Abstract
Background
Liver fibrosis is an irreversible liver destruction. Apigenin (API) has different pharmacological properties as anticancer, anti-inflammatory, and antioxidant; however, API hepatoprotective and therapeutic effects are not often studied. This study assesses protective and therapeutic API effects on hepatic injuries produced by thioacetamide (TAA) in rats.
Methods
Forty-nine rats were sorted into seven groups (7 in each): negative control (G1), positive control (G2, TAA), API group (G3), TAA+API group (G4), TAA+SL group (G5), API+TAA group (G6), and SL+TAA group (G7). API and SL effects on TAA-induced hepatotoxicity were examined by determined body weights, liver weights, complete blood count picture (white blood cells, red blood cells, hemoglobin, hematocrit, and platelets counts), liver function tests (alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, gamma glutamyl transferase, alkaline phosphatase, total proteins, albumin, and globulin), and oxidative stress markers (malonaldehyde, catalase, superoxide dismutase, and reduced glutathione) in serum and liver histological was assessed.
Results
TAA decreased red blood cells, platelets, hemoglobin content, and hematocrit (p <0.001) and increased white blood cells count (p <0.001) versus control. Serum values of alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, gamma glutamyl transferase, alkaline phosphatase, and malondialdehyde significantly elevated (p <0.001); meanwhile, total protein, albumin, globulin, catalase, superoxide dismutase, and glutathione S transferase decline (p <0.001) versus negative control. Hepatic structure of TAA group revealed fibrosis and hepatocyte destruction. Therapeutic or protective treating TAA-rats with API or SL ameliorate hematological values, liver functions, oxidative stress, and histological alterations especially therapeutic effects on hematological changes, liver function tests, and oxidative stress markers.
Conclusions
Apigenin had therapeutic and protective effects on liver fibrosis due to its antioxidant activity with therapeutic better than protective effects.
Similar content being viewed by others
Introduction
The liver has various biological functions as drugs metabolism, proteins formation, and regulation of glucolipid metabolism. Acute or chronic liver injury can lead to hepatic fibrosis that manifested by an excessive extracellular matrix (ECM) formation and alteration of hepatic functions. In injured liver, hepatic stellate cells (HSCs) produced ECM [1]. Thioacetamide (TAA) is substance that contains thiono-sulfur. It is used as an organic solvent, a fungicide, a rubber vulcanization accelerator, and motor oil stabilizer [2]. Animals treated with TAA developed hepatic fibrosis and ultimately cirrhosis, with biochemical and structural alterations as those reported in human diseases. Because of TAA effects on RNA, DNA, and gamma-glutamyl transpeptidase activity, it is a model hepatotoxicity. When TAA is given in different doses orally for long period, it causes histologically alterations similar to viral hepatitis infection, large hepatic nodules, liver cell adenomas, cholangiomas, and hepatocarcinomas [3]. TAA poisoning produced as a result of a mixed-function oxidase system’s bioactivation of substance, mainly by flavin adenine dinucleotide (FAD) monooxygenases and CYP2E1 [4]. After TAA is metabolically activated, reactive metabolites produced, including radicals formed from reactive oxygen species (ROS) and TAA-S-oxide produced as intermediates. Reactive metabolites combined to cellular macromolecules covalently cause oxidative stress [5, 6].
There are currently no completely effective medications that provide the organ with entire protection or support hepatic cell regeneration, despite significant improvements in modern medicine. Natural antioxidants, especially phytochemicals, are employed in liver diseases treatment caused by oxidative stress [7]. Apigenin (API) (4′,5,7-trihydroxyflavone) obtained from Matricaria chamomilla is a naturally occurring flavonoid with significant antioxidant potential without having noticeable harmful effects. Apigenin is found in large quantities in different vegetables and fruits as celery, parsley, onions, chamomile, oranges, propolis, honey, thyme, garlic, and spices [8]. It has a variety of therapeutic potentials, including anti-inflammatory, anti-cancer qualities, and anti-oxidant [8]. Previous researches demonstrated that API had chemoprotective, hepatoprotective, and anti-genotoxic actions versus several models of chemically induced hepatic damage [9, 10]. Apigenin has a protective outcome on ischemia/reperfusion-induced rat hepatic necrosis via Fas/FasL pathway regulation [11], suppression of inflammation, oxidative stress, and apoptosis [12]. Goudarzi et al. reported that API can reduce oxidative stress and inflammation to prevent methotrexate-induced hepatotoxicity in rats [13]. Also, Zhao et al. reported that by controlling the SIRT1-p53 axis, API protects against acetaminophen-induced liver damage by encouraging acetaminophen-induced autophagy and reducing inflammatory reactions and oxidative stress damages [14]. Meanwhile, studies did not evaluate protective or therapeutic API effects in liver fibrosis.
