468 cells was 1067 and 2323 µg/mL [13]. IC50 values of their major ingredients, i.e., carvone, were obtained as 3657 and 6038 µg/mL against A-375 and MDA-MB-468 cells [13]. Moreover, IC50 values of clove EO against A-375 and MDA-MB-468 were 545 and 243 µg/mL [14]. Besides, IC50 values of Anethum graveolens, Citrus limon, and Zingiber officinale EOs against MCF-7 were 1908, 201, and > 500 µg/mL; their efficacy on MDA-MB-468 cells were 403, 210, and 775 µg/mL [15]. However, the efficacy of Zataria multiflora Bioss. EO was more potent than the mentioned EO; its IC50 values against A-375, MCF-7, and MDA-MB-468 were obtained as 59, 76, and 302 µg/mL [8, 16]. Therefore, this EO was selected for further investigation in the current study. ChiNPs containing Z. Multiflora EO were thus first investigated, and their efficacy was then investigated on melanoma and breast cancer cells (A-375, MCF-7, and MDA-MB-468). Besides, a polycaprolactone–polyethylene oxide electrospun scaffold was proposed as dressing after topical treatment with the nanoparticles.
2 Methods
2.1 Materials
The cell lines were purchased from the Pasteur Institute of Iran; A-375 (ATCC CRL-1619), MCF-7 (ATCC HTB-22), and MDA-MB-468 (ATCC HTB-132). Polycaprolactone (PCL 80.000 Da), polyethylene oxide (PEO 20.000 Da), acetic acid, phosphate-buffered saline tablets, tween 20, sodium-tri-polyphosphate (TPP), chitosan low molecular weight, and 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazo-lium bromide (MTT) were purchased from Sigma-Aldrich (USA). Fetal bovine serum was purchased from Gibco (USA). Dulbecco’s Modified Eagle’s Media (DMEM) cell culture medium, trypsin, penicillin–streptomycin, and dimethyl sulfoxide were obtained from a Chinese company, Shellmax.
2.2 Preparation of chitosan nanoparticles containing Z. multiflora EO
Chitosan powder (0.25% w/v) was dissolved in a 1% acetic acid aqueous solution (4 h, 2000 rpm, ambient temperature). In order to prepare ChiNPs containing Z. multiflora EO, a modified ionic gelation technique was employed [17]. In the first step, the EO (0.5% w/v) and tween 20 (0.25% w/v) were mixed at room temperature for 3 min while the rotation speed was 2000 rpm (Fig. 1). In the next 30 min, the chitosan solution was added and stirred. Then, a syringe pump was employed to add 1 mL/h TPP (0.15% w/v) aqueous solution. The mixture was stirred for 40 min (2000 rpm) to stabilize the ChiNPs containing Z. multiflora EO. The same methodology was used for preparing free chitosan nanoparticles; only no EO was used.
2.3 Preparation of PCL–PEO scaffold
PCL granules and PEO powder (10%:4% w/v) were dissolved in hexafluoroisopropanol (overnight/ 2000 rpm/ room temperature). The solution was poured into a 10 mL syringe connected to a blunted needle (23 G) and was situated in a syringe pump of the electrospinning machine (Fanavaran nano-meghyas, Iran). Instrumental factors were optimized for preparing the beadles nanofibers with the nano-sized diameter; 0.7 mL/h the injection rate, 20 kV applied DC voltage between needle and collector, and 120 mm distance between needle and collector. In order to separate the formed nanofibrous scaffold, the cylindrical collector (diameter 7.5 cm) was covered using a thin layer of aluminum foil. The collector was rotated during the preparation of scaffolds (110 rpm).
2.4 Characterizations of the prepared nanostructures
The dynamic light scattering (DLS) technique (K-ONE NANO. LTD, Korea) analyzed the particle size of ChiNPs containing Z. multiflora EO. D50 and d90-d10/d50 were considered particle size and size distribution (SPAN). D is the diameter, and 10, 50, and 90 show the percentile of particles with a smaller diameter than these specified diameters. Transmission electron microscopy (LEO 906E Zeiss, Germany) confirmed the particle size of ChiNPs containing Z. multiflora EO and determined their morphology. The sample was first concisely diluted using distilled water twice, and one drop was then located on a 200-mesh carbon-coated copper grid and applied to the device. ChiNPs nanoparticles and preparation of PCL–PEO scaffold were confirmed using Fourier transform infrared analysis (Bruker Company, Model Tensor II, Germany). The spectra of ChiNPs containing Z. multiflora EO, the scaffold, and their ingredients were recorded in 400–4000/cm.
