Abstract
Background
Cirrhotic cardiomyopathy, an intricate and multifaceted complication of end-stage liver disease, manifests as systolic and diastolic dysfunction in patients without previously diagnosed cardiac disease. Our study aims to investigate the prevalence of systolic and diastolic function in patients with cirrhotic cardiomyopathy in our region.
Methods
We conducted a cross-sectional study on 68 patients with established cirrhosis, and no overt cardiac manifestations, who consequently underwent 2D echocardiography to quantify systolic and diastolic dysfunction, as defined by the 2019 Cirrhotic Cardiomyopathy Consortium. The severity of cirrhosis was determined using various validated scoring systems.
Results
A total of 19 out of 68 (28%) had systolic dysfunction, while 6/68 (9%) had evidence of diastolic dysfunction. Overall prevalence of cirrhotic cardiomyopathy was 23/68 (34%), and the presence of hepatitis C was strongly associated with systolic dysfunction with p-value of 0.007. However, it was not significantly associated with diastolic dysfunction, p-value = 0.59. Logistic regression analysis did not show any significant association between cardiac dysfunction and the severity of liver cirrhosis, as assessed by Child–Pugh, MELD, ALBI, PALBI, portal hypertension, and FIB-4 score (R2 = 3.66, F (13, 39) = 1.33, p = 0.234).
Conclusion
Our study reveals a remarkable prevalence of cirrhotic cardiomyopathy, which despite being a frequently occurring phenomenon often goes unrecognized. Lack of correlation with the severity of liver cirrhosis, based on currently available scoring system, suggests either a still poorly understood pathological mechanism or requires the development of a new validated reliable scoring system through multi-center longitudinal studies.
Similar content being viewed by others
Introduction
First described by Kolwaski et al. [1] in 1953, the intricate link between liver disease and cardiovascular abnormalities has been irrevocably established [2, 3]. Cirrhotic cardiomyopathy occurs in patients without preexisting cardiac disease and is unrelated to the etiology of cirrhosis [2, 4]. It consists of a hyperdynamic circulation characterized by decreased systemic vascular resistance, increased stroke volume, and ultimately increased cardiac output [2]. Once developed, it can predispose patients to other complications such as hepatorenal syndrome, increased mortality with liver transplantation, and worsened outcomes associated with surgery, infection, and hemorrhage [5, 6].
According to the proposed pathophysiology, cirrhosis impedes the forward flow of blood resulting in backward portal hypertension [7, 8]. Additionally, metabolic activities of the liver get compromised leading to the accumulation of vasodilatory substances such as nitric oxide and carbon monoxide, which contribute to the dilation of the mesenteric vasculature, a phenomenon known as forward portal hypertension [8,9,10]. The consequent mesenteric congestion results in the translocation of gut bacteria leading to endotoxemia. This heralds the production of inflammatory cytokines like tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β), which impair cardiac contractility. Concomitantly, vasodilators tend to activate the sympathetic nervous system resulting in the overstimulation of cardiac beta-adrenergic receptors and ultimately rendering them down-regulated, desensitized, and defunct [10, 11].
The initial criteria for diagnosing cirrhotic cardiomyopathy proposed by the Montreal World Congress of Gastroenterology in 2005 consisted of systolic and diastolic dysfunctional indices and supportive findings such as electrophysiological variations, biomarkers, and structural changes [12]. However, advancements in imaging techniques along with revisions in concepts of heart failure led to the emendation of these criteria by a multidisciplinary team that devised the Cirrhotic Cardiomyopathy Consortium in 2019 as summarized in Table 1 [13].
Although there is no definitive treatment for cirrhotic cardiomyopathy [6, 7], several pharmaceutical agents have proven to be beneficial and improve survival, including nonselective beta-blockers and novel ion-channel blockers like ivabradine [19,20,21]. Liver transplantation remains the gold standard. However, this is a double-edged sword as cardiac dysfunction accounts for 17.2% of peri- and postoperative deaths [22].