The flavonolignans known as silymarin are derived from “milk thistle,” Silybum marianum. Flavonoid isomers like silidianin, isosilibinin, silibinin, and silichristin are mixed together in it. Silymarin is utilized to treat hepatitis and liver cirrhosis caused by alcohol [15]. Silymarin decline hepatic fibrosis by 30–35%, and in little cases, it reversed liver fibrosis [16]. Silymarin had free radical scavenging characteristics and capability to increase endogenous anti-oxidant defense systems in vivo [17]. Silymarin possess anti-metastatic and anti-inflammatory actions; it has protective action against radiotherapy and chemotherapy toxicity [15].
The aim of the current work was to study protective and therapeutic effects of apigenin in TAA-induced hepatotoxicity rat model by estimating complete blood count (CBC), serum liver function tests, and oxidative stress markers and histological assessment of liver tissue and to investigate possible mechanisms of action.
Materials and methods
Chemicals
Thioacetamide (CH3CSNH2, ACS reagent, ≥99.0%) was obtained from Sigma-Aldrich group (SKU #163678), St. Louis, MO, USA. TAA stock solution (0.03% w/v) prepared by liquefying 30 mg solid crystals in 100 ml distilled water. Apigenin, with 80% purity, was obtained from I Herb, USA (product code FOA-66022). Silymarin (product code 14605), with 80% purity, was purchased from local pharmacy in Jeddah, Saudi Arabia. Before utilization, apigenin and silymarin were dissolved in distilled water (10% w/v) and mixed well till powder dissolved and given orally to rats.
Animals
Animal house at King Fahd Medical Research Center (KFMRC), King Abdul-Aziz University (KAU), Jeddah, Saudi Arabia, provided 49 adult male Wister Albino rats with weights ranging from 180 to 250 g. The male rats only were used to avoid hormonal changes that occurs in female rats during estrus cycle. Before the studies began, rats were housed for 1 week, 7 rats in each cage, in wide cable bases polypropylene cages to prevent coprophagy to allow them to acclimatize to the typical laboratory conditions of temperature (20°C), humidity (55–65%), and light/dark (12/12h). Water and normal ordinary rodent laboratory pellets were freely available to the rats. Study was approved by Ethical Committee of KAU Medical Research Center, Jeddah, KSA. The care and handling of experimental animals were performed according to ARRIVE guideline for care and use of laboratory animals.
Study design
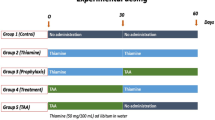
The rats were randomly divided into seven equal groups.
-
1.
Negative control group (G1): rats took 0.9% NaCl (vehicle) daily intraperitoneal (i.p.) injections for 4 weeks.
-
2.
TAA group (G2): to cause liver fibrosis in rats, TAA (200 mg/kg) injected i.p. three times/week for 4 weeks [18].
-
3.
API group (G3): rats received apigenin (50 mg/kg) orally daily for 3 weeks [19].
-
4.
TAA+API therapeutic experimental group (G4): rats injected i.p. with TAA (200 mg/kg) 3 times/week for 4 weeks then received API (50 mg/kg) orally daily for 3 weeks.
-
5.
SL+API therapeutic experimental group (G5): rats injected i.p. with TAA (200 mg/kg) 3 times/week for 4 weeks then received silymarin (100 mg/kg) orally daily for 3 weeks [20].
-
6.
API+TAA protective experimental group (G6): rats took apigenin (50 mg/kg) orally daily for 3 weeks then injected with TAA (200 mg/kg) 3 times/week for 4 weeks.
