Abstract
Background
Pro-inflammatory cytokines such as interleukin-5 (IL-5) and tumor necrosis factor-alpha (TNF-α) as well as immunoglobulin-E (IgE) appear to play a role in asthma. N-acetylcysteine (NAC), an antioxidant, might have clinical benefits in asthma prevention. The possible preventive effects of NAC against experimentally induced asthma in rats are investigated. The rats were allocated into five groups: a normal control, asthma control, a standard dexamethasone (DEXA, 1 mg/kg, orally) group, and two NAC groups (300 and 500 mg/kg, orally, respectively). Ovalbumin (OVA) sensitization was used to trigger asthma, which was then followed by an intra-nasal challenge. Test gents were administrated for 14 days before the challenge and during the three challenge days (20, 21, and 22). The tidal volume (TV) and peak expiratory flow rate (PEFR) as respiratory functions were determined. The pro-inflammatory cytokines as IL-5 and TNF-α were evaluated in lung homogenate. Serum IgE and absolute eosinophil count (AEC) in bronchoalveolar lavage fluid (BALF) were measured. In addition, the oxidative markers in lung tissue and nitrosative marker in BALF were assessed; finally, lungs were isolated for histopathological study.
Results
NAC restored lung functions, inhibited the asthma-dependent increase in TNF-α, IL-5, IgE, AEC, nitric oxide, and malondialdehyde levels. NAC further re-established lung glutathione content and superoxide dismutase activity, resulting in milder overall lung pathology.
Conclusions
Experimental bronchial asthma may be protected by NAC. The anti-asthmatic potential of NAC may be explained by its suppressant influence on IgE antibody formation, pro-inflammatory cytokines production, eosinophil infiltration, and oxidative stress.
Similar content being viewed by others
1 Background
Bronchial asthma is an inflammatory condition of the conducting airways that causes distinct structural and functional alterations in the airways, resulting in non-specific bronchial hyper-responsiveness (BHR) and fluctuating airflow restriction [1,2,3]. Reversible bronchospasm and chronic inflammation of the airway passages characterize asthma, which is caused by different types of cells, such as eosinophils, mast cells, and T lymphocytes. As a result of the inflammation, airway responsiveness to a range of stimuli increases [4].
It was reported that reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as superoxide anion, hydroxyl radicals, hydrogen peroxide, hypochlorous acid, and peroxynitrite, play a crucial role in bronchial asthma [5]. Furthermore, a rise in ROS productivity is inversely proportional with forced expiratory volume (FEV1) [6]. Airway inflammatory cells are the likely source of these increases. For instance, airway macrophages from asthmatic patients produce more superoxide than those from the normal subject [7]. Additionally, antigen challenge causes more pronounced spontaneous ROS production from airway eosinophils in patients with asthma [8].
When IgE attaches to membrane receptors in peripheral blood monocytes, they produce superoxide, and eosinophils separate from asthmatic patients create greater hydrogen peroxide 24 h after antigen exposure [9]. Multiple investigators have shown that increases in ROS that occur during asthma are associated with damage to a wide range of biological molecules in the lung [10].
The inflammatory response is mediated by oxidants either inhaled and/or released by the activated neutrophils, alveolar macrophages, eosinophils, and epithelial cells leading to the production of ROS and membrane lipid peroxidation. This leads to activation of transcription of pro-inflammatory cytokine and chemokine genes, up-regulation of adhesion molecules, and increased release of pro-inflammatory mediators which mediate inflammatory responses in patients with asthma and chronic obstructive pulmonary disease [10].
Airway remodeling is the structural changes in the airway including subepithelial fibrosis, increased smooth muscle mass, enlargement of glands, neovascularization, and epithelial alterations. Local factors, such as airway structural cells and the matrix, respond to inflammation in a predictable, organized manner, which could be a try to correct the injury produced by local inflammation and to maintain the airway intact [11].
N-acetylcysteine (NAC) has a range of important pharmacological actions that could explain its putative therapeutic roles. NAC is an antioxidant as it acts as a precursor of cysteine, the rate-limiting component in glutathione formation [12]. NAC has been purported to have anti-inflammatory properties [13]. NAC inhibits the induction of the pro-inflammatory transcription factors as nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1). The induction of these transcription factors in response to oxidative stress supports the theory that NAC's anti-inflammatory activities are related to its antioxidant mechanism of action [14].
