Abstract
Background
Helminth infection and infestation in fishes are detrimental and have a major effect on fish health and fish production. Among various factors, parasitic infections are known to modulate antioxidant defences in fish. Similar to other aerobic animals, fish are also susceptible to the effect of reactive oxygen species and thus have well established intrinsic and efficient antioxidant defences. ‘Oxidative stress markers are an important indicator of the physiological state of the parasite and its host’. Indian catfish, Wallago attu is a freshwater fish that serves as the definitive host of the adult piscine trematode Isoparorchis hypselobagri. Our two years prevalence data signifies the intensity of the problem revealing a minimum of 5.5% and a maximum of 54% I. hypselobagri infection in Indian catfish W. attu (unpublished data). The present study aimed to achieve baseline data attributed to changes in some oxidative markers due to parasitic infection.
Results
During the present study, the level of enzyme activities of Catalase (CAT), Glutathione reductase (GR), Glutathione-S-transferase (GST), Glutathione peroxidase (GPx), Superoxide dismutase (SOD) and lipid peroxidation was investigated to explore the pathogenic impact on the fish host. The level of these oxidative stress markers was monitored in the swim bladder, liver, intestine and muscle of the host. We also recorded the enzyme activities in the parasite I. hypselobagri. Analysis of data revealed an elevation in GST, SOD, GR, GPx and CAT activity in the infected host tissue as compared to the non-infected fish. Further, we observed presence of GST, SOD, GR and GPx enzymes in the parasite I. hypselobagri while CAT did not show any enzyme activity.
Conclusions
Increased level of enzyme activity in liver, muscle and intestine of infected host has been recorded which indicates increased oxidative stress in the host due to parasitic invasion. The presence of antioxidant enzymes in the parasites suggests an active antioxidant defence system to avoid immune responses to long term survival and establishment in their host.
Similar content being viewed by others
1 Background
Isoparorchis hypselobagri (Billet 1898) is a digenetic trematode parasitizing swim bladder of the Indian catfish Wallago attu and other important food fishes namely Channa, Notopterus, Mystus, Mystacembelus and carps [4, 16, 50] (Rahaman and Manna [ 42]). Moreover, many vertebrates like a whale [54], crocodile [8], frog [43], turtle [45], pig [52] and human being [14, 25] have been reported to be infected with I. hypselobagri. This species has been recorded from India, Bangladesh, Pakistan, Thailand, Japan, China, Australia and Indonesia [18].The infection makes the fish unsuitable for human consumption since it produces black spot disease or ink spot disease in the muscles and visceral organs, causing mortality of fishes and thus resulting in the great economic loss [16, 29]. Human infection with I. hypselobagri has been recorded by Chandler and Read [15] which is very common in Manipur State of India due to eating raw swim bladders of fish. Cribb [18] proposed a three-host life cycle of parasite I. hypselobagri. Eggs of the parasite do not hatch and must be eaten by a gastropod, within the gastropod, it develops into sporocyst, rediae and cercariae. The cercariae leave the gastropod and are consumed by a second intermediate fish host Oxygaster bacaila, Gagata cenia and Gambusia affinis etc., in which the cercariae develop into metacercariae. The second intermediate host is consumed by a catfish and the metacercariae penetrate the intestine and migrate to the swim bladder and develop into an egg-producing adult [18].