-
7.
SL+TAA protective experimental group (G7): rats supplemented with silymarin (100 mg/kg) orally daily for 3 weeks then injected with TAA (200 mg/kg) 3 times/week for 4 weeks.
Body weights of the animals were recorded at start and experimental end. Percentage changes of body weight were calculated by subtract final body weight from initial body weight to get weight gain that was then divided by initial body weight and multiply by 100. At 7 weeks, after 12 h of experimental end, the animals were anesthetized by diethyl ether inhalation and abdominal cavity was opened. The liver was isolated, cleaned, and washed. The livers were weighed; and liver index was calculated by dividing liver weight by final body weight multiplied by 100 (liver index = liver weight/total body weight × 100). The livers were excised into pieces and were kept in isotonic formalin for histological assessment.
Collection of blood samples
At 7 weeks, after 12 h of experimental end, the animals were anesthetized by diethyl ether inhalation. Blood (5 ml) was gathered from orbital venous plexus in two different types of tubes. First tubes contained calcium EDTA for use with an automatic hematology analyzer (BC-2800) to measure count of white blood cells (WBCs), platelets, red blood cells (RBCs), hemoglobin contents (HGB), and hematocrit levels (HCT). The other tubes containing blood samples were left for a brief period of time to permit coagulation. Sera produced by centrifuging at 600 g for 10 min, after which sera were aliquoted and kept at 80°C until needed. With the help of rats ELIZA kits, the obtained serum was used to calculate liver function tests such as aspartate transaminase (AST), alkaline phosphatase (ALP), alanine transaminase (ALT), gamma glutamyl transferase (GGT), lactate dehydrogenase (LDH), total proteins, albumin, and globulin, as well as oxidative stress markers like malondialdehyde (MDA), glutathione S transferase (GST), catalase (CAT), superoxide dismutase (SOD), and using rats ELIZA kits in central laboratory.
Histological examinations of the liver
For any obvious changes, the liver was closely scrutinized. Midsections of the left liver lobes prepared for light microscopy in order to perform a liver histological evaluation. The specimen was first fixed in 10% neutral formalin solution, gradually dehydrated in ethanol (50–100%), cleaned in xylene, and embedded in paraffin. Sections were then cut into 5-m thick pieces, and hematoxylin and eosin was used to stain them. To evaluate tissue alterations, an experienced histopathologist used light microscopy to analyses liver slices.
Statistical analysis
The data were expressed as mean ± standard error of means (SEM). Values were analyzed utilizing SPSS version 22 (Statistical Package for Social Sciences, IBM Corp., Armonk, NY, USA). The normality of data distributions was assessed using the Shapiro-Wilk test. This test was chosen due to its suitability for small sample sizes and its accuracy in detecting deviations from normality. Normality assumptions are crucial for the validity of usage parametric tests for comparison. Result of the test revealed that the parametric data was normally distributed so one-way ANOVA followed by Tukey’s test was utilized to compare data to negative and positive control groups. Statistical significance was defined as P <0.05.
Results
Body weights (grams) and percentage changes in body weight (%)
Final body weights (grams) were significantly increased in API, TAA+SL, API+TAA versus TAA group (P < 0.001, P = 0.005, P = 0.032). Percentage change in body weight (%) was significantly decreased in TAA and API+TAA versus negative control (P < 0.001 and P = 0.029), but were significantly increased in API, TAA+API, TAA+SL versus TAA group (P <0.001 for all) (Table 1).
Liver weight and liver index
The liver weights (grams) were significantly elevated in positive control group versus negative control group (P =0.023). Meanwhile, liver indices (%) were significantly elevated in TAA group versus negative control, API, TAA+API, and TAA+SL (P < 0.001, P < 0.001, P = 0.009. P = 0.021, respectively) (Table 1).