Based on the aforementioned data, this study aims to assess the NAC's ability to prevent the incidence of bronchial asthma in rats. The aim extends to reveal the underlying mechanisms of such anti-asthmatic effect of NAC.
2 Methods
2.1 Animals
Male Wistar rats (150 ± 10 g) were obtained from animal house colonies, housed at a constant temperature of 25 °C with 12-hour/12-h light/dark cycles and free access to tap water and commercial pellets. Animal care and handling were carried out in compliance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978).
2.2 Drugs and chemicals
N-acetylcysteine (NAC), aluminum hydroxide (Al(OH)3), and ovalbumin (OVA; grade III) were purchased from Merck/MilliporeSigma Co., USA. Dexamethasone (DEXA) was purchased from Zhejiang Jingxin Pharmaceuticals Co., LTD, China. ELISA kits of IgE, TNF-α, and IL-5 were purchased from Express Biotech, Ray Biotech, the USA, and Cusabo Biotech Co., LTD, China, respectively.
2.3 Experimental design
Using a random-numbers table, forty rats were grouped into five (n = 8). Group 1 was kept as normal control, non-sensitized and non-treated, obtaining vehicles. Group 2: asthma control, was sensitized and challenged with OVA. Group 3: asthmatic rats were treated with DEXA as a standard drug (1 mg/kg, orally) [15]. Groups 4 and 5: asthmatic rats were treated with NAC (300 and 500 mg/kg, orally, respectively) [16, 17].
2.4 Bronchial asthma induction
2.4.1 Intra-peritoneal sensitization with OVA
On days 1, 2, 3, and 11, animals of all groups except normal were actively sensitized with an intraperitoneal injection of 1 ml suspension of 200 µg OVA/10 mg Al(OH)3 as previously reported by Shimizu [18]. While normal control received vehicle intra-peritoneally.
2.4.2 Intranasal challenge with OVA
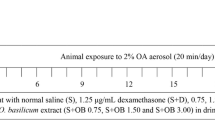
On days 20, 21, and 22, thiopental (50 mg/kg, intraperitoneally) was used to anesthetize the rats of all groups then, all except normally challenged intra-nasally with 1.5 mg of OVA suspended in 300 µl of saline, while normal received intranasal saline only (Fig. 1). The challenge method was carried out as previously described [3].
2.4.3 Assessment of respiratory function
One hour after the last OVA challenge dose, respiratory function was assessed for all groups. The non-anesthetized rat was put in the main chamber of the whole body plethysmograph (AD Instruments spirometer, ML140, England) to measure tidal volume (TV) and peak expiratory flow rate (PEFR), rat heads were protruded into a chamber connected to a spirometer via a latex neck collar as mentioned previously [3].
2.4.4 Handling of blood samples
On day 23, blood samples were obtained from a vein of the retro-orbital plexus for serum separation. Thereafter, blood samples were allowed to clot before being centrifuged for 10 min at 1500 rpm. Samples of serum were maintained at − 20 °C for IgE level analysis [19].
2.4.5 Collection of bronchoalveolar lavage fluid (BALF)
After the blood sampling on the same day, all rats were killed under anesthesia by cervical dislocation, and their lungs were lavaged with two aliquots of ice-cooled saline, each of 5 ml. A cooling centrifuge (Sigma 3-30k, USA) was used to centrifuge BALF for 10 min at 400 rpm, 4 °C). The supernatant liquid was maintained at − 80 °C till the time of total nitrate/nitrite (NOx) analysis. For absolute eosinophil count (AEC), the cell pellets were re-suspended in 50 µl of phosphate-buffered saline [20].
2.4.6 Handling of lung tissue
The lungs of rats were isolated, cleaned, and dried with filter paper. The left lung was examined histopathologically, and the right lung was homogenized with a homogenizer (yellow line, DI18 basic, Germany), to form a 25% w/v homogenate. Lung homogenates were centrifuged (2000 rpm for 20 min at 4 °C), then maintained at − 80 °C for superoxide dismutase (SOD), reduced glutathione (GSH), TNF-α, also interleukin-5 (IL-5) analysis.