Our study aims to investigate the prevalence of systolic and diastolic function in patients with cirrhotic cardiomyopathy in our region according to the Cirrhotic Cardiomyopathy Consortium.
Methods
Ethical statement
The study was carried out with the approval of the Institution Review Board (610/FRC/CMH/LMC). This was a cross-sectional study conducted between August 13, 2021, and June 20, 2023, following the principles of the Helsinki Declaration. All patients provided written consent to participate in the study.
Study aims
The study aimed to investigate the correlation between liver cirrhosis and cardiomyopathy by assessing patients for systolic and/or diastolic dysfunction.
Additionally, the study endeavored to determine if the severity of liver cirrhosis, as established by Child–Pugh classes B and C, increasing scores of MELD (model for end-stage liver disease), FIB-4 (fibrosis-4), ALBI (albumin-bilirubin), PALBI (platelet-albumin-bilirubin), and PH (portal hypertension), was associated with cardiomyopathy. The diagnosis of cirrhotic cardiomyopathy (CCM) was based on 2019 criteria, proposed by Cirrhotic Cardiomyopathy Consortium, which defines it as a chronic cardiac dysfunction in patients with end-stage liver disease without any other cardiac disease with impaired systolic or diastolic function [13].
Study procedure
The study was a collaboration between the gastroenterology and cardiology departments within the hospital.
The study included patients who had given informed consent and were registered from the gastroenterology wards, outpatients, or endoscopy suite in Combined Military Hospital (CMH) Lahore. The patients had liver cirrhosis caused by either viral hepatitis or metabolic dysfunction-associated steatotic liver disease (MASLD), previously known as nonalcoholic fatty liver disease (NAFLD) and were over 18 years old. They were selected using a non-probability consecutive sampling technique. The patients were then referred to the cardiology department for echocardiography, which was performed by a senior cardiologist to evaluate the presence and severity of cardiomyopathy.
We did not include patients who refused to give consent or were under 18 years of age. Additionally, we excluded those with a previously diagnosed structural heart disease unrelated to liver cirrhosis, coronary artery disease, arterial hypertension, hepatocellular carcinoma outside Milan criteria, portal vein thrombosis, and those with transjugular intrahepatic portosystemic shunt (TIPS). The study enrolled 68 patients who met the inclusion criteria.
The patient’s demographic details and clinical history were taken, including their current medical conditions, past history, comorbid conditions, especially cardiovascular disease, and detailed drug treatment history, including use of beta-blockers. The parameters were recorded to determine if the patient had clinical symptoms or signs of heart failure.
Assessment of liver cirrhosis
The blood sample of study participants was analyzed for serum bilirubin, ALT (alanine aminotransferase), AST (aspartate aminotransferase), albumin, platelet count, hemoglobin, INR (international normalized ratio), serum sodium and creatinine level, etc. An ECG (electrocardiogram) of the patients was done and interpreted. Patients underwent an abdominal ultrasound examination to determine the liver’s echo-texture, splenic size and presence/absence of ascites. Patients with liver cirrhosis routinely undergo upper GI endoscopy at our hospital. Findings of endoscopy were noted to determine whether patients had esophageal varices or portal hypertensive gastropathy.
Cirrhosis was diagnosed by consistent clinical, hematological (reduced platelet count, raised INR), biochemical (low albumin, raised bilirubin, and AST > ALT), or radiological parameters (the presence of nodular liver or heterogenous liver parenchyma with irregular margins or features of liver decompensation or portal hypertension like ascites, dilated portal vein, or splenomegaly). Severity of liver cirrhosis was assessed according to the aforementioned scores.
Assessment of cardiomyopathy
Next, all study participants underwent echocardiography on a Vivid E95 cardiac ultrasound machine.
Evaluation of left ventricular systolic function
Stroke volume (SV) was calculated automatically by the machine as was the left ventricular ejection fraction (LVEF) in the two-dimensional motion mode (2D M-mode).
Systolic dysfunction was defined as left ventricular ejection fraction ≤ 50%.
Evaluation of left ventricular diastolic function
Various hemodynamic and Doppler parameters were determined to evaluate diastolic dysfunction.