Diseases in fish are one of the major factors which impede the successful development of the fish industry including the safety of fish products [34]. Helminth infection can cause a major effect on the fish trade due to the harmful effect of several helminth species. Many commercially important fish species are infected by helminth parasites, which may have a considerable impact on physiological and biochemical processes in the host organisms and are harmful to their health [49]. Humans get fish borne helminth infection by ingesting raw or undercooked fish containing infective parasitic larvae [51] (Chai and Lee [13]). Parasitic infection may also harm the function, growth, reproduction and survival of its host [46]. Financial losses due to parasitic diseases in the fish sector were estimated at 9.6 billion US dollars per year at the global level [44]. World Health Organization (WHO) has estimated that ‘the number of people currently infected with fish-borne trematodes exceeds 18 million, and many more are at risk’ [40]. Parasites can affect the fish population by causing mechanical, physiological as well as reproductive damage which may lead to a decline in the stock [30]. Morphological changes including partial necrosis of fin tissue, scale loss, loss of pigmentation, damage to the viscera, especially the gonads, and the abdominal muscles, on fish Channa punctata infected with I. hypselobagri, has been reported by Mahajan et al., [35]. Reduced feeding behaviour and a high mortality rate have been observed in infected fish. Infection by helminths causes ‘oxidative stress in their host [25] which as a result generates reactive oxygen species (ROS) including superoxide anion radicals, hydroxyl radicals, singlet oxygen, hydrogen peroxide and hypochlorous acid [21]. These are mainly produced as a part of the immune defence mechanism against foreign particles [39]. Oxidative stress is described as an imbalance state between pro-oxidants and antioxidants, resulting in higher production of ROS and free radicals which causes detrimental effects [38] on the host. Production of ROS is increased due to parasitic infection, which is responsible for killing or expulsion of parasites from their hosts and also prevents the establishment of infection [5, 11, 47]. The antioxidant system protects against the damage by scavenging free radicals. The main enzymatic antioxidants are catalase, GPx and SOD while reduced glutathione, vitamin C, vitamin E, β carotene, ceruloplasmin and bilirubin are non-enzymatic factors [21]. Superoxide dismutase converts superoxide radicals into hydrogen peroxide and molecular oxygen, glutathione peroxidase and catalase convert hydrogen peroxide into water. Hence, two toxic species, superoxide radical and hydrogen peroxide are converted into water [53].
The oxidative stress due to I. hypselobagri infection in its host and the oxidative stress experienced by the parasites have not been reported earlier. Therefore, the present study aims to estimate the levels of some antioxidant enzymes and lipid peroxidation in the parasite I. hypselobagri and the liver, swim bladder, intestine and muscle tissues of the host Wallago attu. The intestine has an important function in food digestion and nutrient absorption, however, the intestinal tract is prone to suffering from oxidative damage by the oxidants in foods or the larval stages of helminth parasites migrating through the intestine. The muscle (skeletal muscle) is crucial in maintaining ROS below a threshold level to keep up redox homeostasis. Since, the juvenile worms of Isoparorchis hypselobagri penetrate the intestine, liver and swim bladder before transforming into an adult stage, we hypothesise that the migrating juvenile worms cause tissue damage that could result in the down-regulation of antioxidant enzymes.
2 Methods
2.1 Collection of samples
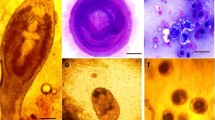
Indian catfish W. attu, (Fig. 1a) were purchased from the local fish market and brought to the laboratory. Mature I. hypselobagri (Fig.1c, d) were collected from the infected swim bladder (Fig. b) and washed several times with 50 mM phosphate buffer solution, pH 7.4 to remove host-related debris. The liver, intestine, muscle and swim bladder of both infected and non-infected fishes were collected to determine the level of antioxidant enzymes.
2.2 Homogenate preparation
Tissue samples were subjected to 10% (w/v) homogenization in chilled phosphate-buffered saline, (pH7.0) using pestle and mortar. The homogenate was centrifuged at 10,000×g for 10 min at 4 °C; then the supernatant was collected and stored at − 20 °C till further use.
2.3 Protein estimation
The protein concentration was estimated by the Bradford dye-binding method (1976) as modified by Spector [48] using bovine serum albumin (BSA) as standard. Coomassie Brilliant Blue G-250 dye was prepared by dissolving 0.01% (w/v) in 5% Ethanol (v/v) and 10% Orthophosphoric acid (v/v). The optical density was recorded at 595 nm on a UV spectrophotometer.
2.4 Estimation of glutathione-S-transferase (GST)
GST enzyme activity was assayed spectrophotometrically at 340 nm following the method of Habig et al. [28]. A total of 3.0 ml reaction mixture contained 50 µl of sample, 2.640 ml of 0.1 M phosphate buffer (pH,6.5), 0.3 ml of 1 mM reduced glutathione and 10 µl of 1 mM 1 chloro-2,4 dinitrobenzene (CDNB). The enzyme activity was expressed as nmol/mg protein/min (molar extinction coefficient = 9.6 × 103 M/cm).