Complete blood count
WBC count (10^3/uL) was significantly elevated in TAA, API+TAA, and SL+TAA versus negative control (P < 0.001, P =0.008, P < 0.001), but was significantly decreased in API, TAA+ API, TAA+SL, API+TAA, and SL+TAA versus TAA group (P < 0.001 for all). TAA intake led to significant decreased in RBC count (10^6/uL), HBG content (g/dL), and HCT value (%) in TAA group versus negative control, API, TAA+API, TAA+SL, API+TAA, and SL+TAA (P <0.001 for all). Admiration of TAA led to significant decreased in platelets count (10^6/uL) in TAA, API+TAA, and SL+TAA versus negative control (P <0.001 for all) (Table 2).
Liver function tests
Serum values of ALT (U/L), AST (U/L), ALP (U/L), GGT (U/L), and LDH (U/L) were elevated in TAA group versus negative control, API, TAA+API, TAA+SL, API+TAA, and SL+TAA (P <0.001for all). Also, AST (U/L), ALT (U/L), and GGT (U/L) were elevated in API+TAA versus negative control (P < 0.001, P < 0.001, and P = 0.001). In SL+TAA, AST (U/L) and ALT (U/L) serum levels were significantly increased versus negative control (P < 0.001 and P = 0.017). TAA intake led to significant decreased in total proteins (g/L), albumin (g/L), and globulin (g/L) in TAA group versus negative control, API, TAA+API, TAA+SL, API+TAA, and SL+TAA (P <0.001 for all) (Table 3).
Oxidative stress markers
MDA serum levels (nmol/ml) were significantly elevated in TAA group versus negative control, API, TAA+API, TAA+SL, API+TAA, and SL+TAA (P <0.001 for all). Meanwhile, antioxidant serum values of CAT (nmol/ml) and SOD (ng/ml) were significantly declined in TAA group versus negative control, API, TAA+API, TAA+SL, API+TAA, and SL+TAA. Also, CAT levels (nmol/ml) were significantly decreased versus control in TAA+API, API+TAA, and SL+TAA. SOD values (ng/ml) were decline versus control in TAA+SL, API+TAA, and SL+TAA. GST serum values (ng/ml) were decreased in TAA versus negative control, API, TAA+API, TAA+SL, and API+TAA. GST serum values (ng/ml) were still significantly declined in therapeutic groups API+TAA and SL+TAA versus negative control (Table 4).
Histological results
Negative control group (G1)
Examination of gross morphology of control rat liver revealed the normal appearance, even, and smooth surfaces of the liver without micronodules (Fig. 1a). Using HX & E-stained sections of liver of healthy negative control rats showed normal hepatic architecture, in which liver divided into ill-defined classic hepatic lobules. Lobule was formed of cords of hepatocytes radiating from central vein to lobule periphery (Fig. 1b). The hepatocytes were polyhedral in shape with strongly eosinophilic cytoplasm containing basophilic granules, and distinct vesicular nuclei. Hepatic sinusoids observed in between the hepatic cell cords lined with von Kupffer cells and endothelial cells (Fig. 1c). At lobules’ periphery, branches of hepatic artery, portal vein, and bile duct were found in portal tracts (Fig. 1d).
The liver of negative control group (G1) shown. a Image shows the macroscopic appearances of liver with regular smooth surface. b Photomicrograph shows normal appearance of hepatic parenchyma: notice the thin capsule (black thick arrow). The boundaries of liver lobules are ill-defined but can be identified by presence of central veins (thin black arrows) and portal areas (white arrow) (HX&E ×100). c A photomicrograph shows the thin-walled central vein (V) and normal hepatocytes (H) with rounded vesicular nuclei and eosinophilic cytoplasm with basophilic granules. Note: thin-walled blood sinusoids (arrow) between the hepatocyte cell cords and lined with endothelial cells and von Kupffer cells (×400 HX&E). d A photomicrograph shows that the portal area contained branch of hepatic artery (A), branch of portal vein (V), and bile duct (D) (×400 HX&E)
Positive control group (G2)
Gross morphology in positive control group took TAA and showed irregular-shaped liver with uniform micronodule formations (whitish granules) and uneven granular surface (Fig. 2a). Examination of HX&E-stained sections of TAA-treated group showed massive changes in liver histology as proved by presence of leucocytic cell infiltration, necrotic tissue, and deposition of connective tissue fibers. The presence of nodular hepatocytes was clearly observed (Fig. 2b). The nodules were surrounded by thick fibrous connective tissue septa that divide the liver into pseudo lobules. Dilated central vein was also detected, and massive, dilated portal vein and bile duct proliferation. Hepatocytes had deep acidophilic cytoplasm with small dark nuclei and leucocytic cellular infiltrations were seen. Some hepatocytes showed that different degenerative alterations like hydropic degeneration; cloudy swelling; vacuolization, and necrosis were observed. In addition, the portal area manifested inflammatory cell infiltration and fibrosis surrounding bile duct (Fig. 2c).