The left ventricular end-diastolic diameter (LVEDD) and interventricular septum diameter were measured in the 2D M-mode, while the right ventricular end-diastolic diameter (RVEDd) was estimated in the 2D-parasternal long-axis view. Doppler echocardiography was used to calculate the cardiac index (CI). Left ventricular longitudinal function was determined via the mitral annular plane systolic excursion (MAPSE). Tricuspid annular plane systolic excursion (TAPSE) was used to evaluate right ventricular function using the 2D M-mode. The isovolumic relaxation time (IVRT) was calculated in the apical five-chamber view, whereas the tricuspid regurgitation velocity (TR velocity) and deceleration time (DT) were measured in the apical four-chamber view using doppler echocardiography. The E/a ratio, the ratio of early diastolic peak velocity of blood flow caused by left ventricular relaxation (E) to late diastolic peak velocity of blood flow caused by atrial contraction (a), which measures the diastolic function of the left ventricle, was quantified in the apical four-chamber view of the heart. Medial e is the early diastolic mitral annulus velocity in the medial commissure. The E/e′ ratio, which is a measure of the left ventricular filling pressure, is the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annulus velocity (e′). Finally, the left atrial volume index (LAVI), which is a measure of the size of the left atrium, was determined by dividing the volume of the left atrium by the body surface area. This measurement was taken using the four-chamber view of the heart but could not be determined in all patients due to time constraints and technical difficulties.
Diastolic dysfunction was defined, according to CCM consortium as ≥ 3 of the following:
-
E/e′ ratio ≥ 15
-
e′ septal < 7 cm/s
-
TR (tricuspid regurgitation velocity) > 2.8 m/S
-
LAVI (left atrial volume index) > 34 mL/m2
Statistical analysis
Data was entered in Statistical Package of Social Sciences (SPSS) version 25 of Windows. Quantitative variables like age and values of laboratory tests like AST, ALT, bilirubin, albumin, and ejection fraction are presented as means with standard deviation. Qualitative variables like gender, symptoms of heart failure, and portal hypertension are presented as frequency and percentages. To assess percentage comparisons, we employed both the chi-square test and Fisher’s exact test. The variable of interest was cardiomyopathy, and logistic regression analysis was used to determine the effects of advancement in liver cirrhosis, as expressed by rising Child–Pugh or MELD score on cardiac function (expressed as systolic or diastolic dysfunction). Multivariate Cox regression was used to examine cirrhosis and cardiovascular disease association. Statistical significance was determined by a p-value of ≤ 0.05.
Results
Demographic and baseline characteristics of the patients, n = 68, are shown in Table 2.
Mean age of the patients was 58.8 ± 9.6, while the majority of the patients were male (n = 43; 63%). The major comorbid illness was diabetes mellitus, which was present in 19/68 (28%) of the patients, while one patient was diabetic as well as hypothyroid (1.5%).
The echocardiographic parameters to determine systolic and diastolic dysfunction are presented in Table 3.
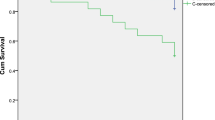
A total of 19 out of 68 (28%) had systolic dysfunction, while 6/68 (9%) had evidence of diastolic dysfunction. Overall prevalence of cirrhotic cardiomyopathy was 23/68 (34%) (Fig. 1).
Out of the patients with diastolic dysfunction, 7% were males (3/43), while 12% were females (3/25), p-value = 0.48. The presence of hepatitis C was strongly associated with systolic dysfunction with a p-value of 0.007. However, it was not statistically significantly associated with diastolic dysfunction, p-value = 0.59.
Logistic regression analysis was used to assess the association of advancement of liver cirrhosis, as assessed by Child Pugh, MELD, ALBI, PALBI, portal hypertension, FIB-4 score, on cardiac dysfunction. The overall regression was statistically non-significant (R2 = 3.66, F (13, 39) = 1.33, p = 0.234) (Table 4).