2.5 Biochemical estimations
Within 1 h of BALF collection, AEC was counted in the pellets with the aid of a hemocytometer and Dunger's diluting fluid that prepared fresh. Dunger's diluting fluid was made by adding 0.5 ml of 95% acetone, 0.5 g Eosin Yellow and 0.5 ml of 40% formalin to 99 ml of bidistilled water. As Dunger's diluting fluid lyses RBCs and other WBCs, it stained the eosinophilic granules brightly and clearly [21].
Using the ELISA technique, serum samples were analyzed for IgE by the manufacturer's guidelines [22]. The content of NOx in BALF was determined as previously described [23] briefly, NOx level assessment based on the reduction of any nitrate to nitrite by vanadium followed by the detection of total nitrite by Griess reagent. The chromophoric azo derivative can be measured colorimetrically at 540 nm. Lung homogenates were assessed for the oxidative stress biomarkers as GSH, MDA, and SOD by chemical methods as previously reported [24,25,26]. Pulmonary content of TNF-α [27] and IL-5 [28] was assessed with ELISA kits according to the manufacturer's guidelines as previously described using the Sandwich technique, and then, the yield yellow color is measured at 450 nm.
2.6 Histopathological study
Soon after killing, left lungs of rats were isolated, washed from blood, dried then fixed in 10% formal saline for 48 h, dehydrated through ascending grades of alcohol, cleared in xylene for 4 h followed by embedding in paraffin wax at 58 °C to prepare 5 µm thick paraffin sections, finally stained with hematoxylin and eosin (H&E) as previously described [29], as firstly stained with hematoxylin for 3 min followed by eosin for 45 s and covered. Then, the scores of perivascular and peribronchiolar inflammation were assessed for 5 rats/group as follows: scores of 0 given to normal sections, 1 given to sections with few inflammatory cells, 2 given to sections with a one-cell layer ring of inflammatory cells, 3 given to a ring of two to four cells deep of inflammatory cells, finally, 4 given to a ring of inflammatory cells that is more than four cells deep [30].
2.7 Statistical analysis
All data of respiratory functions and biochemical parameters were expressed as mean ± SEM (standard error of the mean). The statistical package for social sciences (SPSS) software was used to conduct the analysis (version 16). One-way analysis of variance (ANOVA), followed by the Bonferroni test, was used to evaluate the statistical significance of differences in each parameter among the different groups, and a P-value < 0.05 was considered statistically significant. The Chi-square test was used to perform perivascular and peribronchiolar inflammation scores in a histological examination.
3 Results
3.1 Effect of administration of NAC in the lower and higher dose levels on the pulmonary function tests TV and PEFR in asthmatic rats
Rats that received intranasal OVA showed a significant decrease in TV and PEFR to 17% and 19%, respectively, as compared with the normal rats, while DEXA pretreatment significantly increased TV and PEFR to 542% and 496%, respectively, compared with the asthma control group. Also, NAC pretreatment in the lower and higher dose levels induced a significant elevation in TV to 349% and 529%, respectively, and also elevate PEFR to 238% and 487%, respectively, concerning asthma control rats (Table 1).
3.2 Effect of administration of NAC in the lower and higher dose levels on AEC and NOx of BALF in asthmatic rats
Induction of bronchial asthma was associated with a significant increase in AEC and NOx levels in BALF to 824% and 268%, respectively, concerning normal rats. DEXA pretreatment induced a significant decrease in AEC and NOx to 23% and 60%, respectively. Furthermore, NAC administration in the lower and higher dose levels induced a significant reduction of AEC to 32% and 17%, respectively, and reduced NOx to 62% and 41%, respectively, concerning the asthma control rats (Table 2).
3.3 Effect of administration of NAC in the lower and higher dose levels on GSH, SOD, and MDA levels in asthmatic rats
Rats that received intranasal OVA showed a significant decrease in GSH and SOD to 27% and 58%, respectively, and induced a significant elevation in MDA to 240% concerning the normal rats, while DEXA pretreatment significantly elevated GSH and SOD to 372% and 175%, respectively, and significantly decreased MDA to 29% concerning asthma control rats. Additionally, NAC pretreatment in the lower and higher dose levels induced a significant elevation of GSH to 339% and 429%, SOD to 112% and 143%, and decreasing MDA to 34% and 28%, respectively, concerning asthma control rats (Table 3).