Scanning electron microscopy was used to investigate the morphology and size of the PCL–PEO scaffold (Scanning Electron Microscopy, Vega 3, TESCAN, Czech Republic). The scaffolds were punched and coated with gold vapor (sputtering coater, Q150R-ES, Quorum Technologies, UK) before subjecting to the scanning electron microscopy instrument. Besides, the wettability of the prepared scaffold was evaluated by determining the contact angle of deionized water with its surface. A five μL volume of deionized water was injected into the surface of the scaffold, and the contact angle was measured.
2.5 Investigation of the anticancer activity
The MTT assay was used to investigate the anticancer activity of ChiNPs containing Z. multiflora EO. The cell lines were cultured in 25 cm2 culture flasks using DMEM medium cell culture (supplemented with 10% and 1% of fetal bovine serum and penicillin–streptomycin) and incubated at 37 °C in an air/CO2 mixture (95:5%). First, the cells (A-375, MDA-MB-468, and MCF-7) were separated by trypsin; then, they were seeded (1 × 104 cells per well) in 96 well plates and incubated overnight for attachment. The next day, the culture media was discarded, and a 75 µL complete fresh medium was added to each well. Finally, concentrations were fixed at 1200, 600, 300, 150, and 75 µg/mL by adding appropriate amounts of ChiNPs containing Z. multiflora EO. Moreover, a piece (0.5 cm) of PCL–PEO scaffold was got into other wells to investigate their cytotoxicity.
The treated plates were incubated for 24 h at 37 °C with CO2 5%. Then, their content was discarded, and wells were washed with 100 μL phosphate-buffered saline to remove the nanoformulations' milky color and non-degraded scaffold. In the next step, 100 μL MTT reagent (0.5 mg mL−1) was added to each well and incubated for another 4 h; dimethyl sulfoxide then dissolved created formazan crystals (100 μL/well). Finally, by an ELISA plate reader, the absorbance of the wells was measured at 570 nm; the cell viability at each concentration was calculated using the following equation; (mean absorbance sample/mean absorbance control) × 100. Noteworthy, the control group (six-well/plate) was not treated.
3 Results
3.1 Characterization of chitosan nanoparticles containing Z. multiflora EO
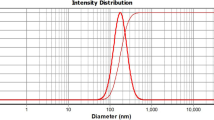
DLS diagram of ChiNPs containing Z. multiflora EO with particle sizes of 177 ± 10 nm is depicted in Fig. 2A. The SPAN value is less than 1 (0.96) [18], so its narrow particle size distribution was confirmed. In addition, the presence of a peak sharp in nanoformulations is also indicated narrow particle size distribution. Finally, the transmission electron microscopy verified the morphology of ChiNPs containing Z. multiflora EO; it revealed a cubic shape (Fig. 2B).
3.2 Loading of Z. multiflora EO in chitosan nanoparticles
Fourier transform infrared analysis is popular optical spectroscopy for identifying the molecular structure and possible interactions between the main components of polymeric nanoparticles or scaffolds [19]. Spectra of free chitosan nanoparticles, Z. multiflora EO, ChiNPs containing Z. multiflora EO, as well as PCL, PEO, and PEO–PCL scaffold spectra, are shown in Fig. 3A.