The liver of rats treated with TAA (G2) show. a Macroscopic appearances of liver show irregular whitish macro-and micronodules. b A photomicrograph stained with HX&E stain shows lymphocytic infiltrations (arrow), degeneration, and necrosis in the liver cells and pseudo lobules (L) with thick fibrous septa (FS). Note: dilatation of the central vein (CV) (HX&E ×200). c A photomicrograph stained with HX&E stain showing cytoplasmic vacuolization, hepatocyte degeneration, necrosis, infiltration of lymphocytes, expanded portal tracts, bile duct proliferation (bd), and deposition of connective tissue fibers (CT). Note: dilatation of the portal vein (PV) (HX&E ×400)
Apigenin-treated group (G3)
Gross liver morphology of rats treated with apigenin (API) revealed normal architecture of the liver with smooth even surface without micronodules as negative control group (Fig. 3a). Regarding HX&E-stained sections, the structure of the liver appeared nearly similar to the negative control group with relatively normal hepatocytes exhibiting rounded vesicular nuclei and eosinophilic cytoplasm with basophilic granules. Thin-walled blood sinusoids lined with endothelial cells and von Kupffer cells were observed between hepatocyte cell cords (Fig. 3b).
The liver of rats treated with apigenin (API) (G3) shown. a Macroscopic appearances of the liver normal architecture of the liver with smooth surface. b A photomicrograph stained with HX&E stain shows that the structure of the liver appeared nearly similar to the negative control group with normal hepatocytes (H) exhibited rounded vesicular nuclei and eosinophilic cytoplasm with basophilic granules. Note: the central vein (CV) (×400 HX&E)
Apigenin therapeutic experimental group (G4)
Gross liver morphology of rats treated with TAA and API revealed normal hepatic architecture with smooth uneven surface more or less as negative control group (Fig. 4a). Liver sections stained with HX&E revealed the ameliorating action of apigenin versus TAA-induced liver damage. Treatment with apigenin showed partially preserved hepatocyte’s structure and architecture. Small areas of hepatocytes showed mild degeneration and necrosis (Fig. 4b). The blood sinusoids showed slight dilatation and hemorrhage. In addition, hemorrhage and few cellular infiltrations around the central vein were still observed (Fig. 4c).
The liver of rats treated with TAA and API (G4) shown. a The macroscopic appearances of the liver with normal architecture with smooth surface nearly similar to that of the negative control group. b A photomicrograph stained with HX&E stain shows partially preserved hepatocytes structure and architecture with small areas of mild hepatocytes necrosis (N) and leucocytic infiltration around the central vein (arrow) (×200 HX&E) c A photomicrograph stained with HX&E stain shows partially preserved hepatocyte structure and architecture with small areas of mild hepatocyte necrosis (arrow) and leucocytic infiltration around central vein (arrowhead). Note: slight dilatation and hemorrhage of the blood sinusoids were observed (×400 HX&E)
Silymarin therapeutic experimental group (G5)
Gross liver morphology of rats treated with TAA and silymarin revealed normal hepatic shape with nearly smooth uneven surface more or less as negative control group (Fig. 5a). Silymarin-fed groups displayed significant protection of the hepatic cells from apoptotic death versus widespread liver damages initiated in TAA-treated group (Fig. 5b). Liver sections of silymarin-treated group revealed many hepatocytes with vesicular nuclei and eosinophilic granular cytoplasm. Dilated and slightly congested central vein was still detected in some areas of this group (Fig. 5c). Silymarin alleviate inflammation, edema, leucocyte cell infiltration, necrotizing hepatocytes, and connective tissue fiber propagation caused by TAA. As a result, the liver tissue retained its almost typical hepatic lobular architecture with central veins and radiating hepatic cell cords (Fig. 5c). This group appeared more improved in histological structure compared to the G7. Silymarin showed hepatoprotection and ameliorated TAA-induced liver damage when given after treatment with TAA.
