Abstract
Background and purpose of the study
Hepatic steatosis (HS) is a common and important histologic finding in patients with chronic hepatitis C virus (HCV) infection. However, little is known about this finding in HCV patients co-infected with human immunodeficiency virus (HIV).
Purpose of the study
To evaluate the prevalence and predictors of HS among HIV patients with and without HCV.
Methods
A cross-sectional including 47 HIV mono-infected and 50 HIV/HCV patients. Detailed demographic, laboratory and clinical data were collected. A transient elastography (TE) examination with the controlled attenuation parameter (CAP) was performed for all patients. Steatosis was scored on a scale from 0 to 3 according to the percentage of steatosis involving hepatocytes.
Results
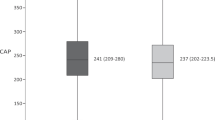
HS was detected in 18 /50 (36%) HIV/HCV co-infected patients and 22 /47 (46.8%) HIV mono-infected patients. In addition, HCV mono-infected patients were more likely to have grade 2 steatosis as compared with HIV/HCV co-infected individuals (25.5% vs. 12%). LSM was significantly higher among HIV/HCV co-infected patients (p = 0.003). When dividing HIV/HCV co-infected and HIV mono-infected group according to CAP values. BMI, abdominal wall thickness, and bright liver were significantly associated with SHS (CAP ≥ 238 dB/m) in both groups. Diabetes was only significantly associated with SHS in HIV/HCV co-infected group BMI was the only independent predictor of SHS among the whole group and subgroup analysis.
Conclusion
The prevalence of HS was lower in HIV/HCV co-infected patients compared to HIV mono-infected patients; we cannot say that HCV has a protective role against HS in co-infected patients. More fibrosis progression was common in the HIV/HCV co-infected patients.
Similar content being viewed by others
Introduction
Human immune deficiency virus (HIV) and hepatitis C Virus (HCV) infections are among the most significant burden of morbidity and mortality globally. HIV-infected individuals may be at higher exposure risk to HCV with geographical variations [1]. Globally, 37 million people living with HIV (PLHIV) and 115 million infected with HCV; about 2.3 million people PLHIV are co-infected with HCV [2].
The interaction between HIV and HCV infections affects the transmission and natural history of HCV infection. The transmission efficiency of HCV increases in the presence of HIV infection [3]. PLHIV without treatment are less likely to spontaneously clear HCV infection, have higher HCV viral loads [4], and experience more rapid HCV disease progression than those without HIV infection, with higher rates of cirrhosis, hepatic decompensation and hepatocellular carcinoma (HCC) [5].
Nonalcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease that lead to liver fibrosis and cirrhosis. Another emerging challenge is hepatocellular cancer which increasingly arises in pre-cirrhotic stages. There is increasing concern that patients with chronic HIV infection may be at increased risk of NAFLD, which can evolve into nonalcoholic steatohepatitis (NASH) and cirrhosis. Multiple factors have been hypothesized to be necessary for the development and progression of this condition including metabolic derangements, chronic inflammation, hepatitis co-infection, and treatment with certain nucleoside reverse transcriptase inhibitors (NRTIs). HIV-associated conditions such as hyperlactatemia and lipodystrophy frequently overlap with fatty liver disease [6].
Hepatic steatosis (HS) has also been reported in HIV/HCV co-infection. However, little is known about steatosis formation or the risk factors in such patients [7].
Herein, we aimed to evaluate the prevalence and predictors of HS among HIV patients with and without HCV.
Patients and methods
Selection of patients
This cross-sectional study recruited HIV infected patients with and without chronic HCV infection from Imbaba Fever Hospital who underwent transient elastography (TE) examination at Kasr Al-Aini Viral Hepatitis Center (KAVHC)—Faculty of Medicine—Cairo University. Eligible HIV patients with and without HCV co-infection were enrolled according to the following criteria (1): adult patients (age ≥ 18years); 2) diagnosis of HIV infection was established by the national reference laboratories; 3) chronic HCV infection was established by the presence of seropositivity for HCV antibodies and detectable HCV RNA levels (Cobas Amplicor, HCV Roche, Branchburg, NJ, USA, v 2.0, with a lower limit of quantification (LLOQ) of 15 IU/mL) for at least six months in HIV/HCV co-infection group; 4) the acceptance to undergo TE examination. Exclusion criteria were: 1) co-infection with hepatitis B virus; 2) evidence of other liver disease (autoimmune hepatitis, primary biliary cholangitis, and metabolic liver diseases); 3) manifestations of hepatic decompensation (e.g. ascites); 4) a history of HCC; 5) failure of TE examination or unreliable LSM. 5) Patients with chronic HCV infection who were on treatment with direct acting antiviral agents (DAAs) or just finished their treatment course. Patients were categorized into 2 groups: Group (A): HIV/HCV co-infected patients who were on antiretroviral therapy (ART) for HIV and did not start treatment for HCV (n = 50). Group (B); HIV mono-infected patients on ART (n = 47).