Discussion
The findings of this study reveal that cirrhotic cardiomyopathy is a complex condition with an elusive clinical presentation. Due to the lack of a universal criteria for diagnosing this condition, the 2019 cirrhotic cardiomyopathy consortium [13] was implemented in our study as it is current, comprehensive, and consistent with the 2016 ASE/EACVI guidelines [23]. In our case, cirrhotic cardiomyopathy was found in 34% patients. This is in concordance with the findings of similar studies conducted in this region where the frequency of CCM has been reported between 28 and 76% [24,25,26,27]. There was no significant association between demographic characteristics such as age, gender, and CCM. Similarly, the etiologies of cirrhosis were found to be insignificantly associated with CCM as well, except hepatitis C which was present in 30% patients with systolic dysfunction (p < 0.05).
The degree of cirrhosis was assessed using various scoring systems such as Child Pugh, MELD (model for end-stage liver disease), FIB-4 (fibrosis-4), ALBI (albumin-bilirubin), PALBI (platelet-albumin-bilirubin), and PH (portal hypertension) in order to ascertain the affiliation between worsening liver cirrhosis and cardiac dysfunction, which turned out to be insignificant in our case. While this is concurrent with most studies of a similar nature [28, 29], a recent investigation by Dash et al. in 2023 [24] reported a positive correlation between systolic dysfunction and the severity of cirrhosis. However, they were unable to uncover any significant connection with diastolic dysfunction. Further research may be required to validate these findings and establish a definitive relationship between CCM and worsening cirrhosis.
Systolic dysfunction is assessed via LVEF and is defined by an ejection fraction < 50% [13]. It was prevalent in 28% of our sample size. Systolic dysfunction is quiescent in CCM and often manifests as an inability to increase LVEF under stress. However, the ability of cirrhotic patients to exercise is diminished and consequently limits echocardiographic stress testing [30]. The use of dobutamine is restricted, while the use of beta-blockers is common in these patients which renders it ineffective [31]. In spite of these shortcomings, a study by Donovan et al. [32] unveiled ventricular dysfunction in less than 10% patients who underwent dobutamine stress testing. Alternatively, a plethora of studies have unmasked the presence of systolic dysfunction at rest as well. While Hammami et al. [28] failed to discover a correlation between systolic dysfunction and the severity of cirrhosis, they discovered systolic dysfunction in 17.5% patients at rest. Similarly, Kazankov et al. found both systolic and diastolic function to be impaired at rest [33].
Diastolic dysfunction is a commonly encountered problem in CCM. It usually manifests as LVDD. While its pathophysiology remains unclear, it is presumed to occur due to myocardial stiffness and impaired ventricular relaxation [33]. Some studies have implicated titin abnormalities of sarcomere in this process [34,35,36]. Previous studies have declared the prevalence of diastolic dysfunction to be between 43 and 70% [34,35,36,37], whereas it was found to be 9% in our case. This can be attributed to the discordance between the various diagnostic criteria used by different studies and the lower occurrence of diastolic dysfunction associated with the 2019 cirrhotic cardiomyopathy consortium as opposed to the 2005 Montreal recommendations [38]. The lack of harmony between the aforementioned diagnostic criteria and the echo-algorithm proposed by the American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) [23] was explored by Razpotnik et al. in 2020 who concluded that the prevalence of systolic dysfunction was higher with the 2019 consortium, while that of diastolic dysfunction was higher with the 2005 Montreal criteria, an observation that has been reinforced by our results [38]. Moreover, Lancelotti et al. [39] and Anderson et al. [40] compared the 2009 ASE/EACVI criteria with the 2016 ASE/EACVI criteria and found the latter to be more reliable, clinically relevant, and consummate, which not only establishes the superiority of the 2019 consortium but also substantiates our findings [41]. Additionally, diastolic dysfunction may be under diagnosed via typical echocardiography which tends to show a pseudo-normal pattern and may be confined by load-dependent E/A. These drawbacks can be resolved via tissue doppler imaging, an option that is endorsed by the American Society of Echocardiography criteria for diastolic dysfunction [42]. While newer techniques like speckle tracking echocardiography and cardiac magnetic resonance exist, they have not been proven to be better, and their use is limited due to cost and availability [43]. Diastolic dysfunction was not significantly associated with etiology in our study with its concordance with the findings of prior investigations [24, 44]. Similarly, it was also not associated with the severity of cirrhosis, another finding that corroborates the results from previous studies [24, 45]. Additionally, many studies have highlighted the association between diastolic dysfunction and a poor prognosis [30].