3.4 Effect of administration of NAC in the lower and higher dose levels on the level of serum IgE in asthmatic rats
Rats that received intranasal OVA challenge showed a significant elevation of IgE level to 1270% concerning the normal control rats. While DEXA administration significantly lowered serum IgE level to reach 13% concerning the asthma control group. Also, NAC administration in the lower and higher dose levels induced a significant lowering in IgE level to 15% and 11%, respectively (Fig. 2a).
NAC effects on the levels of a serum IgE, b lung TNF-α and c lung IL-5 in asthmatic Rats. The results were presented as % of asthma control (N = 8 rats). One-way analysis of variance (ANOVA) was used in the statistical analysis, followed by the Bonferroni test. aat p < 0.05, considered significantly different from normal control. bat p < 0.05, considered significantly different from the asthma control group. cat p < 0.05, considered significantly different from the DEXA group
3.5 Effect of administration of NAC in the lower and higher dose levels on TNF-α and IL-5 levels in asthmatic rats
Rats that received intranasal OVA challenge significantly increased the levels of TNF-α and IL-5 to 741% and 474%, respectively, concerning the normal control rats, while DEXA pretreatment significantly lowered the levels of TNF-α and IL-5 to 29% and 30%, respectively, concerning asthma control rats. Also, NAC pretreatment in the lower and higher dose levels significantly lowered both TNF-α to 41% and 30%, respectively, and IL-5 to 32% and 23%, respectively, concerning asthma control rats (Fig. 2b, c).
3.6 Effect of administration of NAC in the lower and higher dose levels on the histopathological study
In photomicrographs examination, the pulmonary architecture of normal rats' lungs was normal, with no perivascular or peribronchiolar inflammatory infiltration (Fig. 3a, b). In contrast, lung sections from rats that received intranasal OVA showed severe injury in the lung architecture, with severe perivascular and peribronchiolar inflammatory cell infiltration (Fig. 3c, d).
Lung sections from DEXA pretreated rats revealed more or less normal lung architecture, and mild perivascular and peribronchiolar inflammatory cell infiltration (Fig. 3e, f).
Also, lung sections of NAC pretreated rats in the lower dose level revealed moderate perivascular and peribronchiolar inflammatory cell infiltration and moderate bronchial and alveolar architectures injury (Fig. 3g, h). Likewise, sections obtained from NAC pretreated rats in the higher dose level revealed mild lung architecture injury and mild inflammatory cell infiltration perivascularly and peribronchiolar (Fig. 3i, j).
The rats of the asthma control group revealed a significant increase in perivascular and peribronchiolar inflammatory scores concerning normal rat scores. On the other hand, the scores were significantly decreased in the DEXA and the two NAC pretreated groups (Fig. 4).
Peribronchiolar and perivascular inflammation score (N = 5). The Chi-square test was performed at p < 0.05. aat p < 0.05, considered significantly different from normal control. bat p < 0.05, considered significantly different from asthma control group. cat p < 0.05, considered significantly different from DEXA group
4 Discussion
In a previous study, we used OVA sensitization followed by a challenge as a model of bronchial asthma [2]. In the current work, intranasal OVA administration induced a significant decrease in TV and PEFR as compared with normal rats. On the other side, NAC showed a significant increase in TV and PEFR compared to asthma control values. Moreover, NAC in the higher dose level (500 mg/kg) precipitated TV and PEFR values not significantly different from those obtained by DEXA, the reference standard treatment, indicating attenuation of airway hyper-responsiveness.
According to the results of the present investigation, NAC administration to rats with OVA-induced bronchial asthma was found to reduce eosinophil penetration into the BALF, a finding coming in agreement with the previous investigation [31]. A suggested mechanism is the NAC’s capability to inhibit NADPH oxidase translocation to eosinophils’ membrane [32]. Additionally, the model of allergic asthma in a guinea pig reported an early increase in mucus (MUC5AC) expression before eosinophil infiltration [33], suggesting a potential inhibitory effect for NAC on the expression of MUC5AC and consequently on eosinophils infiltration.
Besides its direct effect on eosinophils, NAC in the present study showed an inhibitory effect on immune cell attractive chemokines including IL-5 as compared to asthma control, reaching values not significantly different from those of the DEXA group. As explained in a previous study, IL-5 is an eosinophil recruiting cytokine [34]. In agreement, NAC was reported in a previous study to decrease the number of eosinophils in the lungs via reducing IL-5 [35].