Data acquisition
Data on demographics variables (age, gender, weight, and height to calculate body mass index [BMI] (kg/m2), history of diabetes or hypertension, laboratory investigations (complete blood picture, liver and renal functions, HbA1c for diabetic patients, and HBsAg), HIV-related variables (HIV treatment status, type of ART used, HIV viral load, and CD4+ cell counts), and HCV-related parameters (presence of chronic liver disease, HCV treatment status, and HCV viral load by a PCR-based method) were collected. Abdominal ultrasonography was performed using real time scanning Ultrasound System device with convex transducer, 1.5 – 6.0 MHz. The following was examined: liver size and texture, splenic size and texture, the presence of ascites or abdominal lymph nodes, and abdominal wall thickness.
HIV patients with and without HCV co-infection receiving ART were required to have HIV RNA of < 50 copies/mL and a CD4+ cell count ≥ 100 cells/mm3. ART regimens can contain combinations of the following drugs: darunavir–ritonavir, atazanavir–ritonavir, lopinavir–ritonavir, efavirenz, nevirapine, raltegravir, tenofovir, emtricitabine, abacavir, lamivudine, and zidovudine.
Transient elastography examination
TE using a FibroScan® device (Echosens, Paris) and the built in controlled attenuation parameter (CAP) software were performed to measure liver stiffness (LS) and HS. It was performed with a standard M probe and XL probe (for obese patients). Based on the manufacturer’s instructions, measurements were performed through the intercostal spaces in the right lobe. At least 10 valid measurements were obtained and the median would be the result. A success rate (SR) of ≥ 60% and the ratio of the interquartile range (IQR) of LS to the median (IQR/MLSM) ≤ 30% were considered reliable and were used for the final analysis [8]. The CAP was measured only on validated measurements according to the same criteria used for liver stiffness measurement (LSM), which measured the same liver area measured by LSM. The final CAP value was the median of 10 individual CAP values regardless of the SR. The LS was expressed in Kilopascal (kPa) and HS was expressed in units of decibels per meter (dB/m). HS grades were assigned as follows: S0 (no steatosis, CAP 0–237 dB/m), S1 (mild steatosis, CAP 238–260 dB/m), S2 (moderate steatosis, CAP 261–289 dB/m) and S3 (severe steatosis, CAP ≥ 290 dB/m) [9]. Furthermore, a cutoff of 238 dB/m identifies the presence of significant hepatic steatosis (SHS), which is hepatic steatosis affecting at least 10% hepatocytes, and was selected by Sasso et al. [9].
Statistical analysis
Descriptive statistics were done; categorical variables were presented as number (percentage) and numerical variables as mean (SD). Shapiro–Wilk test was used to assess the normality of data distribution. Comparison between the 2 groups was done using the Student’s t-test for normally distributed data or the Mann–Whitney test for non-normal data. The Chi-square test or Fischer’s exact test were used to compare categorical variables. Logistic regression analysis was done for predictors of SHS (CAP ≥ 238 dB/m). P values < 0.05 was deemed significant. STATA 15 was used for the analysis.
Results
A total of 97 HIV-infected individuals were consecutively recruited in the study, whereas HCV co-infection was present in 51.5% (50/97). The demographic features of the enrolled patients were shown in Table 1. The HIV co-infected patients were younger than those with HIV/HCV co-infection. The median age was 33 years and 35 years for HIV mono-infected patients and HIV/HCV co-infected patients respectively. Male gender was predominant across the two studied groups. There was a statistical significant difference in the BMI between both groups. Obesity and overweight was more among HIV mono-infected patients. Regarding self- reported risk factor of HIV infection, the most commonly self-reported risk factor of HIV infection by the HIV/HCV co-infected participants was intravenous drug user (IVDU) whereas in HIV mono-infection group, the main mode of transmission of infection is sexually transmission. Furthermore, the majority of patients in both groups were on treatment with tenofovir (TDF) and emtricitabine (FTC) as a part of NRTI backbone along with NNRTI regimen (efavirenz) (47 patients in HIV/HCV co-infection and 43 patients in HIV mono-infection). No statistical significant difference between both groups regarding the received treatment and treatment duration.