In the spectrum of free chitosan nanoparticles, the strong bond at about 1700 cm−1 can correspond to carbonyl stretching of the secondary amide band of the pure chitosan and carbonyl group in tween. The characteristic peak at 1094 cm−1 relates to symmetric and anti-symmetric stretching vibrations in the PO2 group. The strong band at 1020 cm−1 belongs to symmetric and anti-symmetric stretching vibrations in the PO3 group. After the crosslinking process, two bands at 1280 and 1152 cm−1 belonging to anti-symmetric stretching vibrations of PO2 groups in TPP ions appeared. This new peak showed the formation of ionic crosslinks between protonated amino groups of chitosan and tripolyphosphate anionic groups [20]. The Z. multiflora EO spectrum showed broadband between 3200 and 3600 cm−1, characteristic of the hydroxyl functional group, and a band at 3019 cm−1, attributed to the stretching vibration of =C–H groups from olefins. In addition, peaks at 2959, 2925, and 2869 cm−1 relate to –CH's stretching vibrations and the absorption peak around 1737 cm−1 relates to C=O. The absorption peaks around 1619 and 1420 cm−1 are attributed to C=C, peaks at 1222 and 1175 cm−1 relate to (C–O–C) bonds, and the ones at 809 cm−1 are attributed to angular deformations of CH2 groups. In spectra of ChiNPs containing Z. multiflora EO, two bands at 1280 and 1098 cm−1 belong to the linkage between phosphoric groups of TPP and the ammonium group of chitosan. This new peak showed the formation of ionic crosslinks between protonated amino groups of chitosan and TPP anionic groups. The other characteristic peaks are similar to Z. multiflora EO.
3.3 Physicochemical characteristics of PCL–PEO scaffolds
The spectra of PCL and PEO and the PEO–PCL scaffold are shown in Fig. 3B. The main characteristic peaks of pure PEO at 2878 and 1466 cm−1 are attributed to the asymmetric stretching and asymmetric bending of CH2. The peaks at 1341 and 1359 cm−1 are associated with the bending vibration of –CH2. The triplet peaks at 1144, 1094, and 1059 cm−1 are related to the C–O–C vibration and are also assigned to the existence of the crystalline PEO. The peaks at 960 cm−1 and 841 cm−1 are associated with the CH2 rocking vibrations of the methylene (–CH3) group [21, 22]. In the case of PCL, the prominent peak at 1722 cm−1 is associated with the carbonyl (C=O) group. The characteristic peaks located at 2943 and 2865 cm−1 are assigned to the stretching vibration of –CH2. The FTIR spectrum of PCL also exhibited absorption bands at 1292 cm−1 for C–O and C–C stretching and at 1164 and 1236 cm−1 for COC symmetric and asymmetric stretching, respectively [23]. Importantly, the main characteristic bands of each compound (PEO and PCL) appeared in the FTIR spectrum of the PEO–PCL scaffold, thus suggesting that both PEO and PCL were present in the prepared scaffolds [24]. The PCL's C=O stretching vibration at 1722 cm−1 gets shifted to 1704 cm−1 in the PCL–PEO scaffold with lower intensity. The absorption peaks at 2943 and 2865 cm−1 corresponding to stretching vibration of –CH2 in PCL are decreased in intensity in the PCL–PEO scaffold. The shifting of the main peaks indicates the molecular interaction of PEO and PCL in the final scaffold [22, 24].
The wettability of solid surfaces is investigated with a water contact angle meter. If the measured contact angle is above 90 degrees, the solid has poor wetting and is called hydrophobic [25]. The water contact angle of the PCL–PEO scaffold was high as 136.5° (Fig. 4A). Besides, Fig. 4B shows randomly oriented and beadles PCL–PEO nanofibrous with a mean diameter of 246 ± 39 nm.
3.4 In vitro anticancer activity of chitosan nanoparticles containing Z. multiflora EO
Figure 5 indicates the dose-dependent effects of the ChiNPs containing Z. multiflora EO on all examined cell lines, including the A-375 melanoma cell line and two breast cancer cell lines, MCF-7 and MDA-MB-468. Interestingly, the viability of A-375 and MCF-7 more than 90% were reduced after treatment with ChiNPs containing Z. multiflora EO 600 and 1200 µg/mL. Besides, the viability of cells after treatment with free ChiNPs 10–15% was reduced. Moreover, the scaffold did not significantly affect three cell lines (data not shown).
Furthermore, IC50 values of ChiNPs containing Z. multiflora EO against A-375, MCF-7, and MDA-MB-468 were obtained as 32 (12–84), 46 (32–67), and 105 (85–131) µg/mL, respectively.