As supportive criteria, such as QTc prolongation, is not diagnostic for CCM, it was not included in our findings. However, previous studies have estimated its prevalence to be between 30 and 60% [35]. Its association with the severity of cirrhosis has emerged in certain studies, but no concrete conclusions have been reached thus far [18]. Despite the ambiguity, patients with QTc prolongation are at increased risk of arrhythmias [10] due to the use of diuretics, vasoactive agents, and prophylactic fluoroquinolones [31]. While beta-blockers may help alleviate this risk, they might precipitate refractory ascites in the process. Thus, such patients must be monitored carefully for electrolyte imbalances and the use of QT-interval prolonging drugs [10, 18, 31].
A study by Lee et al. discovered that CCM is linked to an unfavorable prognosis and independently predicts survival in patients with decompensated cirrhosis [30]. While many treatment options exist, liver transplantation is preferred as it can rescind the effects of CCM. However, it is not completely unimpeachable as studies have shown the risk of cardiovascular events after transplant ranges between 25 and 70% [34, 35]. A study by Sonny et al. surprisingly discovered deteriorating diastolic dysfunction 41 months after transplantation in their cohort [7]. It ameliorated by more than 1 grade in about 40% of patients, while LV mass index demonstrated a significant increase in others [7]. Similarly, a single-center, retrospective cohort study by Izzy et al. in 2020 followed 141 liver transplantation patients over a median of 4.5 years and uncovered that application of the new 2019 criteria increased the likelihood of posttransplant events by twofold [4, 46].
Conclusion
In conclusion, despite the burgeoning prevalence of CCM, it is still fairly underdiagnosed in patients with cirrhosis. Our study, in concordance with other studies of its ilk, highlights the need to incorporate a universal and standardized criteria for diagnosing CCM in these patients. It also illuminates the need to explore possible correlations between the severity of cirrhosis and cardiac dysfunction. Our extensive perusal of available literature on the subject also underscored the need to conduct future researches to evaluate novel and innovative treatment approaches and identify prognostic indicators and risk-stratification tools for diagnosing CCM.
Limitations
The current study had a few limitations such as its confinement to a single center and therefore a smaller pool of patients which could have led to potential biases in patient selection. Moreover, the inability to deploy the latest diagnostic modalities as well as stress echocardiography could have caused a few cases to escape undetected. Furthermore, brain natriuretic peptide (BNP) was not estimated in our current study as it is not exclusive to cardiac dysfunction arising from cirrhosis [24]. Due to certain constraints, we were unable to measure LAVI in all of our subjects which could have undermined the accuracy of our study. It is important to acknowledge these limitations as they provide invaluable insights for future researches and reinforce the need to conduct longitudinal, multi-center studies to address them.
Availability of data and materials
Data is available on demand.