On studying the laboratory data of the studied patients, the hemoglobin level was significantly higher in HIV/HCV co-infection group compared to HIV mono-infection group (p = 0.002). However, the rest of laboratory data showed no significant difference between both groups, as shown in Table 2.
As outlined in Table 3, liver by ultrasound was bright in 52% of HIV/HCV co-infected patients versus 40.4% in HIV mono-infection group (p = 0.03). LSM was significantly higher among HIV/HCV co-infected patients (p = 0.003). Moreover, the prevalence of HS in HIV/HCV co-infection group was 36% (18/50) while in HIV mono-infection group was 46.8% (22/47). In addition, HCV mono-infected patients were more likely to have grade 2 steatosis as compared with HIV/HCV co-infected individuals (25.5% vs.12%).
Participants were further subdivided into two groups according to the presence or absence of significant hepatic steatosis (SHS) (CAP ≥ 238 dB/m). We noticed that BMI and abdominal wall thickness were higher in patient with SHS (CAP ≥ 238 dB/m) compared to patient with less SHS(CAP < 238 dB/m). Percentage of female patients were higher among patients with (CAP ≥ 238 dB/m). Liver was enlarged and bright among patients with (CAP ≥ 238 dB/m) as shown in Table 4. When dividing HIV/HCV co-infected and HIV mono-infected group according to CAP values, again BMI, abdominal wall thickness, and presence of bright liver was significantly associated with SHS (CAP ≥ 238 dB/m) in both groups. Diabetes was only significantly associated with SHS in HIV/HCV co-infected group as shown in Table 5.
Logistic regression analysis was done to identify the predictors of SHS among HIV patients with and without HCV. Among the whole cohort, BMI was the independent predictor of SHS, for each unit increase in BMI, the odds of having SHS increases by 1.23 folds. Subgroup analysis, BMI was again the independent predictor of SHS in both groups as presented in Table 6.
Discussion
As a result of shared transmission routes, HCV infection is common in patients infected with HIV. The immunosuppression induced by HIV accelerates the natural history of HCV-related liver disease and the progression of chronic hepatitis C to cirrhosis and end-stage hepatic disease [10]. HS, defined by the accumulation of lipid droplets in hepatocytes, is present in 24–75% of HIV and HCV co-infected patients. Some factors contributing to the development of HS in the general population, such as visceral obesity, alcohol consumption, hypertriglyceridemia, hypertension and diabetes mellitus remain hugely discrepant during co-infection. In HIV and HCV co-infected patients, HS may occur because of the HIV infection or because of concomitant HCV infection, in addition to metabolic factors as diabetes, obesity, or ART which could induce metabolic syndrome, lipodystrophy or lactic acidosis due to mitochondrial damage [11].
Our results showed a statistically significant male predominance among the studied patients especially in the HIV-HCV co-infected group, as 96% of the group were males. This matches with Chromy et al., 2019 who studied HS in HIV/HCV co-infected patients and 75% of their patients were males [12]. The results are also similar to those of Li Vecchi et al. who studied hepatic steatosis in HCV/HIV co-infected patients and HCV mono-infected patients, and the majority of their patients were males [13]. Male predominance may be attributed to their high risk of exposure to different diseases and also they easily seek medical advice but on the other hand, females may be away from health services especially in rural areas leading to underestimation in their diseases prevalence.
As regard the modes of transmission of infection in HIV/HCV co-infected group, IVDU was the commonest mode of transmission in 66% of patients, however in HIV mono-infected group, the main mode of transmission was sexual transmission. The parenteral transmission is a common mode for entry of both HIV and HCV viruses and this was in agreement with previous studies that showed a high prevalence of HCV co-infection in HIV-infected IVDU (14 and 15).
Our results show that there was no statistically significant difference in the presence of diabetes and hypertension between both groups. Prevalence of diabetes was 24% in HIV-HCV co-infected patients versus 34% in HIV mono-infected patients. It was reported in a study done by Chromy et al., 2019 that only 3% was diagnosed with DM [12]; this difference in numbers may be due to the higher prevalence of diabetes and metabolic syndrome among Egyptian population compared to other populations.