4 Discussion
Zataria multiflora (Lamiaceae family) is one of the most common medicinal plants that grows in Pakistan, Afghanistan, and southern Iran [26]. Its EO possesses some biological properties, such as antibacterial, antioxidant, and anticancer effects [27, 28]. In this study, the anticancer effect of ChiNPs containing Z. multiflora EO on the viability of melanoma (A-375) and breast cancer (MCF-7 and MDA-MB-468) cells was examined. A-375 human melanoma cell maintains typical cutaneous melanoma characteristics and is a suitable in vitro model for studying human cancers' most aggressive, treatment-resistant, and chemo-resistant form [29, 30]. MCF-7 cell is a suitable in vitro model for investigating breast cancer pathogenesis and anticancer drugs and is the most studied ER-positive cell line globally [31, 32]. MDA-MB-468, as an ER-negative breast cancer cell line, is a target cell line for evaluation in vitro invasive and metastatic cancer models [33]. Since the 1970s, incidence rates for many cancers like lung cancer have decreased, but breast and skin cancers are the most commonly diagnosed cancers with high incidence and poor survival rates [34]. Therefore, further research and more effective chemotherapeutic agents are needed to achieve the best outcomes for treating these cancers.
Plant-derived substances originate about 50% of the clinically active chemotherapeutic agents [35, 36]. At least four classes of herbal anticancer agents are on the market today, vinca alkaloids (vincristine, vindesine, and vinblastine), epipodophyllotoxins (teniposide and etoposide), taxanes (docetaxel and paclitaxel), and the camptothecin derivatives (irinotecan and camptothecin) [37]. Moreover, many attempts have been made to exploit EOs as antioxidant, antimicrobial, and anticancer drugs. Nevertheless, besides low water solubility, EOs main compounds have low absorption, low efficiency, and low plasma membrane permeability, which has the limited clinical application of these herbal compounds [29, 38]. Therefore, the preparation of EO-based nanoformulation is a promising approach that increases cellular uptake, solubility, and biological and pharmacological activities. Nanoformulation could also reduce the dosage use, toxicity, and side effects and increase the noticeable efficacy of EOs [39]. For example, nanoformulated Mentha piperita EO, as noted by Abedinpour et al., increases noticeably cytotoxic and efficacy of Mentha piperita EO against human breast cancer cell lines [18]. It has also been indicated that lipid nanoparticles increased the release rate of EO compared to pure EO. Furthermore, Poladi et al. have shown that Artemisia EO inhibits cancer cell viability, which is more effective by nanoformulation [40]. Furthermore, emerging evidence suggests that ChiNPs can penetrate cancerous cells and induce DNA damage, and finally disrupt cancer cell growth and metabolism [41, 42]. ChiNPs containing EOs have thus been widely used to improve the EOs’ therapeutic and pharmacological effects. For instance, Soltani et al. designed a cytotoxicity study against liver hepatocellular carcinoma cells by loading Boswellia sacra EO in ChiNPs with a particle size of 80.13 nm [43]. Another group proposed ChiNPs containing green tea EO with a mean particle size of 30.7 ± 1.13 nm as a natural drug delivery system to cancer against hepatocellular breast and colon carcinoma cells [44].
In the present study, preclinical cancer models (MCF-7, MDA-MB-468, and A-375) investigated the anticancer effect of ChiNPs containing Z. multiflora EO with a particle size of 177 ± 10 nm. In vitro treatment of both ER-positive (MCF-7), negative breast cancer (MDA-MB-468), and melanoma (A-375) cells by ChiNPs containing Z. multiflora EO inhibited growth percentages in cancer cells. The obtained IC50 value for ChiNPs containing Z. multiflora EO against A-375 melanoma cells (IC50 = 32 μg/mL) lower than ER-negative MDA-MB-468 cells (IC50 = 105 μg/mL). ER-positive MCF-7 breast cancer cells (IC50 = 46 μg/mL) was sensitive compare to the ER-negative MDA-MB-468 cells (IC50 = 105 μg/mL). Our results agreed with the findings of previous studies indicating that Z. multiflora EO has anticancer properties on breast, melanoma, and colon cancers and has the potential to be used in cancer treatment [45, 46]. The results demonstrated that A-375 melanoma cells are more sensitive toward ChiNPs containing Z. multiflora EO than ER-positive and –negative breast cancer. It can be concluded that ChiNPs containing Z. multiflora EO has a cell line-dependent anticancer activity. In agreement with these results, Yerlikaya et al. showed that chitosan could reduce cell proliferation in melanoma cell lines (A-375, SKMEL28, and RPMI7951) with different mechanisms and cells line-dependent manner [47]. It is important to note that, the potency of ChiNPs containing Z. multiflora EO in our study was more potent than non-formulated Z. multiflora EO in previous studies against these cells; A-375 (IC50 = 59 µg/mL), MCF-7 (IC50 = 76 µg/mL), and MDA-MB-468 (IC50 = 302 µg/mL) [8, 16]. The findings reported in this study and previous results give depth to our understanding of cell line-dependent anticancer activity of ChiNPs.