The liver of rats treated with TAA and silymarin (G5) shown. a Macroscopic appearances of the liver show normal architecture with smooth surface nearly similar to the negative control group. b A photomicrograph stained with HX&E stain shows partially preserved hepatocyte structure and architecture with little leucocytic infiltration around the central vein (×200 HX&E). c A photomicrograph shows partially preserved hepatocyte structure and architecture with small areas of mild necrosis (arrow) and few leucocytic infiltrations especially around the central vein (arrowhead) (×00 HX&E)
API protective experimental group (G6)
Gross liver morphology of rats treated with TAA and API revealed normal liver architecture with smooth uneven surface more or less as negative control group and also to group 4 (Fig. 6a). Using HX&E-stained sections revealed the protective effect of API with partially preserved hepatocyte’s structure and architecture. Small areas of hepatocytes showed mild degeneration and necrosis (Fig. 6b). The blood sinusoids showed slight dilatation and hemorrhage. In addition, hemorrhage and few cellular infiltrations around the central vein were still observed (Fig. 6c). This group is nearly similar to group 4 in histological structure.
The liver of rats treated with API and TAA (G6) shown. a Macroscopic appearances of the liver show normal architecture with smooth surface nearly similar to the negative control group. b A photomicrograph stained with HX&E stain shows improvement in the histological structure of the liver (×200 HX&E). c A photomicrograph stained with HX&E stain shows improvement in the histological structure of the liver with slight hemorrhage around the central vein (CV) and in the dilated blood sinusoids (S) (×400 HX&E)
Silymarin protective experimental group (G7)
Gross liver morphology of rats treated with silymarin and TAA preserved their liver’s nearly normal anatomical form and appearance with smooth even surface and prevented the growth of micro nodules (Fig. 7a). Liver section taken from group 7 and stained with HX&E showed preserved and improvement in hepatocyte structure and architecture with few cells still showed necrosis compared to positive control group (Fig. 7b). Dilation and congestion of the blood sinusoids and hemorrhage around the dilated central vein were also observed more obvious than group 5 (Fig. 7c).
Liver of rats treated with silymarin and TAA (G7) shown. a The macroscopic appearances of the liver reveal normal architecture of the liver with smooth surface nearly similar to the negative control group. b A photomicrograph stained with HX&E stain shows partially preserved hepatocyte structure and architecture with small areas of cell necrosis and hemorrhage around the dilated central vein (CV) (×200 HX&E). c A photomicrograph stained with HX&E shows preserved hepatocyte structure and architecture with few cells shows necrosis (arrow) and hemorrhage around the dilated central vein (CV). Note: the dilated blood sinusoids with slight hemorrhage (S) (×400 HX&E)
Discussion
Liver has various biological functions as drug metabolism, protein synthesis, and glucolipid metabolism regulation. Hepatotoxins also were produced as a result of excess medications, chemicals, including those found in factories and labs, natural chemicals (such as microcystins), and herbal medicines [21]. Recent researches stressed upon hepatoprotective actions of plants [22, 23]. Meanwhile, the hepatoprotective and therapeutic actions of apigenin are not sufficiently supported by researches. This study aimed to investigate the hepatoprotective and therapeutic effects of apigenin’s in rat model of TAA-induced hepatotoxicity. Data from the literature indicate that rat animal models are useful for understanding the pathogenesis and etiology of hepatotoxicity since their physiology is similar to that of humans [24].