References
Kowalski HJ, Abelmann WH (1953) The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest 32:1025–1033. https://doi.org/10.1172/jci102813
Nakashima M, Nakamura K, Nishihara T et al (2023) Association between cardiovascular disease and liver disease, from a clinically pragmatic perspective as a cardiologist. Nutrients 15(3):748. https://doi.org/10.3390/nu15030748. Published 2023 Feb 1
El Hadi H, Di Vincenzo A, Vettor R et al (2020) Relationship between heart disease and liver disease: a two-way street. Cells 9(3):567. https://doi.org/10.3390/cells9030567. Published 2020 Feb 28
Izzy MJ, VanWagner LB (2021) Current concepts of cirrhotic cardiomyopathy. Clin Liver Dis 25(2):471–481. https://doi.org/10.1016/j.cld.2021.01.012
Kelbæk H, Rabøl A, Brynjolf I et al (1987) Haemodynamic response to exercise in patients with alcoholic liver cirrhosis. Clin Physiol. 7(1):35–41. https://doi.org/10.1111/j.1475-097x.1987.tb00631.x
Bodys-Pełka A, Kusztal M, Raszeja-Wyszomirska J et al (2021) What’s new in cirrhotic cardiomyopathy?—Review article. J Pers Med 11(12):1285. https://doi.org/10.3390/jpm11121285
Sonny A, Ibrahim A, Schuster A et al (2016) Impact and persistence of cirrhotic cardiomyopathy after liver transplantation. Clin Transplant 30(9):986–993. https://doi.org/10.1111/ctr.12778
Chayanupatkul M, Liangpunsakul S (2014) Cirrhotic cardiomyopathy: review of pathophysiology and treatment. Hep Intl 8(3):308–315. https://doi.org/10.1007/s12072-014-9531-y
Fukui H (2020) Leaky gut and gut-liver axis in liver cirrhosis: clinical studies update. Gut Liver. https://doi.org/10.5009/gnl20032
Yoon KT, Liu H, Lee SS (2020) Cirrhotic cardiomyopathy. Curr Gastroenterol Rep. 22(9). https://doi.org/10.1007/s11894-020-00783-1
Zardi EM, Abbate A, Zardi DM et al (2010) Cirrhotic cardiomyopathy. J Am Coll Cardiol 56(7):539–549. https://doi.org/10.1016/j.jacc.2009.12.075
Gassanov N, Caglayan E, Semmo N et al (2014) Cirrhotic cardiomyopathy: a cardiologist’s perspective. World J Gastroenterol 20(42):15492–15498. https://doi.org/10.3748/wjg.v20.i42.15492
Izzy M, VanWagner LB, Lin G et al (2020) Redefining cirrhotic cardiomyopathy for the modern era. Hepatology 71(1):334–345. https://doi.org/10.1002/hep.30875
Kaur H, Premkumar M (2022) Diagnosis and management of cirrhotic cardiomyopathy. J Clin Exp Hepatol 12(1):186–199. https://doi.org/10.1016/j.jceh.2021.08.016
Dourakis SP, Geladari E, Geladari C et al (2021) Cirrhotic cardiomyopathy: the interplay between liver and cardiac muscle. How does the cardiovascular system react when the liver is diseased? Curr Cardiology Rev 17(1):78–84. https://doi.org/10.2174/1573403x15666190509084519
Stundiene I, Sarnelyte J, Norkute A et al (2019) Liver cirrhosis and left ventricle diastolic dysfunction: systematic review. World J Gastroenterol 25(32):4779–4795. https://doi.org/10.3748/wjg.v25.i32.4779
Zambruni A, Di Micoli A, Lubisco A et al (2007) QT interval correction in patients with cirrhosis. J Cardiovasc Electrophysiol. 18(1):77–82. https://doi.org/10.1111/j.1540-8167.2006.00622.x
Bernardi M, Maggioli C, Dibra V et al (2012) QT interval prolongation in liver cirrhosis: innocent bystander or serious threat? Expert Rev Gastroenterol Hepatol 6(1):57–66. https://doi.org/10.1586/egh.11.86
Henriksen JH, Bendtsen F, Hansen EK et al (2004) Acute non-selective β-adrenergic blockade reduces prolonged frequency-adjusted Q-T interval (QTc) in patients with cirrhosis. J Hepatol 40(2):239–246. https://doi.org/10.1016/j.jhep.2003.10.026
Sinha R, Lockman KA, Mallawaarachchi N et al (2017) Carvedilol use is associated with improved survival in patients with liver cirrhosis and ascites. J Hepatol 67(1):40–46. https://doi.org/10.1016/j.jhep.2017.02.005
Premkumar M, Rangegowda D, Vyas T et al (2019) Carvedilol combined with ivabradine improves left ventricular diastolic dysfunction, clinical progression, and survival in cirrhosis. J Clin Gastroenterol 54(6):561–568. https://doi.org/10.1097/mcg.0000000000001219