The wettability of biomaterials is one of the most important properties considered in tissue engineering for skin diseases such as metastatic melanoma [48, 49]. Pathogenic infections increase patient mortality because of a weakened immune system in cancer patients [50]. This study prepared a PCL–PEO scaffold to create a skin coating for melanoma patients after treatment with ChiNPs containing Zataria multiflora EO. The scaffold could be used as a protective coating in melanoma patients for inhibiting the entry of environmental pathogens into the site, allowing air exchange [51, 52].
5 Conclusions
The findings of in vitro cancer models suggest that the ChiNPs containing Z. multiflora EO could act as a drug supplement to inhibit cancer cell proliferation (breast and melanoma cells). The comparative study showed that the anticancer effect of ChiNPs containing Z. multiflora EO was more pronounced in melanoma cells than in breast cancer cells. The PCL–PEO scaffold was also proposed as a skin coating after treatment with the nanoformulation. The prepared prototype could be considered for further investigations in in vivo studies.
Availability of data and materials
All data are given in the current report.
Abbreviations
- ChiNPs:
-
Chitosan nanoparticles
- Eos:
-
Essential oils
- MTT:
-
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyltetrazo-lium bromide
- PCL:
-
Polycaprolactone
- PEO:
-
Polyethylene oxide
- DLS:
-
Dynamic light scattering
- TPP:
-
Sodium-tri-polyphosphate
References
Cancer (2021) https://www.who.int/news-room/fact-sheets/detail/cancer
Nagai H, Kim YH (2017) Cancer prevention from the perspective of global cancer burden patterns. J Thorac Dis 9(3):448–451. https://doi.org/10.21037/jtd.2017.02.75
Bhushan A, Gonsalves A, Menon JU (2021) Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics 13(5):723. https://doi.org/10.3390/pharmaceutics13050723
Spoerl S, Spanier G, Reiter E, Gerken M, Haferkamp S, Grosse J, Drexler K, Ettl T, Klinkhammer-Schalke M, Fischer R, Spoerl S, Reichert TE, Klingelhoffer C (2021) Head and neck melanoma: outcome and predictors in a population-based cohort study. Head Face Med 17(1):45. https://doi.org/10.1186/s13005-021-00295-x
Zito PM, Scharf R (2021) Melanoma of the head and neck. StatPearls, Treasure Island
Nurgali K, Jagoe RT, Abalo R (2018) Editorial: adverse effects of cancer chemotherapy: anything new to improve tolerance and reduce sequelae? Front Pharmacol 9:245. https://doi.org/10.3389/fphar.2018.00245
Sharifi-Rad J, Ozleyen A, Tumer TB, Oluwaseun Adetunji C, El Omari N, Balahbib A, Taheri Y, Bouyahya A, Martorell M, Martins N, Cho W (2019) Natural products and synthetic analogs as a source of antitumor drugs. Biomolecules 9(11):679. https://doi.org/10.3390/biom9110679
Valizadeh A, Khaleghi AA, Roozitalab G, Osanloo M (2021) High anticancer efficacy of solid lipid nanoparticles containing Zataria multiflora essential oil against breast cancer and melanoma cell lines. BMC Pharmacol Toxicol 22(1):52. https://doi.org/10.1186/s40360-021-00523-9
Alipanah H, Farjam M, Zarenezhad E, Roozitalab G, Osanloo M (2021) Chitosan nanoparticles containing limonene and limonene-rich essential oils: potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement Med Ther 21(1):186. https://doi.org/10.1186/s12906-021-03362-7
Wu J, Shu Q, Niu Y, Jiao Y, Chen Q (2018) Preparation, characterization, and antibacterial effects of chitosan nanoparticles embedded with essential oils synthesized in an ionic liquid containing system. J Agric Food Chem 66(27):7006–7014. https://doi.org/10.1021/acs.jafc.8b01428