In general, the low prevalence of DM may have contributed to the lack of a significant association between DM and HS. Moreover, the low prevalence of DM indicates a low frequency of metabolic syndrome, which could explain why hypertension determinants were not predictive for HS [16]. Results of our study showed that BMI was significantly higher among HIV mono-infected patients being more obese and overweight compared to patients with HIV/HCV co-infection. This differs from Chromy and his colleagues' results who found no change in BMI of HIV/HCV after receiving anti-HCV treatment and achieving SVR [12]. In addition, on the contrary of our study, no significant difference in BMI was shown between HCV/HIV co-infected patients and HCV mono-infected patients as reported by Li Vecchi and his colleagues [13].
In our study, the prevalence of steatosis in HIV/HCV co-infected patients was 36% versus 46.8% in HIV mono-infected patient. Although less HS was observed in co-infected patients, no statistical significant difference between both groups was observed. Similarly, a study conducted by Pembroke and his colleagues who studied 726 HIV-infected patients (22.7% HCV co-infected), showed that the prevalence of any grade of HS did not differ between HIV mono-infected and HIV/HCV co-infected patients (36.1% vs. 38.6%, respectively) [17]. On the contrary, significantly higher CAP values after clearance of HCV in previously HIV/HCV co-infected patients were reported by Chromy and his colleagues [12]. However, the role of HIV infection in causing hepatic steatosis has been controversial. In the present study, duration of HIV disease, presence of HIV infection, and CD4 + cell count were not statistically significant differences between HIV/HCV co-infected and HIV mono-infected group according to CAP values. This was in agreement with Lui and his colleagues who concluded that duration of HIV disease, nadir or current CD4 + cell count, or prior AIDS were not associated with fatty liver [18]. Again, Fernandez-Botran and his colleagues demonstrated that no statistically significant associations were found between HIV viral load history, CD4 + T-cell count and biomarkers, including ART use, on CAP measurements in HIV patients [19].
Results of our work showed that LSM in patients with HIV/HCV co-infection was significantly higher compared to patients with HIV mono-infection. This matches with Chromy and his colleagues results who noticed a significant decline in liver stiffness measurements after clearance of HCV in previously HIV/HCV co-infected patients [12]. Since steatosis is closely linked to the development and progression of fibrosis. However, we observed that HIV/HCV co-infected patients had higher LSM and lower prevalence of HS than HIV mono-infected patients in our study. This might be explained that HIV/HCV co-infected patients exhibited low BMI index and abdominal wall thickness than HIV mono-infected patients which represented as surrogate indicators of HS in addition to liver fibrosis progresses faster in HIV/HCV co-infected patients, a slower HS progression could be partly explained by fat loss due to higher rates of advanced fibrosis [20]. There is increasing concern that patients with chronic HIV infection may be at increased risk of NAFLD, which can evolve into NASH and cirrhosis. Multiple factors have been hypothesized to be necessary for the development and progression of this condition including metabolic derangements, chronic inflammation, hepatitis co-infection, and treatment with certain NRTIs. In our study, the majority of patients in both groups were on treatment with TDF and FTC as a part of NRTI backbone along with NNRTI regimen (efavirenz) (47 patients in HIV/HCV co-infection and 43 patients in HIV mono-infection).We noticed no statistically significant difference between both groups regarding the received treatment or treatment duration. Our results were supported by findings reported by Martinez et al., 2012 as they concluded that neither the type of ART nor the duration of exposure to a specific drug or class of drug was related to HS [10]. In a meta-analysis of the risk factors associated with HS in HIV/HCV patients, failed to find any association with ART of any class and HS as in our study [21].
On studying the patients based on their stage of hepatic steatosis, we found that there was a statistically significant female predominance among patients with SHS. On the contrary, a study was done by Chromy et al. mentioned that the majority of their patients with SHS were males [12]. Also we noticed that BMI and abdominal wall thickness were higher in patients with SHS compared to patients with less SHS in both studied groups. Similar results were observed by Chromy et al., 2019 who noticed that BMI and DM were significantly associated with SHS [12]. We also found that the prevalence of enlarged and bright liver was significantly higher in patients with SHS compared to patients with less SHS in both studied groups. The well-known risk factors of HS among patients with HIV infection are the use of HAART, high BMI [22], diabetes mellitus [23], and alcohol intake.