The current study found that an OVA challenge resulted in a substantial increase in serum IgE levels. IgE antibody is a key mediator in atopic asthma, which is generated by OVA that induced sensitization. As it can elicit an allergic response and increase the serum IgE levels in susceptible people after sensitization. IgE antibodies bind to the Fc epsilon receptor I, a high-affinity IgE receptor found on mast cells. As a result of allergens cross-linking related IgE–FcεRI complexes, cytoplasmic vesicles containing histamine degranulate, resulting in the synthesis of reactive oxygen species and eicosanoids. As previously mentioned, this causes smooth muscle contraction, vasodilation, and mucous secretion all that are asthma hallmarks [36]. This elevation was also observed in another study [2]. In vivo, however, there is little evidence that NAC has a suppressive effect on allergen-specific IgE production; our results revealed that NAC significantly attenuated IgE level by elevating the cellular GSH content in comparison with asthma control rats. This is in line with previous research suggesting that the thiol compound NAC can serve as a free radical scavenger as well as its role in GSH biosynthesis as a precursor [37,38,39]. According to the theory, IgE formation necessitates the activation of the NF-kB signaling pathway in antigen-presenting cells or B and T lymphocytes, and ROS enhances this pathway. It was also discovered that a higher level of GSH inhibited the increase of the Th-2 cytokine, which is responsible for IgE formation in cells [40]. In addition, the increased TNF-α level in asthma control rats was decreased in NAC-pretreated rats. This result is in agreement with the idea that the levels of GSH control, TNF-α production in vivo, as well as the observation of TNF-α inhibition by NAC in several studies [41]. Side by side, TNF-α production increased the production of ROS, which was inhibited by NAC in previous studies [42, 43].
The findings of the histopathological analysis corroborated the biochemical estimates. Sections prepared from rats receiving the lower dose of NAC (300 mg/kg) showed moderate perivascular and peribronchiolar inflammatory infiltration and moderate distorted bronchial and alveolar architectures. Alternatively, the sections from rats pretreated with the higher dose of NAC (500 mg/kg) revealed a slight injury in lung architecture with mild inflammatory cell infiltration perivascularly and peribronchiolar.
However, one previous clinical study demonstrated that the addition of NAC to usual asthma medication for only five days showed no significant effect in the treatment of asthma [44]. Another research found that NAC therapy improved the clinical course and lung function in patients with fibrosing alveolitis [45]. Despite these conflicts, we believe the findings of this study support the current concept that NAC could be a promising treatment for bronchial asthma due to its inhibitor effect on oxidative and nitrosative stress, IgE antibody formation, inflammatory cytokines release, eosinophil infiltration, and airway remodeling. Additionally, further clinical studies are needed to prove the mechanistic role of NAC on asthma due to its effect on IgE level.
5 Conclusions
N-Acetyl cysteine may protect bronchial asthma induced experimentally. NAC's anti-asthmatic ability can be explained by its suppressant effect on IgE antibody formation, inflammatory cytokines release, infiltration of eosinophil, and oxidative stress.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AEC:
-
Absolute eosinophil count
- BALF:
-
Bronchoalveolar lavage fluid
- DEXA:
-
Dexamethasone
- ELISA:
-
Enzyme-linked immunosorbent assay
- FEV:
-
Forced expiratory volume
- GSH:
-
Reduced glutathione
- H&E:
-
Hematoxylin and eosin
- IgE:
-
Immunoglobulin E
- IL-5:
-
Interleukin-5
- MDA:
-
Malondialdehyde
- NAC:
-
N-acetylcysteine
- NOx:
-
Total nitrate/nitrite
- OVA:
-
Ovalbumin
- PEFR:
-
Peak expiratory flow rate
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TNF-α:
-
Tumor necrosis factor-alpha
- TV:
-
Tidal volume
References