Esmaeili A, Asgari A (2015) In vitro release and biological activities of Carum copticum essential oil (CEO) loaded chitosan nanoparticles. Int J Biol Macromol 81:283–290. https://doi.org/10.1016/j.ijbiomac.2015.08.010
Roozitalab G, Yousefpoor Y, Abdollahi A, Safari M, Rasti F, Osanloo M (2022) Antioxidative, anticancer, and antibacterial activities of a nanoemulsion-based gel containing Myrtus communis L. essential oil. Chem Pap. https://doi.org/10.1007/s11696-022-02185-1
Alipanah H, Rasti F, Zarenezhad E, Dehghan A, Sahebnazar B, Osanloo M (2022) Comparison of anticancer effects of carvone, carvone-rich essential oils, and chitosan nanoparticles containing each of them. Biointerface Res Appl Chem 12(4):5716–5726. https://doi.org/10.33263/BRIAC124.57165726
Valizadeh A, Khaleghi AA, Alipanah H, Zarenezhad E, Osanloo M (2021) Anticarcinogenic effect of chitosan nanoparticles containing syzygium aromaticum essential oil or eugenol toward breast and skin cancer cell lines. BioNanoScience. https://doi.org/10.1007/s12668-021-00880-z
Mahmoud O, Ali G, Ali T (2021) Antioxidant and anticancer activities of Anethum graveolens L., Citrus limon (L.) Osbeck and Zingiber officinale Roscoe essential oils. Tradit Integr Med 6(4):333–347. https://doi.org/10.18502/tim.v6i4.8266
Ghanbariasad A, Osanloo M (2020) Development of two stable green nanoformulations with potent anticancer properties. Nanomed Res J 5(3):234–244. https://doi.org/10.22034/NMRJ.2020.03.004
Osanloo M, Sedaghat M, Sereshti H, Rahmani M, Saeedi Landi F, Amani A (2019) Chitosan nanocapsules of tarragon essential oil with low cytotoxicity and long-lasting activity as a green nano-larvicide. J Nanostruct 9(4):723–735. https://doi.org/10.22052/JNS.2019.04.014
Abedinpour N, Ghanbariasad A, Taghinezhad A, Osanloo M (2021) Preparation of nanoemulsions of mentha piperita essential oil and investigation of their cytotoxic effect on human breast cancer lines. BioNanoScience 11(2):428–436. https://doi.org/10.1007/s12668-021-00827-4
Ghosal K, Manakhov A, Zajíčková L, Thomas S (2017) Structural and surface compatibility study of modified electrospun poly (ε-caprolactone)(PCL) composites for skin tissue engineering. AAPS PharmSciTech 18(1):72–81. https://doi.org/10.1208/s12249-016-0500-8
Hussain Z, Sahudin S (2016) Preparation, characterisation and colloidal stability of chitosan-tripolyphosphate nanoparticles: optimisation of formulation and process parameters. Int J Pharm Pharm 8(3):297–308
Anilkumar K, Jinisha B, Manoj M, Jayalekshmi S (2017) Poly (ethylene oxide)(PEO)–Poly (vinyl pyrrolidone)(PVP) blend polymer based solid electrolyte membranes for developing solid state magnesium ion cells. Eur Polym J 89:249–262. https://doi.org/10.1016/j.eurpolymj.2017.02.004
Zhou Y, Qi P, Zhao Z, Liu Q, Li Z (2014) Fabrication and characterization of fibrous HAP/PVP/PEO composites prepared by sol-electrospinning. RSC Adv 4(32):16731–16738. https://doi.org/10.1039/C3RA47168C
Eldurini S, Abd El-Hady BM, Shafaa MW, Gad AAM, Tolba E (2020) A multicompartment vascular implant of electrospun wintergreen oil/polycaprolactone fibers coated with poly (ethylene oxide). Biomed J. https://doi.org/10.1016/j.bj.2020.04.008
Eskitoros-Togay ŞM, Bulbul YE, Tort S, Korkmaz FD, Acartürk F, Dilsiz N (2019) Fabrication of doxycycline-loaded electrospun PCL/PEO membranes for a potential drug delivery system. Int J Pharm 565:83–94. https://doi.org/10.1016/j.ijpharm.2019.04.073