Also, HCV infection may induce HS in HIV patients via several molecular mechanisms such as a direct cytopathic effect of the HCV virus and HIV acts synergistically with HCV, causing steatosis by disruption of lipid metabolism [13]. In this study, BMI was the independent predictor of SHS in both groups. However, we noticed that the prevalence of SHS in HIV/HCV co-infected patients was lower than HIV mono-infected patients (13/50, 26% VS. 15/47, 32%) this might be explained that HIV/HCV co-infected patients exhibited low BMI index and abdominal wall thickness than HIV mono-infected patients which represented as surrogate indicators of HS (28.1 vs. 32.5 kg/m2 and 1.8 vs. 2.5 cm, respectively). This matches with results of Macias et al., 2014 who studied steatosis in HIV infected patients and HIV/HCV co-infected patients, and concluded that BMI was the only independent predictor of HS [24]. Similarly, Chromy et al., 2019 found that higher BMI was significantly associated with HS in HIV/HCV co-infected patients [12], and Pembroke and his colleagues mentioned that BMI was independent predictor of HS [17].
There are some limitations of our study. First, the cross-sectional design of the study. Second, self-reported risk factor of HIV infection could subject to bias; however, it was important to combat intravenous drug abuse for prevention of HIV/HCV co-infection, as it was the main mode of transmission in our study. Third, the absence of liver biopsy to determine the stage of liver steatosis and fibrosis; nevertheless, international guidelines now recommend using TE with CAP in place of a standard liver biopsy to determine the stages of liver steatosis and fibrosis (25). Fourth, number of included patients was relatively small. Therefore, more studies including larger number of patients to evaluate impact of HCV and HIV on HS.
Conclusion
From our study, we concluded that however, the prevalence of steatosis was lower in HIV/HCV co-infected patients compared to HIV mono-infected group; we cannot say that HCV has a protective role against steatosis in co-infected patients. More fibrosis progression was common in the HIV/HCV co-infected patients. Therefore, non-invasive screening for the existence of steatosis and fibrosis should be taken into account in the routine clinical care of HIV patients with or without HCV co-infection. This would enable prompt detection of chronic liver disease and enable the implementation of preventative measures in such patients.
Availability of data and materials
The data supporting the results are available from the corresponding author upon reasonable request.
Abbreviations
- ART:
-
Antiretroviral therapy
- BMI:
-
Body mass index
- CAP:
-
Controlled attenuation parameter
- DAAs:
-
Direct acting antiviral agents
- dB/m:
-
Decibels per meter
- FTC:
-
Emtricitabine
- HbA1c:
-
Hemoglobin A1C
- HBsAg:
-
Hepatitis B surface antigen
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- HS:
-
Hepatic steatosis
- IQR:
-
Interquartile range
- IVDU:
-
Intravenous drug user
- kPa:
-
Kilopascal
- LLOQ:
-
Lower limit of quantification
- LS:
-
Liver stiffness
- LSM:
-
Liver stiffness measurement
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- NRTIs:
-
Nucleoside reverse transcriptase inhibitors
- PCR:
-
Polymerase chain reaction
- PLHIV:
-
People living with HIV
- RNA:
-
Ribonucleic acid
- SHS:
-
Significant hepatic steatosis
- SR:
-
Success rate
- TDF:
-
Tenofovir
- TE:
-
Transient elastography
References
Kim AY, Onofrey S, Church DR. An epidemiologic update on hepatitis C infection in persons living with or at risk of HIV infection. J Infect Dis. 2013;207 Suppl 1:S1–6. https://doi.org/10.1093/infdis/jis927
Platt L, Easterbrook P, Gower E, McDonald B, Sabin K, McGowan C, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis. 2016;16(7):797–808.