2.5 Estimation of superoxide dismutase (SOD)
SOD enzyme activity was assayed spectrophotometrically at 420 nm by the method of Marklund and Marklund [36]. A total of 3.0 ml reaction mixture contained 50 µl of the sample, 2.85 ml of 50 mM Tris cacodylate buffer and 0.1 ml of 0.2 mM pyrogallol. One unit of enzyme activity is defined as the amount of enzyme required to cause 50% inhibition of the rate of pyrogallol auto-oxidation. The enzyme activity was expressed as units/mg protein/min.
2.6 Estimation of glutathione peroxidase (GPx)
Glutathione peroxidase enzyme activity was assayed spectrophotometrically at 340 nm by the method of Flohé’s and Günzler [26]. The reaction mixture (1.0 ml) contained 500 µl of 0.1 M potassium phosphate buffer (pH7.0) containing 1 mM EDTA, 100 µl of 10 mM GSH (prepared in water), 100 µl of 1.5 mM NADPH (prepared in 0.1% NaHCO3), 100 µl of enzyme sample and 100 µl substrate (H2O2). The enzyme activity is expressed as nmol/mg protein/ml.
2.7 Estimation of glutathione reductase (GR)
Glutathione reductase enzyme activity was assayed spectrophotometrically at 340 nm by the method of Carlberg and Mannervik [12]. The reaction mixture (1.0 ml) contained 500 µl of 0.2 M potassium phosphate buffer (pH 7.0) containing 2 mM EDTA, 50 µl of 2 mM NADPH (prepared in 10 mM Tris HCl pH 7.0), 50 µl of 20 mM GSSG, 350 µl distilled water. The reaction was initiated by adding 50 µl of the sample. The enzyme activity is expressed as nmol/mg protein/min.
2.8 Estimation of catalase (CAT)
Catalase activity was assayed spectrophotometrically at 240 nm by the method of Aebi [1]. A total of 3.0 ml reaction mixture contained 2.85 ml of 50 mM potassium phosphate buffer (pH 7) and 0.05 ml sample. The reaction was initiated by adding 0.1 ml of 30 mM H2O2. One unit of enzyme activity is defined as the amount of enzyme needed for degradation of 1 μm of H2O2 per min. The enzyme activity is expressed as units/mg protein/min.
2.9 Estimation of lipid peroxidation (LPO)
Lipid peroxidation was assessed by determining MDA (a thiobarbituric acid reactive species: TBARS) spectrophotometrically following the method of Beuge and Aust [11]. The reaction mixture contained 1 ml of sample and 2 ml of TBA-TCA-HCl reagent (0.37% thiobarbituric acid, 0.25 N hydrochloric acid and 15% trichloroacetic acid), placed in boiling water bath for 15 min, cooled at room temperature and centrifuged for 10 min. The pink chromogen formed by the MDA-TBA complex was detected at 535 nm. The level of lipid peroxidation was expressed in nmoles of malondialdehyde (MDA) formed/mg protein.
2.10 Statistical analysis
Statistical analysis was done by student’s t-test followed by Prism 8.0.1.244. All the values are mean of three independent replicates ± SEM (Standard error mean). Values of p < 0.05, p < 0.01, p < 0.001 and p < 0.0001 were considered to be significant.
3 Results
During the present study, we observed both qualitative and quantitative differences in the activity of antioxidant enzymes (Figs. 2, 3, 4, 5, 6). An overall comparison of enzyme activities in the infected and non-infected liver, swim bladder, muscle and intestine of Indian catfish Wallago attu revealed a unique pattern in their order of activity. We recorded the higher enzyme activity of SOD followed by Catalase, GPx, GR and GST in the swim bladder, muscle and intestine, while in the liver tissues, Catalase revealed higher activity followed by GPx, SOD and GST enzymes (Figs. 2, 3, 4, 5, 6).In general, we observed that the fishes infected with I. hypselobagri demonstrated an elevation in the level of the antioxidant enzyme as compared to non-infected fish.
