Abstract
Background
Echinococcosis, or hydatid disease, is caused by the larval forms of taeniid cestodes belonging to the genus Echinococcus. Echinococcus granulosus and E. multilocularis are the primary species responsible for human echinococcosis, and mostly they affect the liver. The disease course is typically slow, and the patients tend to remain asymptomatic for many years.
Case presentation
A case of 19-year-old male Somali from Medina, Saudi Arabia, was presented to the Mogadishu Somali Turkey Recep Tayyip Erdogan Training and Research Hospital with the complaint of right upper quadrant abdominal pain and dysuria for 1 month. A thorough physical examination, laboratory examination, and imaging investigations, including abdominal sonography and computed tomography (CT), were conducted.
Both ultrasound and CT scans revealed a cystic lesion in the right lobe of the liver, confirming the diagnosis of a hydatid cyst. Consequently, the patient underwent exploratory laparotomy. It was successfully managed surgically, and the definitive diagnosis was provided by the pathologist, confirming degenerated echinococcal cysts that contain abundant debris. Additionally, the patient received oral albendazole both before and after the surgery and after 7 months and is currently symptom-free.
Conclusions
Hydatid disease in the liver may persist without symptoms and often goes undiagnosed due to the slow growth of the cysts. The diagnosis needs careful history reporting, physical examination, and appropriate imaging investigations.
Similar content being viewed by others
Background
Echinococcosis in humans, also known as hydatidosis or hydatid disease, is caused by the larval forms of dog tapeworm parasites belonging to the genus Echinococcus (family Taeniidae). Human echinococcosis is caused by the primary species of Echinococcus granulosus and E. multilocularis [1]. Echinococcus granulosus has been identified as the most common cause of hydatid disease, which forms cysts in any organ or tissue of human [2]. This infection is a major endemic health problem and is highly prevalent in specific regions worldwide, including the Mediterranean region, Eastern Europe, South America, the Middle East, and East Africa [3].
In the life cycle, the mature tapeworm lives in the intestines of their definitive hosts, primarily canines, such as dogs, where it releases excreted eggs in the feces. These eggs are subsequently consumed by intermediate hosts, notably livestock such as sheep [2]. Humans, known as aberrant intermediate hosts, can be infected by ingesting the eggs in food or water contaminated with stool from infected dogs [3].
Hydatid can involve any organ. The liver and lungs are the most frequently affected sites [4, 5]. Hepatic hydatid is a life-threatening disease, mainly differentiated into cystic and alveolar forms, associated with infections of E. granulosus and E. multilocularis, respectively. Cystic hydatid is distributed worldwide, while alveolar hydatid is endemic in North America and several Asian and European countries [6].
Ultrasonography is important in the diagnosis of the disease. The classification of the World Health Organization (WHO), based on ultrasonographic findings, is used for staging the disease and treatment selection. Additionally to the imaging methods, some of the immunological investigations such as indirect hemagglutination (IHA), enzyme-linked immunosorbent assay (ELISA), and immunoblotting are used to support the diagnosis, while the available treatment options for E. granulosus infections include open surgery, percutaneous interventions, and pharmacotherapy. Aggressive surgery is the first-choice treatment, while pharmacotherapy is used as an adjunct to surgery [7].
Two cases of hydatid infections have been documented in Somali patients who were infected by hydatid cyst of the foot and hydatid cyst bone disease [8, 9]. In this study, the case of an immigrant male patient who had hepatic hydatid cysts with no specific symptoms and signs but with typical sonography and computed tomography (CT) findings, including daughter cysts and internal septa, was reported for the first time in Somalia. Accurate diagnosis and, subsequently, surgical intervention along with pre- and post-operative albendazole led to a successful cure.
Case presentation
A 19-year-old man was healthy until he suffered from discomfort in the right upper quadrant abdominal pain, along with a mild fever, abdominal pain, loss of appetite, and headache for about a month before he was admitted to the hospital, where there was no previous hospitalization and surgical interventions.
Previously, he lived in Saudi Arabia before he came to Mogadishu, Somalia. When he visited the outpatient general surgery department, his body temperature was 36.5 °C, and he had mild right upper quadrant abdominal tenderness without evidence of liver enlargement on the vertical span. His cardiovascular system and respiratory parameters were unremarkable. No other symptoms, such as nausea, vomiting, or skin rash, were noted. The respiratory rate, pulse, and blood pressure were normal. His white blood cells (WBCs) count was 7200/mm, red blood cells (RBCs) count was 5880/mm, and hemoglobin level was 15.2 g/dl, with 30.8% neutrophils and 2.1% eosinophils. Urine and stool analyses were unremarkable. Biochemical tests for alkaline phosphatase, gamma-glutamyl transferase, aspartate aminotransferase, glucose, urea, creatinine, and bilirubin were within normal limits. Furthermore, C-reactive protein (CRP) was normal too. Tests for anti-hepatitis C virus antibody, hepatitis B surface antigen (HBsAg), and anti-HBsAg antibody were negative. These tests were conducted according to the guidelines of the hospital in such cases.