Walker C, Virchow J, Bruijnzeel P, Blaser K (1991) T cell subsets and their soluble products regulate eosinophilia in allergic and nonallergic asthma. J Immunol 146:1829–1835
Abdel-Fattah MM, Salama AA, Shehata BA, Ismaiel IE (2015) The potential effect of the angiotensin II receptor blocker telmisartan in regulating OVA-induced airway remodeling in experimental rats. Pharmacol Rep 67:943–951
Abdel-Fattah MM, Messiha BA, Salama AA (2015) Assessment of the mechanistic role of cinnarizine in modulating experimentally-induced bronchial asthma in rats. Pharmacology 96:167–174
Holgate S, Arshad H, Roberts G, Howarth P, Thurner P, Davies D (2010) A new look at the pathogenesis of asthma. Clin Sci 118:439–450
Paola Rosanna D, Salvatore C (2012) Reactive oxygen species, inflammation, and lung diseases. Curr Pharm Des 18:3889–3900
Jarjour NN, Calhoun WJ (1994) Enhanced production of oxygen radicals in asthma. J Lab Clin Med 123:131–136
Calhoun W, Reed H, Moest D, Stevens C (1992) Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis 145:317–325
Andreadis AA, Hazen SL, Comhair SA, Erzurum SC (2003) Oxidative and nitrosative events in asthma. Free Radical Biol Med 35:213–225
Demoly P, Vachier I, Pène J, Michel FB, Godard P, Damon M (1994) IgE produces monocyte superoxide anion release: correlation with CD23 expression: comparison of patients with asthma, patients with rhinitis, and normal subjects. J Allergy Clin Immunol 93:108–116
MacNee W (2001) Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 429:195–207
Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S et al (2011) Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med 364:2006–2015
Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA (2007) N-acetylcysteine—a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol 7:355–359
Zuin R, Palamidese A, Negrin R, Catozzo L, Scarda A, Balbinot M (2005) High-dose N-acetylcysteine in patients with exacerbations of chronic obstructive pulmonary disease. Clin Drug Investig 25:401–408
Pinkus R, Weiner LM, Daniel V (1996) Role of oxidants and antioxidants in the induction of AP-1, NF-κB, and glutathione S-transferase gene expression. J Biol Chem 271:13422–13429
Xie Q-m, Wu X, Wu H-m, Deng Y-m, Zhang S-j, Zhu J-p et al (2008) Oral administration of allergen extracts from Dermatophagoides farinae desensitizes specific allergen-induced inflammation and airway hyperresponsiveness in rats. Int Immunopharmacol 8:1639–1645
Kalimeris K, Briassoulis P, Ntzouvani A, Nomikos T, Papaparaskeva K, Politi A, Batistaki C, Kostopanagiotou G (2016) N-acetylcysteine ameliorates liver injury in a rat model of intestinal ischemia reperfusion. J Surg Res 206:263–272
Annouf Y, Al Laham S, Chatty E (2020) Effect of amlodipine combined with N-acetylcysteine on Crohn’s disease model in rats. J Pharm Sci Med 5:1–12
Shimizu T, Hirano H, Majima Y, Sakakura Y (2000) A mechanism of antigen-induced mucus production in nasal epithelium of sensitized rats: a comparison with lipopolysaccharide-induced mucus production. Am J Respir Crit Care Med 161:1648–1654
Locke NR, Royce SG, Wainewright JS, Samuel CS, Tang ML (2007) Comparison of airway remodeling in acute, subacute, and chronic models of allergic airways disease. Am J Respir Cell Mol Biol 36:625–632
Henderson RF, Muggenburg BA (2004) Use of bronchoalveolar lavage to detect lung injury. Curr Protoc Toxicol 18.14. 11–18.14. 10
Ghai C (2007) The total leukocyte count A textbook of practical physiology, 7th edn. Jaypee Brother Medical Publishers (Pvt) Ltd, New Delhi, pp 64–81
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F et al (2006) Human mesenchymal stem cells modulate B-cell functions. Blood 107:367–372
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Rahman I, Kode A, Biswas SK (2007) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Brouckaert P, Libert C, Everaerdt B, Takahashi N, Cauwels A, Fiers W (1993) Tumor necrosis factor, its receptors and the connection with interleukin 1 and interleukin 6. Immunobiology 187:317–329
Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D et al (2008) Breast milk–mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med 14:170–175
Bancroft JD, Gamble M (2008) Theory and practice of histological techniques: Elsevier Health Sciences