The specific enzyme activity of glutathione-s-transferase (GST) in parasite I. hypselobagri and non-infected and infected liver, swim bladder, muscle and intestine of host Wallago attu. NIL = Non infected liver, IL = Infected liver, NISB = Non infected swim bladder, ISB = Infected swim bladder, NIM = Non infected muscle, IM = Infected muscle, NII = Non infected intestine, II = Infected intestine
A higher level of GST was noticed in the liver and intestine of infected fish but there was no significant increase in muscle and swim bladder of the host (Fig. 2). Elevation in the level of GST by 49.6, 15, 13 and 76.36% in infected liver, swim bladder, muscle and intestine (p < 0.05) were recorded as compared to non-infected fish (Fig. 2). It can be surmised that the higher GST activity helps to re-establish the balance between pro-oxidative damage and antioxidants to ease ROS induced oxidative damage. Figure 3, shows a significant elevation in the enzyme activity of SOD in the liver (p < 0.05), intestine (p < 0.01), muscle and swim bladder (p < 0.05) of the infected host which is an indication of increased oxidative stress due to parasitic infection. Further, we observed a significant increase in the level of GPx activity in the liver (86%) (p < 0.01) and muscle (31%) while the level decreased significantly in the infected intestine (p < 0.01) and swim bladder (p < 0.001) respectively (Fig. 4) indicating that this enzyme failed to influence the expulsion of the parasite. We also observed increased Glutathione reductase (GR) activity in the liver, muscle and intestine of infected fish while the activity decreased in the swim bladder (Fig. 5).GR activity in infected liver (p < 0.01), muscle (p < 0.01) and intestine increased by 3.1, 5 and 1.75 fold respectively while the activity decreased significantly (p < 0.01) by 6.6 fold in the swim bladder (Fig. 5), in comparison to non-infected fish. Figure 6, showed that the enzyme activity of CAT in the infected liver (p < 0.05), swim bladder, muscle (p < 0.05) and intestine increased by 2, 10 and three fold respectively, as compared to the non-infected host. In the present study, we also observed increased lipid peroxidation activity in the liver, intestine and muscle of the infected host whereas a considerable decrease in the enzyme activity was observed in the swim bladder of infected fish (Fig. 7). We found a significant elevation in the level of lipid peroxidation by 1.3, 2.8 and 6.6 fold in the infected liver (p < 0.05), intestine (p < 0.001) and muscle (p < 0.001), respectively whereas considerable decrease by 18.7 fold in swim bladder (p < 0.01) was noticed (Fig. 7).
The specific activity of superoxide dismutase (SOD) enzyme in parasite I. hypselobagri and non-infected and infected host tissues of fish Wallago attu (liver, swim bladder, muscle and intestine). NIL = Non infected liver, IL = Infected liver, NISB = Non infected swim bladder, ISB = Infected swim bladder, NIM = Non infected muscle, IM = Infected muscle, NII = Non infected intestine, II = Infected intestine
The specific enzyme activity of glutathione peroxidase (GPx) in parasite I. hypselobagri and non-infected and infected liver, swim bladder, muscle and intestine of Wallago attu. NIL = Non infected liver, IL = Infected liver, NISB = Non infected swim bladder, ISB = Infected swim bladder, NIM = Non infected muscle, IM = Infected muscle, NII = Non infected intestine, II = Infected intestine
The specific activity of glutathione reductase (GR) enzyme in parasite I. hypselobagri and non-infected and infected liver, swim bladder, muscle and intestine of host Wallago attu. NIL = Non infected liver, IL = Infected liver, NISB = Non infected swim bladder, ISB = Infected swim bladder, NIM = Non infected muscle, IM = Infected muscle, NII = Non infected intestine, II = Infected intestine
The specific enzyme activity of catalase (CAT) in parasite I. hypselobagri and non-infected and infected liver, swim bladder, muscle and intestine of fish Wallago attu. NIL = Non infected liver, IL = Infected liver, NISB = Non infected swim bladder, ISB = Infected swim bladder, NIM = Non infected muscle, IM = Infected muscle, NII = Non infected intestine, II = Infected intestine
The specific activity of lipid peroxidation in parasite I. hypselobagri and non-infected and infected host tissues of fish Wallago attu (liver, swim bladder, muscle and intestine). NIL = Non infected liver, IL = Infected liver, NISB = Non infected swim bladder, ISB = Infected swim bladder, NIM = Non infected muscle, IM = Infected muscle, NII = Non infected intestine, II = Infected intestine
In addition to examining antioxidant enzyme activities in the tissues of infected and non-infected W. attu, we also performed antioxidant enzyme assays in the parasite I. hypselobagri which infects the swim bladder of the fish under investigation. Analysis of the result revealed that the parasite possesses an active antioxidant system showing a higher amount of enzyme activity for SOD followed by GPx, GR and GST (Figs. 2–6). Catalase was also examined in the parasite but it did not show any enzyme activity.