Koshy A, Gow PJ, Han H et al (2020) Cardiovascular mortality following liver transplantation: predictors and temporal trends over 30 years. Eur Heart J Qual Care Clin Outcomes 6(4):243–253. https://doi.org/10.1093/ehjqcco/qcaa009
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29(4):277–314. https://doi.org/10.1016/j.echo.2016.01.011
Dash SC, Rajesh B, Behera SK et al (2023) Is cirrhotic cardiomyopathy related to cirrhosis severity? Rambam Maimonides Med J 14(1):e0001. https://doi.org/10.5041/RMMJ.10488. PMID: 36719669; PMCID: PMC9888483
Naqvi IH, Mahmood K, Naeem M et al (2016) The heart matters when the liver shatters! Cirrhotic cardiomyopathy: frequency, comparison, and correlation with severity of disease. Prz Gastroenterol 11(4):247–256. https://doi.org/10.5114/pg.2016.57962
Bokarvadia R, Jain M, Kedarisetty C, Varghese J, Venkataraman J (2019) Prevalence and clinical presentation of cirrhotic cardiomyopathy: a single centre experience from southern India. Indian J Gastroenterol 38(2):150–157. https://doi.org/10.1007/s12664-019-00946-7
Koushik A, Ganesh P, Shanmuganathan S (2020) Incidence of cirrhotic cardiomyopathy among hundred patients of Tamil Nadu, India- a cross-sectional study. J Clin Diagn Res. https://doi.org/10.7860/jcdr/2020/46768.14278
Hammami R, Boudabbous M, Jdidi J et al (2017) Cirrhotic cardiomyopathy: is there any correlation between the stage of cardiac impairment and the severity of liver disease? Libyan J Med 12(1):1283162. https://doi.org/10.1080/19932820.2017.1283162
Lee SK, Song MJ, Kim SH et al (2018) Cardiac diastolic dysfunction predicts poor prognosis in patients with decompensated liver cirrhosis. Clin Mol Hepatol 24(4):409–416. https://doi.org/10.3350/cmh.2018.0034
Mozos I (2015) Arrhythmia risk in liver cirrhosis. World J Hepatol 7(4):662–672. https://doi.org/10.4254/wjh.v7.i4.662
Torregrosa M, Aguadé S, Dos L et al (2005) Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol 42(1):68–74. https://doi.org/10.1016/j.jhep.2004.09.008
Donovan CL, Marcovitz PA, Punch JD et al (1996) Two-dimensional and dobutamine stress echocardiography in the preoperative assessment of patients with end-stage liver disease prior to orthotopic liver transplantation. Transplantation 61(8):1180–1188. https://doi.org/10.1097/00007890-199604270-00011
Kazankov K, Holland-Fischer P, Andersen NH et al (2011) Resting myocardial dysfunction in cirrhosis quantified by tissue Doppler imaging. Liver Int 31(4):534–540. https://doi.org/10.1111/j.1478-3231.2011.02468.x
Ruiz-del-Árbol L, Serradilla R (2015) Cirrhotic cardiomyopathy. World J Gastroenterol 21(41):11502–11521. https://doi.org/10.3748/wjg.v21.i41.11502
Liu H, Nguyen HH, Yoon KT et al (2022) Pathogenic mechanisms underlying cirrhotic cardiomyopathy. Front Netw Physiol 19(2):849253. https://doi.org/10.3389/fnetp.2022.849253. PMID: 36926084; PMCID: PMC10013066
Glenn TK, Honar H, Liu H et al (2011) Role of cardiac myofilament proteins titin and collagen in the pathogenesis of diastolic dysfunction in cirrhotic rats. J Hepatol 55(6):1249–1255. https://doi.org/10.1016/j.jhep.2011.02.030
Dadhich S, Goswami A, Jain VK et al (2014) Cardiac dysfunction in cirrhotic portal hypertension with or without ascites. Ann Gastroenterol 27(3):244–249
Razpotnik M, Bota S, Wimmer P et al (2021) The prevalence of cirrhotic cardiomyopathy according to different diagnostic criteria. Hernandez‐Gea V, ed. Liver Int 41(5):1058–1069. https://doi.org/10.1111/liv.14769
Lancellotti P, Galderisi M, Edvardsen T et al (2017) Echo-Doppler estimation of left ventricular filling pressure: results of the multicentre EACVI Euro-Filling study. Eur Heart J Cardiovasc Imaging 18(9):961–968. https://doi.org/10.1093/ehjci/jex067