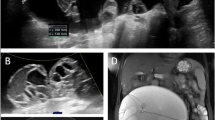
Due to the right upper quadrant abdominal pain, the abdominal sonography was performed. There was a 3 × 3 cm cystic lesion encircling multiple daughter cysts at the level of segment 5 in the right lobe of the liver. At the level of segment 8, there was a dense cystic lesion with a floating membrane (hydatid cyst). Triphasic dynamic abdominal CT was recommended.
The CT scan of the upper quadrant abdomen demonstrated a well-defined 12-cm-sized multi-loculated cystic lesions with peripherally distributed daughter cyst in segment 5 of the liver denoting type 2 hydatid cyst, a 6 × 5 cm rim calcified cyst consistent with type 3 hydatid cyst in segment 8 of the liver (Figs. 1 and 2).
Computed tomography of the abdomen of 19-year-old Somali male infected by hepatic hydatid cyst. A and B Axial section. C Coronal section of contrast-enhanced computed tomography (CT) scan of the abdomen demonstrating large multilocular fluid density cysts, some of them showing septations and daughter cysts as well as soft tissue attenuation area in segment 5
Management and outcomes
Pre-operative oral albendazole 200 mg four times daily was administered for 4 days. Following getting the informed consent, the patient was prepared for exploratory laparotomy, fitness for general anesthesia was confirmed, and surgery was planned. The patient underwent exploratory laparotomy, and two huge cysts interconnected just under the liver were found. A calcified one is on the superior surface of the liver. The surgeon tried to free the difficult cyst, and then he opened the cyst, where many debris and eggs were found inside it (Fig. 3). Then, the cyst was cleaned and washed with hypertonic saline solution and iodine. After that, the surgeon freed the cyst, removed the whole sac, opened the suprahepatic one, and cleaned it as he did with the previous one. Again, he removed the part of the sac that was attached to the liver and cauterized the part connected to the diaphragm. Then, the abdomen was washed with liters of hypertonic solutions, and three drains were installed (Fig. 3) in suprahepatic spaces of Morison’s pouch, pelvis, and hemostasis to drain all the excessive amount of hypertonic solutions that was used in washing the abdomen, and then the abdomen was closed layer by layer.
Histopathologic study of the lesion showed an acellular fibrous wall of the hydatid cyst with laminations and the liver parenchyma infiltrated with mixed chronic inflammatory cells consisting of mononuclear cells, eosinophils, and multinucleated giant cells (Fig. 4). The histopathologic features were compatible with hydatid cysts.
Histopathological examination of hepatic hydatid cyst of infected 19-year Somali male patient. A, B Histopathological examination showing the acellular fibrous wall of the hydatid cyst (Hematoxylin & eosin (H & E) staining). C Liver sections with mixed chronic inflammation consisting of mononuclear cells, eosinophils, and multinucleated giant cells (H & E staining). D Liver cyst wall with laminations (H & E staining)
The patient recovered uneventfully, and there were no post-operative complications except for wound pain, which was treated conservatively. The patient was discharged in good health 2 weeks later. Long-term post-operative albendazole 200 mg four times daily was prescribed for 2 months.
A follow-up laboratory test conducted 1 month later revealed that his parameters were within the normal range. Furthermore, an ultrasound and CT examinations were performed 7 months later, and the previously described two hydatid cysts were not seen.
Conclusions
This study reported the first record of hepatic hydatid cysts of Somali immigrant in Saudi Arabia. Animal hydatid cysts in Somalia are frequently observed in camels (14.82%), seldom in cattle (1.75%), and exceptionally in goats and sheep; a high frequency of E. granulosus infection is found in the stray dogs in Mogadishu (23.4%). Notwithstanding the remarkable rate of infection in camels and the favorable chances of contracting the disease [10], human hepatic hydatid has never been noted in Somalia. Human hydatid is an endemic disease in sheep-raising countries such as Saudi Arabia [4] as the emigration of the patient from an area of endemicity supports a diagnosis of E. granulosus cystic hydatid disease [9].