4 Discussion
Antioxidant enzymes represent the first line of defence which has an important role to restrain the damage done by reactive oxygen molecules (ROS) of parasite origin [17], whereas the survival of parasite may rely on its ability to maintain the necessary balance between oxidation and antioxidation [38]. It is well-established fact that the physiological function of the host is altered by high intracellular ROS concentration which weakens the host immune system and becomes more prone to parasitic diseases [39]. While lower levels regulate several physiological mechanisms including cell differentiation, apoptosis and cell proliferation [53].
Liver and intestine tissues of infected fish revealed an elevation in the GST activity whereas no significant increase in GST was recorded in the muscle and swim bladder of the host. In a study by Radovanović et al. [41], higher glutathione-dependent enzyme activities in barbel (Barbus barbus) infected with intestinal parasite Pomphorhynchus laevis has been observed. Higher antioxidant enzyme activity could be correlated with a greater scale of protection against parasitic infection [27]. Our present study shows a significant elevation of SOD in the liver, intestine, muscle and swim bladder of the infected host. Elevation in the level of SOD in sheep liver infected with Fasciola spp. has also been observed by Assady et al. [2], which is an indication of increased oxidative stress due to parasitic infection. Further, in our study, a significant increase in the level of GPx in the infected liver and muscle and a decrease in the infected intestine and swim bladder of W. attu has been observed. Increased level of peroxidase activity in the liver due to F. hepatica infection has also been observed by Benzer and Ozan [6]. Elevation in the GPx enzyme activity could be suggested as an adaptive change against potential liver injury [21]. The lower level of peroxidase activity in the intestine and swim bladder of infected fish indicated that the GPx enzyme could not compensate for the damage caused by the parasitic invasion. During the present study, infected liver, swim bladder, muscle and intestine revealed a significant increase in CAT enzyme activity. Increased level in the enzyme activity of catalase indicates an accumulation of hydrogen peroxide in infected tissue [27]. Significant elevation of CAT and GST has been reported in the liver and head kidney of Cyprinus carpio infected with Ptychobothryumsp. [21]. Eissa et al. [23] reported increased SOD, CAT, GR, GPx activities and MDA concentration in liver and muscle tissues of Tilapia infected with Diplostomum and Heterophys sp. Kumar et al. [33] reported induction of antioxidant enzymes (GST, SOD and CAT) in gills and liver tissue of fish Pangasianodon hypophthalmus infected with Thaparocleidus sp. Our results showed significant elevation in the level of lipid peroxidation in the infected liver, intestine and muscle whereas a considerable decrease in swim bladder (p < 0.01) was noticed. A similar finding has also been reported by Nabi et al. [39] who observed an increased level of LPO in the liver, intestine and muscle of Schizothorax plagiostomus infected with Pomphorhynchus sp. The enhanced level of LPO protects the parasite from host immune responses. Low level of lipid peroxidation in infected swim bladder may indicate that cells stimulate their maintenance and survival through essential antioxidant defence that upregulate antioxidant proteins resulting in an adaptive stress response [3]. Decreased activity of GR and GPx may be due to protein structure modification leading to change in its function and also responsible for accumulation of peroxides up to toxic level [32].