Qasemi H, Fereidouni Z, Karimi J, Abdollahi A, Zarenezhad E, Rasti F, Osanloo M (2021) Promising antibacterial effect of impregnated nanofiber mats with a green nanogel against clinical and standard strains of Pseudomonas aeruginosa and Staphylococcus aureus. J Drug Deliv Sci Technol 66:102844. https://doi.org/10.1016/j.jddst.2021.102844
Sajed H, Sahebkar A, Iranshahi M (2013) Zataria multiflora Boiss. (Shirazi thyme)—an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol 145(3):686–698. https://doi.org/10.1016/j.jep.2012.12.018
Rabiei GKF (2015) Zataria multiflora: chemical and biological diversity in the essential oil. J Essent Oil Res 30(6):428–436. https://doi.org/10.1080/10412905.2015.1031917
Shokri H, Sharifzadeh A (2016) Zataria multiflora Boiss.: a review study on chemical composition, anti-fungal and anti-mycotoxin activities, and ultrastructural changes. J HerbMed Pharmacol 6(1):1–9
Zhang W, Jiang H, Zhang J, Zhang Y, Liu A, Zhao Y, Zhu X, Lin Z, Yuan X (2014) Liver X receptor activation induces apoptosis of melanoma cell through caspase pathway. Cancer Cell Int 14(1):1–6. https://doi.org/10.1186/1475-2867-14-16
Jerjes W, Theodossiou TA, Hirschberg H, Høgset A, Weyergang A, Selbo PK, Hamdoon Z, Hopper C, Berg K (2020) Photochemical internalization for intracellular drug delivery. From basic mechanisms to clinical research. J Clin Med 9(2):528. https://doi.org/10.3390/jcm9020528
Comşa Ş, Cimpean AM, Raica M (2015) The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Res 35(6):3147–3154
Lee AV, Oesterreich S, Davidson NE (2015) MCF-7 cells—changing the course of breast cancer research and care for 45 years. J Natl Cancer Inst 107(7):djv073. https://doi.org/10.1093/jnci/djv073
Kagan BL, Henke RT, Cabal-Manzano R, Stoica GE, Nguyen Q, Wellstein A, Riegel AT (2003) Complex regulation of the fibroblast growth factor-binding protein in MDA-MB-468 breast cancer cells by CCAAT/enhancer-binding protein β. Cancer Res 63(7):1696–1705
White MC, Peipins LA, Watson M, Trivers KF, Holman DM, Rodriguez JL (2013) Cancer prevention for the next generation. J Adolesc Health 52(5):S1–S7. https://doi.org/10.1016/j.jadohealth.2013.02.016
Pandey BP, Thapa R, Upreti A (2017) Chemical composition, antioxidant and antibacterial activities of essential oil and methanol extract of Artemisia vulgaris and Gaultheria fragrantissima collected from Nepal. Asian Pac J Trop Med 10(10):952–959. https://doi.org/10.1016/j.apjtm.2017.09.005
El Hamdaoui A, Msanda F, Boubaker H, Leach D, Bombarda I, Vanloot P, El Aouad N, Abbad A, Boudyach E, Achemchem F (2018) Essential oil composition, antioxidant and antibacterial activities of wild and cultivated Lavandula mairei Humbert. Biochem Syst Ecol 76:1–7. https://doi.org/10.1016/j.bse.2017.11.004
Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, Bedi YS, Taneja SC, Bhat HK (2008) Medicinal plants and cancer chemoprevention. Curr Drug Metab 9(7):581–591. https://doi.org/10.2174/138920008785821657
Bonifácio BV, da Silva PB, dos Santos Ramos MA, Negri KMS, Bauab TM, Chorilli M (2014) Nanotechnology-based drug delivery systems and herbal medicines: a review. Int J Nanomedicine 9:1–15. https://doi.org/10.2147/IJN.S52634
Ansari S, Islam F, Sameem M (2012) Influence of nanotechnology on herbal drugs: A Review. J Adv Pharm Technol Res 3(3):142–146. https://doi.org/10.4103/2231-4040.101006
Pooladi M, Teimouri M, Odoumizadeh M (2021) Cytotoxicity of Artemisia vulgaris essential oil encapsulated in SLN on breast cancer cell line (MCF7). Arch Adv Biosci 12(3):11–26. https://doi.org/10.22037/aab.v12i3.34543
Pugazhendhi A, Edison TNJI, Karuppusamy I, Kathirvel B (2018) Inorganic nanoparticles: a potential cancer therapy for human welfare. Int J Pharm 539(1–2):104–111. https://doi.org/10.1016/j.ijpharm.2018.01.034