Sulkowski MS (2008) Viral hepatitis and HIV coinfection. J Hepatol 48:353–367
Thomas DL, Astemborski J, Rai RM et al (2000) The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA 284:450–456
Lo Re V, Kallan MJ, Tate JP et al (2014) Hepatic decompensation in antiretroviral-treated patients co-infected with HIV and hepatitis C virus compared with hepatitis C virus-monoinfected patients: a cohort study. Ann Intern Med 160:369–379
Nishikawa K, Iwaya K, Kinoshita M et al (2015) Resveratrol increases CD68 + Kupffer cells colocalized with adipose differentiation-related protein and ameliorates high-fat-diet-induced fatty liver in mice. Mol Nutr Food Res 59(6):1155–1170
Machado MV, Oliveira AG, Cortez-Pinto H (2010) Hepatic steatosis in patients coinfected with human immunodeficiency virus/hepatitis C virus: A meta-analysis of the risk factors. Hepatology 52(1):71–78
Gaia S, Carenzi S, Barilli AL et al (2011) Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol 54:64–71
Sasso M, Miette V, Sandrin L, Beaugrand M (2012) The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using FibroScan. Clin Res Hepatol Gastroenterol 36:13–20
Martinez V, Mokhtari Z, Guiguet M et al (2012) Hepatic steatosis in HIV-HCV coinfected patients receiving antiretroviral therapy is associated with HCV-related factors but not antiretrovirals. BMC Res Notes 5(1):180
Sterling RK, Contos MJ, Smith PG et al (2008) Steatohepatitis: Risk factors and impact on disease severity in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology 47(4):1118–1127
Chromy D, Mandorfer M, Bucsics T et al (2019) Prevalence and Predictors of Hepatic Steatosis in Patients with HIV/HCV Coinfection and the Impact of HCV Eradication. AIDS Patient Care STDS 33(5):197–206
Li Vecchi V, Soresi M, Giannitrapani L et al (2012) Prospective evaluation of hepatic steatosis in HIV-infected patients with or without hepatitis C virus co-infection. Int J Infect Dis 16(5):e397–e402
Burton MJ, Reilly KH, Penman A (2010) Incarceration as a risk factor for hepatitis C virus (HCV) and human immunodeficiency virus (HIV) co-infection in Mississippi. J Health Care Poor Underserved 21:1194–1202
Amon JJ, Garfein RS, Ahdieh-Grant L et al (2008) Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin Infect Dis 46:1852–1858
Alberti KG, Eckel RH, Grundy SM et al (2009) Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645
Pembroke T, Deschenes M, Lebouché B et al (2017) Hepatic steatosis progresses faster in HIV mono-infected than HIV/HCV co-infected patients and is associated with liver fibrosis. J Hepatol 67(4):801–808
Lui G, Wong VW, Wong GL et al (2016) Liver fibrosis and fatty liver in asian HIV-infected patients. Aliment Pharmacol Ther 44(4):411–421
Fernandez-Botran R, Plankey MW, Ware D, Bordon J (2021) Changes in liver steatosis in HIV-positive women are associated with the BMI, but not with biomarkers. Cytokine 144:155573
van der Poorten D, Samer CF, Ramezani-Moghadam M et al (2013) Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology 57:2180–2188
Castera L, Loko MA, Le Bail B et al (2007) Hepatic steatosis in HIV/HCV coinfected patients in France: comparison with HCV monoinfected patients matched for body mass index and HCV genotype. Aliment Pharmacol Ther 26(1112):1489–1498
Amorosa V, Synnestvedt M, Gross R, Friedman H, MacGregor RR, Gudonis D et al (2005) A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr 39:557–561
Lemoine M, Barbu V, Girard PM, Kim M, Bastard JP, Wendum D et al (2006) Altered hepatic expression of SREBP-1 and PPAR gamma is associated with liver injury in insulin-resistant lipodystrophic HIV-infected patients. AIDS 20:387–395
Macias J, Gonzalez J, Tural C et al (2014) Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV infected patients. AIDS 28:1279–1287
EASL-ALEH Clinical Practice Guidelines (2015) Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 63:237–264
Acknowledgements
We acknowledge Cairo University as this manuscript is from the project titled "Hepatitis C virus prevalence among patients infected with human immunodeficiency virus registered for antiretroviral therapy in fever hospitals of Cairo; project ID:51; ethical approval code: N-149-2018" for sponsoring the project and support through-out.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors have substantially contributed to the conception and design, acquisition of data, data analysis and interpretation. All authors have agreed on the content of the manuscript. AE; study design, conception, data analysis and interpretation and manuscript revision: SAA; Fibroscan and abdominal ultrasound operator; SM: data collection and acquisition, SE: data collection and acquisition, AC: data collection and acquisition; GE; Study design, conception and manuscript revision, AM; Fibroscan operator, manuscript writing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was designed to respect all ethical guidelines issued by the 1975 Declaration of Helsinki and was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Cairo University (N-149–2018). Written informed consent form was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
Gamal Esmat: speaker, advisory board member and investigator for Gilead Science while all other authors: nothing to be declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsharkawy, A., Alem, S.A., Moustafa, S. et al. Prevalence and predictors of hepatic steatosis among HIV patients with and without chronic hepatitis C. Egypt Liver Journal 14, 42 (2024). https://doi.org/10.1186/s43066-024-00349-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-024-00349-0