Analysis of antioxidant enzyme assays in the parasite I. hypselobagri under study revealed the presence of an active antioxidant system showing a higher amount of enzyme activity for SOD followed by GPx, GR and GST. Moreover, we observed the absence of catalase activity in the parasite. Our finding is in agreement with the study of Khan et al. [31], who observed that the catalase activity was not present in the somatic extract of the fish infecting parasite, Clinostomum complanatum while GST and SOD enzymes have been observed. In parasites, the production of antioxidant enzymes is helpful to avoid immune responses thereby facilitating long term survival in the host. According to Deger et al. [21], the presence of an elevated level of antioxidant enzyme in the host is to provide a cellular protective role to oppose the oxidative stress generated by parasitic invasion. It is reported that parasitic infection can disturb the metabolic activity of its host [10].The antioxidant defence system of the parasite helps to tackle the host's immune responses for successful establishment and long-term survival in the definitive host. Parasites need protection from reactive oxygen species that arise from host phagocytic cells [19] and [22]. Antioxidant molecules of parasite origin represent a faceted shield in drug resistance, as well as in the modulation of the host immune response. Thus, antioxidants are not only a barrier but an essential factor to establish the host–parasite relationship [17]. The increase or decrease in the activity of different antioxidant enzymes in host tissues infected with I. hypselobagri during the present study can be linked to modulated metabolic activity in the host owing to parasitic load.
5 Conclusions
It is concluded that infection of parasite I. hypselobagri induces oxidative stress which results in the generation of reactive oxygen species in the host Wallago attu, with modulation in the level of essential antioxidant enzymes (GST, SOD, GPx, GR, CAT and LPO). Parasitic infection induces significant oxidative stress which is evident from the increased antioxidant enzymes and lipid peroxidation causing a pathogenic response in the host. Moreover, the antioxidant defence system of the parasite helps to tackle the host's immune responses for successful establishment and long-term survival in the definitive host.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Aebi H (1974) Catalase. In: Methods of enzymatic analysis. Academic Press, pp 673–684
Assady M, Farahnak A, Golestani A, Esharghian MR (2011) Superoxide dismutase (SOD) enzyme activity assay in Fasciola spp. parasites and liver tissue extract. Iran J Parasitol 6(4):17
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 360438:1–31
Bashirullah AK (1972) On the occurrence of the trematode, Isoparorchis hypselobagri (Billet 1898). In: Fishes and notes on its life history. Norwegian J Zool 20:209-212
Ben-Smith A, Lammas DA, Behnke JM (2002) Effect of oxygen radicals and differential expression of catalase and superoxide dismutase in adult Heligmosomoides polygyrus during primary infections in mice with differing response phenotypes. Parasite Immunol 24:119–129
Benzer F, Ozan ST (2003) Lipid peroxidation, antioxidant enzymes and levels of nitric oxide in sheep infected with Fasciola hepatica. Turk J Vet Anim Sci 27(3):657–661
Bhalerao GD (1932) A note on the probability of infection of man and domestic carnivores by Isoparorchis hypselobagri (Billet, 1898). Indian J Vet Sci 11(4):406–407
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Buchmann K, Lindenstrom T (2002) Interactions between monogenean parasites and their fish hosts. Int J Parasitol 32:309–319
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. In: Methods in enzymology, vol 52. Academic Press, pp 302–310
Callahan HL, Crouch RK, James ER (1988) Helminth antioxidant enzymes: a protective mechanism against host oxidants? Parasitol Today 4(8):218–225
Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490
Chai JY, Lee SH (2002) Food-borne intestinal trematode infections in the Republic of Korea. Parasitol Int 51(2):129–154
Chandler AC (1926) The prevalence and epidemiology of hookworm and other helminthic infectionsin India. Part III. Central, Western and Northern Bengal. Indian J Med Res 14(2):195–218