Shanmuganathan R, Edison TNJI, LewisOscar F, Kumar P, Shanmugam S, Pugazhendhi A (2019) Chitosan nanopolymers: an overview of drug delivery against cancer. Int J Biol Macromol 130:727–736. https://doi.org/10.1016/j.ijbiomac.2019.02.060
Soltani M, Etminan A, Rahmati A, Behjati Moghadam M, Ghaderi Segonbad G, Homayouni Tabrizi M (2021) Incorporation of Boswellia sacra essential oil into chitosan/TPP nanoparticles towards improved therapeutic efficiency. Mater Technol. https://doi.org/10.1080/10667857.2021.1976364
Farrag NS, Shetta A, Mamdouh W (2021) Green tea essential oil encapsulated chitosan nanoparticles-based radiopharmaceutical as a new trend for solid tumor theranosis. Int J Biol Macromol 186:811–819. https://doi.org/10.1016/j.ijbiomac.2021.07.077
Valizadeh A, Khaleghi AA, Roozitalab G, Osanloo M (2021) High anticancer efficacy of solid lipid nanoparticles containing Zataria multiflora essential oil against breast cancer and melanoma cell lines. BMC Pharmacol Toxicol 22(1):1–7
Sharififar F, Miri-Moghaddam M, Nematollahi-Mahani S, Forootanfar H, AsgharianRezee M (2017) Cytotoxicity effect of Zataria multiflora Boiss. on two human colon carcinoma cell lines. Res J Pharmacogn 4(4):65–70
Gibot L, Chabaud S, Bouhout S, Bolduc S, Auger FA, Moulin VJ (2015) Anticancer properties of chitosan on human melanoma are cell line dependent. Int J Biol Macromol 72:370–379. https://doi.org/10.1016/j.ijbiomac.2014.08.033
DeLouise LA (2012) Applications of nanotechnology in dermatology. J Invest Dermatol 132(3):964–975. https://doi.org/10.1038/jid.2011.425
Dubey P, Bhushan B, Sachdev A, Matai I, Uday Kumar S, Gopinath P (2015) Silver-nanoparticle-incorporated composite nanofibers for potential wound-dressing applications. J Appl Polym Sci 132(35):42473. https://doi.org/10.1002/app.42473
Wang Y, Qian J, Liu T, Xu W, Zhao N, Suo A (2017) Electrospun PBLG/PLA nanofiber membrane for constructing in vitro 3D model of melanoma. Mater Sci Eng C 76:313–318. https://doi.org/10.1016/j.msec.2017.03.098
Ambekar RS, Kandasubramanian B (2019) Advancements in nanofibers for wound dressing: a review. Eur Polym J 117:304–336. https://doi.org/10.1016/j.eurpolymj.2019.05.020
Simões D, Miguel SP, Ribeiro MP, Coutinho P, Mendonça AG, Correia IJ (2018) Recent advances on antimicrobial wound dressing: a review. Eur J Pharm Biopharm 127:130–141. https://doi.org/10.1016/j.ejpb.2018.02.022
Acknowledgements
Not applicable.
Funding
Fasa University of Medical Sciences supported this study, Grant No. 400168.
Author information
Authors and Affiliations
Contributions
HA performed MTT tests. FY wrote the introduction. FR prepared the scaffold. ShH reviewed the literature. MS interpreted ATR-FTIR spectra. MO designed the study, analyzed data, and prepared chitosan nanoparticles. All authors contributed to the drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the ethical committee of Fasa University of Medical Sciences, Fasa, Iran. Besides, this research did not involve human study; thus, no constant form was used.
Consent for publication
Not applicable.
Competing interests
Researchers have no conflict of interest in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alipanah, H., Yarian, F., Rasti, F. et al. Cytotoxic effects of chitosan nanoparticles containing Zataria multiflora essential oil against human breast and melanoma cells. Beni-Suef Univ J Basic Appl Sci 11, 58 (2022). https://doi.org/10.1186/s43088-022-00241-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00241-z