While the liver is a common site for these infections [9], it is crucial to remember that they can manifest in diverse locations throughout the body, including the lungs, spleen, kidney, brain, and bones [7]. After infection, humans are usually asymptomatic for a long period since the disease progresses slowly [6], the same as in the presenting case where the patient was not complaining of hepatic hydatid symptoms for a long time [7]. The case study was attending the hospital due to mild right upper quadrant abdominal pain. The signs and symptoms of liver hydatidosis include hepatomegaly, right/epigastric pain, nausea, vomiting, hepatitis, cholangitis, and anaphylaxis due to the dissemination of the cyst [6, 7].
Usually, the hepatic cyst growth rate is variable, ranging from 1 to 5 mm in diameter per year. Most primary infections consist of a single cyst, but up to 20–40% of infected people have multiple cysts. This patient had daughter cysts, and the histological examination may indicate an old cyst infection (type 2 and 3). It was reported that the differences between E. granulosus old and young cysts are that the old cysts typically present internal septations and daughter cysts, while the young are spherical, unilocular vesicles consisting of an inner germinal layer and an outer acellular layer [6]. Interestingly, most hydatid patients have a single cyst; 20–40% tend to harbor multiple cysts [6], as in the presenting case.
Mortality from hydatid cysts is usually due to the development of complications and is reported to be 2–4% [11, 12]. Complications of echinococcal disease include allergic reactions to the dissemination of cyst contents due to spontaneous, traumatic, or iatrogenic rupture, secondary infection, and cholangitis [13,14,15].
Considering that the early stages of infection are usually asymptomatic, the diagnosis of hepatic hydatid may often be incidental. In endemic areas, the presence of symptoms suggestive of hepatic hydatid in a person with a history of exposure to sheepdogs supports the suspicion of hydatid [6]. In this case, it was recommended to the patient after his admission to the hospital to perform abdominal sonography. A non-invasive diagnosis of hepatic hydatid is usually accomplished with the combined use of radiologic imaging and immunodiagnostic techniques. Abdominal ultrasonography is considered the gold standard for defining the number, size, dimensions, and vitality of cysts [13, 16], and it is also important to evaluate treatment options. Despite that, ultrasonography is not always able to differentiate hydatid cysts from other space-occupying lesions, like tumors or liver abscesses, so additional imaging techniques, such as magnetic resonance imaging (MRI) and CT scans, are recommended in addition to immunodiagnostic techniques [6, 17].
The goals of hepatic hydatid cyst treatment are the complete elimination of the parasite and prevention of recurrence and minimizing mortality and morbidity risk [14]. To achieve these aims, it is essential to choose the most appropriate treatment concerning disease-specific characteristics (cyst number, size, site, presence of cystobiliary communication), patient clinical conditions, and availability of an experienced surgeon or an interventional radiologist [6]. The improvement of surgical techniques, the introduction of minimally invasive treatments (such as puncture, aspiration, injection, and re-aspiration), and more effective drugs (such as benzimidazoles) have deeply changed the life expectancy and quality of life of patients with hydatid disease. Treatment usually consists of a combination of surgery to remove the cyst and chemotherapy (specifically, albendazole therapy) for 4 days before the procedure. It should be continued for at least 1 month after the operation [13, 18]. Although a wide range of treatment methods have been identified (medical, percutaneous, monitoring, and surgical), a standardized treatment protocol has yet to be defined [7].
In endemic regions, the hepatic echinococcosis can be quite prevalent [1, 3, 6, 7]. Symptoms of hepatic hydatid disease can vary in severity. The clinical presentation may range from asymptomatic form to sudden death. Imaging techniques are mandatory for early detection of the disease. Surgical excision of the cyst without rupture is the modality of choice. Supplemental treatment with albendazole is strongly recommended to decrease the risk of recurrence. Prevention initiatives at local and national levels are the best way to reduce the prevalence of hepatic hydatid disease.
Availability of data and materials
The major data generated or analyzed during this study are included in this published article. All the images and detailed information of this case are available at Somali Turkey Recap Tayyip Erdogan Training and Research Hospital digital patient record and can be submitted upon request.