Chandler AC, Read CP (1961) Introduction to parasitology, vol 428. Wiley, New York, p 469
Chauhan BS (1955) On the taxonomic position and distribution of the trematode genus, Isoparorchis Southwell, 1913. J Zool Soc India 7(1):87–90
Chiumiento L, Bruschi F (2009) Enzymatic antioxidant systems in helminth parasites. Parasitol Res 105(3):593–603
Cribb TH (1988) Two new digenetic trematodes from Australian freshwater fishes with notes on previously described species. J Nat Hist 22(1):27–43
Cross AR, Jones OTG (1991) Enzymic mechanisms of superoxide production. Biochim Biophys Acta BBA Bioenergetics 1057(3):281–298
Dautremepuits C, Betoulle S, Vernet G (2003) Stimulation of antioxidant enzymes levels in carp (Cyprinus carpio L.) infected by Ptychobothrium sp (Cestoda). Fish Shellfish Immunol 15(5):467–471
Deger Y, Ertekin A, Deger S, Mert H (2008) Lipid peroxidation and antioxidant potential of sheep liver infected naturally with distomatosis. Türkiye Parazitol Derg 32(1):23–26
Dzik JM (2006) Molecules released by helminth parasites involved in host colonization. Acta Biochim Pol 53(1):33–64
Eissa AM, Derwa HI, Mona I, Ramadan RA, Mona Z, Nashwa M (2014) Use of enzyme activities as biomarkers for oxidative stress induced by metacercarial affections in some cultured tilapia species. Life Sci 11(3):284–289
Espinal-Carrión T, López-López E (2010) Helminths and lipid peroxidation in Astyanax aeneus (Pisces: Characidae) from a river in the humid subtropics of southeastern Mexico. Dis Aquat Org 88(3):215–224
Faust EC (1930) Human helminthology. A manual for clinicians, sanitarians and medical zoologists. A manual for clinicians, sanitarians and medical zoologists
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–120
Garcia LDO, Becker AG, Bertuzzi T, Cunha MAD, Kochhann D, Finamor IA, Riffel APK, Llesuy S, Pavanato MA, Baldisserotto B (2011) Oxidative stressparameters in silver catfish (Rhamdiaquelen) juveniles infected with Ichthyophthirius multifiliis and maintained at different levels of water pH. Vet Parasitol 178(1–2):15–21
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 249(22):7130–7139
Hora SL (1953) Table for the identification of Indian freshwater fishes, with description of certain families and observation on the relative utility of the probable Larvivorous fishes of India
Iwanowicz DD (2011) Overview of the effects of parasites on fish health. In: Proceedings of the 3rd bilateral conference between Russia and the United States. Bridging America and Russia with Shared Perspectives on Aquatic Animal Health, pp 176–184
Khan S, Ahmed S, Saifullah MK, Abidi SMA (2014) Modulation of oxidative stress in Trichogaster fasciatus caused by infection with Clinostomum complanatum progenetic metacercariae. Funct Biol Biotechnol 81–85
Kolodziejczyk L, Siemieniuk E, Skrzydlewska E (2005) Antioxidant potential of rat liver in experimental infection with Fasciola hepatica. Parasitol Res 96(6):367–372
Kumar S, Raman RP, Prasad KP, Srivastava PP, Kumar S, Rajendran KV (2017) Modulation of innate immune responses and induction of oxidative stress biomarkers in Pangasianodon hypophthalmus following an experimental infection with dactylogyrid monogeneans. Fish Shellfish Immunol 63:334–343
Lebedeva SN, Ayurzhanayeva AB, Tyheev AA, Kutyrev IA, Bazhenova BA, Zhamsaranova SD (2021) The effect of D. dendriticum invasiveness on the antioxidant system of omul from the Selenga River population. In: IOP conference series: earth and environmental science, vol 640(3). IOP Publishing, pp 032–037
Mahajan CL, Agrawal NK, John MJ, Katta VP (1979) Effect of the digenean Isoparorchis hypselobagri (Billet, 1898) on an air-breathing fish Channa punctatus (Bloch) with particular reference to biochemical and haematological changes. J Fish Dis 2(6):519–528
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47(3):469–474
Matés JM, Pérez-Gómez C, De Castro IN (1999) Antioxidant enzymes and human diseases. Clin Biochem 32(8):595–603
Mordvinov VA, Ponomarev DV, Pakharukov YV, Pakharukova MY (2021) Anthelmintic activity of antioxidants: in vitro effects on the liver fluke Opisthorchis felineus. Pathogens 2021(10):284