Andersen OS, Smiseth OA, Dokainish H et al (2017) Estimating left ventricular filling pressure by echocardiography. J Am Coll Cardiol 69(15):1937–1948. https://doi.org/10.1016/j.jacc.2017.01.058
Edvardsen T, Smiseth OA (2018) Evaluation of diastolic function by echocardiography: important progression, but issues to be resolved. Eur Heart J Cardiovasc Imaging 19(4):387–388. https://doi.org/10.1093/ehjci/jex319
Nagueh SF, Appleton CP, Gillebert TC et al (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133. https://doi.org/10.1016/j.echo.2008.11.023
Sampaio F, Pimenta J (2016) Left ventricular function assessment in cirrhosis: current methods and future directions. World J Gastroenterol 22(1):112–125. https://doi.org/10.3748/wjg.v22.i1.112
Somani PO, Contractor Q, Chaurasia AS et al (2014) Diastolic dysfunction characterizes cirrhotic cardiomyopathy. Indian Heart J 66(6):649–655. https://doi.org/10.1016/j.ihj.2014.06.001
Behera MK, Narayan J, Sahu MK et al (2021) Factors predicting cardiac dysfunction in patients with liver cirrhosis. Middle East J Dig Dis 13(3):216–222. https://doi.org/10.34172/mejdd.2021.228
Izzy M, Soldatova A, Sun X et al (2021) Cirrhotic cardiomyopathy predicts posttransplant cardiovascular disease: revelations of the new diagnostic criteria. Liver Transpl 27(6):876–886. https://doi.org/10.1002/lt.2600
Acknowledgements
The authors would like to thank Ms. Maryam Qadeer, Medical Imaging and Echo-Technologist, for her invaluable support and assistance while gathering data for this research.
Funding
No special grant or funding was acquired for this study.
Author information
Authors and Affiliations
Contributions
Mahnam Khizer, MD: Substantial contributions to the acquisition of data for the work, drafting the work, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. Hala Mansoor, FRCP (Edin), FCPS Medicine and Gastroenterology: Substantial contributions to the design of the work; the acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. Aneela Afreen, FCPS Cardiology and MRCP: Substantial contributions to the design of the work; the acquisition, analysis, and interpretation of data for the work; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. Noor Masood Sadiq, MD: Substantial contributions to the design of the work; the acquisition, analysis, and interpretation of data for the work; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. Aamir Habib, FCPS Medicine: Substantial contributions to the design of the work; the acquisition, analysis, and interpretation of data for the work; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. Shafqat Ali, M.B.B.S: Substantial contributions to the design of the work; the acquisition, analysis, and interpretation of data for the work; revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. Asim Raza, PhD Scholar (Public Health): Substantial contributions to the design of the work, analysis and interpretation of data for the work, revising it critically for important intellectual content; final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work. Tayyaba Hafeez, MD: Substantial contributions to the design of the work, the acquisition of data for the work, revising it critically for important intellectual content, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was carried out with the approval of the Institution Review Board (610/FRC/CMH/LMC), in accordance with principles of Helsinki, after getting informed consent from patients.
Consent for publication
Consent for publication is provided by all authors to Egyptian Liver Journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mansoor, H., Khizer, M., Afreen, A. et al. Systolic and diastolic impairment in cirrhotic cardiomyopathy: insights from a cross-sectional study. Egypt Liver Journal 14, 60 (2024). https://doi.org/10.1186/s43066-024-00367-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-024-00367-y