Abbreviations
- CT:
-
Computed tomography
- WHO:
-
World Health Organization
- E.:
-
Echinococcus
- WBCs:
-
White blood cells
- RBCs:
-
Red blood cells
- CRP:
-
C-reactive protein
- HBsAg:
-
Hepatitis B surface antigen
- MRI:
-
Magnetic resonance imaging
- H & E:
-
Hematoxylin & eosin
References
Gessese A (2020) Review on epidemiology and public health significance of hydatidosis. Vet Med Int 2020:8859116
Akrim Y, Babokh F, El Hakkouni A (2022) Cardiac echinococcosis with hepatic involvement in a child: a case report. Cureus 14(10):e30390
Grosso G, Gruttadauria S, Biondi A, Marventano S, Mistretta A (2012) Worldwide epidemiology of liver hydatidosis including the Mediterranean area. World J Gastroenterol 18(13):1425–1437
Krige J, Beckingham I (2001) ABC of diseases of liver, pancreas, and biliary system. BMJ 322:537–540
Guo J, Ma C, Song X, Tang F, Guo L, Mao J et al (2022) Hepatocellular carcinoma complicated by echinococcal cyst: a case report. Front Surg 8:816501
Nunnari G, Pinzone M, Gruttadauria S, Celesia B, Madeddu G, Malaguarnera G et al (2012) Hepatic echinococcosis: clinical and therapeutic aspects. World J Gastroenterol 18(13):1448–1458
Mihmanli M, Idiz U, Kaya C, Demir U, Bostanci O, Omeroglu S et al (2016) Current status of diagnosis and treatment of hepatic echinococcosis. World J Hepatol 8(28):1169–1181
Ewnte B (2020) Hydatid cyst of the foot: a case report. J Med Case Rep 14(1):6
Mackowiak P, Babady PB, Walker R, Rosenblatt J, Binnicker A (2009) A 48-year-old Somali woman with hip pain. Clin Infect Dis 49(5):803–805
Macchioni G, Arispici M, Lanfranchi P, Testi F (1987) Experimental infection of sheep and monkeys with the camel strain of Echinococcus granulosus. In: Kumar V, Brandt J, Geerts S (eds) Helminth zoonoses. Current Topics in Veterinary Medicine and Animal Science, vol 43. Springer, Dordrecht
Junghanss T, da Silva A, Horton J, Chiodini P, Brunetti E (2008) Clinical management of cystic echinococcosis: state of the art, problems, and perspectives. Am J Trop Med Hyg 79:301–311
Belhassen-García M, Romero-Alegria A, Velasco-Tirado V, Alonso-Sardón M, Lopez-Bernus A, Alvela-Suarez L et al (2014) Study of hydatidosis-attributed mortality in endemic area. PLoS ONE 9:e91342
Pawlowski Z, Eckert J, Vuitton D, Ammann R, Kern P, Craig P (2001) Echinococcosis in humans: clinical aspects, diagnosis and treatment. In: Eckert J, Gemmell M, Meslin F, Pawlowski Z (eds) WHO/OIE Manual on Echinococcosis in humans and animals. Office International des Epizooties, Paris, pp 20–71
Brunetti E, Kern P, Vuitton D (2010) Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114:1–16
Torgerson P, Keller K, Magnotta M, Ragland N (2010) The global burden of alveolar echinococcosis. PLoS Negl Trop Dis 4:e722
Shambesh M, Craig P, Macpherson C, Rogan M, Gusbi A, Echtuish E (1999) An extensive ultrasound and serologic study to investigate the prevalence of human cystic echinococcosis in northern Libya. Am J Trop Med Hyg 60:462–468
Hosch W, Junghanss T, Stojkovic M, Brunetti E, Heye T, Kauffmann G et al (2008) Metabolic viability assessment of cystic echinococcosis using high-field 1H MRS of cyst contents. NMR Biomed 21(7):734–754
Hira P, Shweiki H, Lindberg L, Shaheen Y, Francis I, Leven H et al (1988) Diagnosis of cystic hydatid disease: role of aspiration cytology. Lancet 2:655–657
Acknowledgements
The authors wish to acknowledge the support and cooperation of the management of Somali Turkey Recap Tayyip Erdogan Training and Research Hospital. We sincerely appreciate your kind assistance during this study.
Funding
No funds were used to write this case report.
Author information
Authors and Affiliations
Contributions
MAM and AAO analyzed and interpreted the patient data. MAM, IGI, MHA, and AE were major contributors in writing and revising the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The ethical board review of the hospital has approved the publication of this case report. Facial images describing the patient discussed will not be used. The patient has provided written informed consent both to undergo the operation as well as to get the case published.
Consent for publication
Written informed consent was obtained from the patient to publish this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamud, M.A., Omar, A.A., Adam, M.H. et al. A rare case of hepatic hydatid cyst in Somalia: a case report. Egypt Liver Journal 14, 54 (2024). https://doi.org/10.1186/s43066-024-00363-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-024-00363-2