Nabi S, Tanveer S, Ganie SA (2017) Glutathione-S-transferase, Superoxide Dismutase (GST, SOD) Levels, Protein Content and Lipid Peroxidation in Schizothorax plagiostomus under the Infection of Pomphorhynchus in Nallah Sukhnag of Kashmir Valley. Pak J Biol Sci 20(9):442–446
Park CW, Kim JS, Joo HS, Kim J (2009) A human case of Clinostomum complanatum infection in Korea. Korean J Parasitol 47(4):401
Radovanović TB, Prokić MD, Gavrić JP, Despotović SG, Gavrilović BR, Borković-Mitić SS, Pavlović SZ, Saičić ZS (2015) Glutathione-dependent enzyme activities and concentrations of glutathione, vitamin E and sulfhydryl groups in barbel (Barbus barbus) and its intestinal parasite Pomphorhynchus laevis (Acanthocephala). Ecol Ind 54:31–38
Rahaman A, Manna B (2000) On the digestive physiology of Isoparorchis hypselobagri (Billet, 1898) (Digenea: Trematoda), IV Tegumental specializations and its functional role. J Parasit Appl Anim Biol 9(2):75–88
Rao LN, Kameswari M, Rao GRH (1979) A yet undefined host of Isoparorchis hypselobagri Billet, 1898. Curr Sci 48:320
Shinn AJ, Pratoomyot J, Bron J, Paladini G, Brooker E, Brooker A (2015) Economic impacts of aquatic parasites on global finfish production. Global Aquac Advocate 58–61
Simha SS (1958) Studies on the trematode parasites of reptiles found in Hyderabad State. Z Parasitenkd 18(3):161–218
Sindermann CJ (1987) Effects of parasites on fish populations: practical considerations. Int J Parasitol 17(2):371–382
Smith NC, Bryant C (1989) Free radical generation during primary infections with Nippostrongylusbrasiliensis. Parasite Immunol 11(2):147–160
Spector T (1978) Refinement of the Coomassie blue method of protein quantitation: a simple and linear spectrophotometric assay for ≤ 0.5 to 50 μg of protein. Anal Biochem 86(1):142–146
Skuratovskaya E, Zav’yalov A, Rudneva I (2018) Health parameters and antioxidant response in Black Sea whiting Merlangius merlangus euxinus (Nordmann, 1840) parasitized by nematode Hysterothylacium aduncum (Rud., 1802). Commun Sci 9(4):700–709
Srivastava CB (1977) Isoparorchis hypselobagri (Billet, 1898) (Trematoda: Isoparorchiidae), its hosts, distribution and relationships. In: Excerta parasitologicaen memoria del Dr. Eduardo Caballero y Caballero. Universidad Nacional Autdnoma de Mexico, Mexico, pp 325–333
Tada I, Otsuji Y, Kamiya H, Mimori T, Sakaguchi Y, Makizumi S (1983) The first case of a human infected with an acanthocephalan parasite, Bolbosoma sp. J Parasitol 205–208
Varma TK, Ahluwalia SS (1980) An unusual record of Isoparorchis hypselobagri (Billet, 1898) a trematode parasite of fishes from the bile duct of a pig. Indian Vet J 57:688–689
Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5(1):51–66
Zmeev GI (1936) Les trematodes et les cestodes des poisson de L, Amour. InstyletPodstawowychProblemowTechnikiPolskiejAkademiiNauk/Instituteof Fundamental Technological Research Polish Academy of Sciences 3:253–259
Acknowledgements
The authors are thankful to the Chairperson, Department of Zoology, Aligarh Muslim University, Aligarh for providing the necessary laboratory facilities. The first author is also thankful to the University Grants Commission (UGC) for providing UGC Non-NET fellowship.
Funding
The University Grants Commission (UGC) Govt. of India provided UGC Non-NET fellowship to the first author.
Author information
Authors and Affiliations
Contributions
MKS (mentor) and AS designed the experiment. AS collected the sample from the fish market, isolated the parasite from swim bladder of the fish, prepared the host and parasite sample for enzyme assays. AS and KF assayed various enzymes and estimated protein in parasite and tissue samples. AS and KF analysed the data. MKS and TZ contributed in ‘writing the manuscript’.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. Since we purchased dead fishes from the local fish market to collect the parasite Isoparorchis hypselobagri (from the swim bladder), needs no approval from the Ethical committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sahreen, A., Fatima, K., Zainab, T. et al. Changes in the level of oxidative stress markers in Indian catfish (Wallago attu) infected with Isoparorchis hypselobagri. Beni-Suef Univ J Basic Appl Sci 10, 85 (2021). https://doi.org/10.1186/s43088-021-00174-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-021-00174-z