Tong J, Bandulwala HS, Clay BS, Anders RA, Shilling RA, Balachandran DD et al (2006) Fas-positive T cells regulate the resolution of airway inflammation in a murine model of asthma. J Exp Med 203:1173–1184
Blesa S, Cortijo J, Martinez-Losa M, Mata M, Seda E, Santangelo F et al (2002) Effectiveness of oral N-acetylcysteine in a rat experimental model of asthma. Pharmacol Res 45:135–140
Martinez-Losa M, Cortijo J, Juan G, O’Connor J, Sanz M, Santangelo F et al (2007) Inhibitory effects of N-acetylcysteine on the functional responses of human eosinophils in vitro. Clin Exp Allergy 37:714–722
Li Y, Martin LD, Minnicozzi M, Greenfeder S, Fine J, Pettersen CA et al (2001) Enhanced expression of mucin genes in a guinea pig model of allergic asthma. Am J Respir Cell Mol Biol 25:644–651
Corren J (2012) Inhibition of interleukin-5 for the treatment of eosinophilic diseases. Discov Med 13:305–312
Eftekhari P, Foster PS, Hajizadeh S, Hansbro N, Li JJ, Masjedi MR et al (2013) Preventive effect of N-acetylcysteine in a mouse model of steroid resistant acute exacerbation of asthma
Hamid Q, Tulic M (2009) Immunobiology of asthma. Annu Rev Physiol 71:489–507
Rushworth GF, Megson IL (2014) Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther 141:150–159
Hsieh C-C, Hsieh S-C, Chiu J-H, Wu Y-L (2014) Protective effects of N-acetylcysteine and a prostaglandin E1 analog, alprostadil, against hepatic ischemia: reperfusion injury in rats. J Tradit Complement Med 4:64
Prakash A, Kalra JK, Kumar A (2015) Neuroprotective effect of N-acetyl cysteine against streptozotocin-induced memory dysfunction and oxidative damage in rats. J Basic Clin Physiol Pharmacol 26:13–23
Bando N, Yamanishi R, Terao J (2003) Inhibition of immunoglobulin E production in allergic model mice by supplementation with vitamin E and β-carotene. Biosci Biotechnol Biochem 67:2176–2182
Sadowska A, Manuel-Y-Keenoy B, De Backer W (2007) Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther 20:9–22
Koo TY, Kim YJ, Yang WS, Park JS, Han NJ, Lee JM et al (2013) Mycophenolic acid regulates spleen tyrosine kinase to repress tumour necrosis factor-alpha-induced monocyte chemotatic protein-1 production in cultured human aortic endothelial cells. Cell Biol Int 37:19–28
Talasaz AH, Khalili H, Jenab Y, Salarifar M, Broumand MA, Darabi F (2013) N-acetylcysteine effects on transforming growth factor-β and tumor necrosis factor-α serum levels as pro-fibrotic and inflammatory biomarkers in patients following ST-segment elevation myocardial infarction. Drugs R&D 13:199–205
Aliyali M, Amiri AP, Sharifpoor A, Zalli F (2010) Effects of N-acetylcysteine on asthma exacerbation. Iran J Allergy Asthma Immunol 9:103
Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C (1997) Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis: adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med 156:1897–1901
Acknowledgements
The authors are grateful to Dr. El-Shima N. Ahmed Associate professor of pathology and Dr. Hamdy Helmy Kamal, Professor of clinical pathology department, Faculty of Veterinary, Beni-Suef University for providing helping in histopathological study and absolute eosinophil count, respectively.
Funding
There is no funding body for this work.
Author information
Authors and Affiliations
Contributions
MMA performed the experimental design, induced the disease, followed the experiment till the end, and performed the biochemical and histological assessments. AAS analyzed and interpreted the results of the biochemical analysis. BAS was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal housing and handling were performed according to the recommendations of Beni-Suef University Institutional Animal Care and Use Committee (IACUC) with ethical approval had serial No: REC-A-PhBSU-20008 which followed the recommendations of the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Fattah, M.M., Salama, A.A.A. & Messiha, B.A.S. Immunomodulatory and anti-inflammatory effects of N-acetylcysteine in ovalbumin-sensitized rats. Beni-Suef Univ J Basic Appl Sci 11, 2 (2022). https://doi.org/10.1186/s43088-021-00188-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-021-00188-7