This study’s findings revealed that, when compared to negative control group, TAA administration led to decline in final body weight and elevation in liver weight and liver index. These alterations can be the result of TAA’s harmful effects on bodily metabolism and liver. The current study proved that TAA caused changes in measured hematological and biochemical parameters. The obtained hematological findings demonstrated that TAA exposure resulted in decline in RBC count, hemoglobin content, hematocrit value, and platelet count, while a significant increase in WBC count. These results were in line with those obtained by Al-Attar who reported that rats’ exposure to TAA caused statistical decline in values of RBCs, hemoglobin and hematocrit, while the value of WBC was significantly elevated versus health control [25]. Hematological examination was employed in the identification of many diseases and pathologies caused by various environmental contaminants, toxicants, and medications in both animals and humans [26]. The hematopoietic system is prone to destruction by TAA exposure, which leads to decline in RBCs and platelet counts. Reduced hemoglobin concentration may be caused by increased RBC lysis, reduced RBC synthesis by bone marrow, and/or reduced liver production of proteins. Immune system activation due to tissue destruction or increased inflammation brought on by TAA exposure may led to current elevation in WBC count [25]. The current study’s findings also revealed that serum values of liver enzymes as ALT, GGT, AST, and LDH elevated while levels of total proteins, albumin, and globulin were lowered. As indicators of liver injury and illnesses, liver enzymes like AST, ALT, ALP, and GGT are routinely employed [27,28,29]. Following the administration of TAA, cytoplasmic enzymes known as transaminases are released into the systemic circulation, signaling a loss of structural and functional liver integrity. Results of earlier experimental studies also showed that animals exposed to TAA had significantly higher levels of liver enzymes [30,31,32]. According to findings of present study, TAA-induced liver damage resulted in a considerable drop in total protein, which may have been caused by defect in RNA formation [33] and changes in lipid, protein, and carbohydrate metabolisms as a result of TAA-induced liver damage [34]. The results of the current study demonstrated that TAA caused oxidative stress as revealed by a significant decreased in serum levels of antioxidants like SOD, CAT, and GST and an elevation of oxidative stress marker as MDA. TAA caused oxidative stress in earlier experimental studies, which were supported by the considerable changes in oxidative indicators including SOD and GSH [35, 36]. Microsomal cytochrome P450E1 and/or flavin-containing monooxygenase systems are involved in TAA bioactivation, which results in generation of TAA sulfoxide, TAA-S, S-dioxide, and lipid peroxidation at the plasma membrane. The S-oxide metabolite (TASO2) forms acetylimidolysine derivatives, which have hepatotoxic effects, by covalently attaching to liver macromolecules. Calcium was released from intracellular reserves as a result of TAA. It was found that a number of pathways involved in cell damage or proliferation are activated by both ROS and calcium [37]. Increased permeability, mitochondrial inner membrane damage, and inhibition of mitochondrial respiration were caused by elevation of ROS production and calcium homeostasis disturbance [38].
In the present research, TAA intake to rats led to severe histological changes in the liver as revealed by presence of leucocyte cell infiltrations, increase in necrotic tissues, and deposition of connective tissue fibers. Also, marked dilated portal vein and proliferation of bile duct were observed. Nodular hepatocytes surrounded by thick fibrous connective tissue septa were clearly observed indicates hepatic cirrhosis. Selim et al. reported that liver examination of TAA rats showed swelling and vacuolar degeneration in hepatocytes with leucocytes inflammatory cell aggregation in liver parenchyma [39]. Sadasivan et al. reported hepatic destruction, after TAA intake that ranged from necrosis of parenchymal cell and proliferation of liver cell to formation of nodular cirrhosis and pseudo lobules [40]. Chen et al. reported that livers of rats given TAA showed periportal inflammatory cell infiltrations with severe centrilobular necrosis, hepatocyte degeneration with vacuolar cytoplasm, and congestion of blood sinusoids and central veins [41]. Also, Emam et al. [42] and Hammam et al. [43] noticed hepatic inflammatory infiltration, partial distortion of lobular hepatic architecture, moderate hydropic hepatocyte degeneration, constricted portal vein, thick fibrous tissue in the portal canal, and serious liver destruction of TAA rat model.
The experimental findings of this research revealed that in apigenin-treated groups, final body weight was increased while liver weight and liver index were decreased compared to TAA positive control group. These finding were same as those obtained by Jeyabal et al. who reported that i.p. apigenin admiration (25 mg/kg) for 14 days significantly increased body weight and decreased liver weight and index in N-nitrosodiethylamine (NDEA)-induced hepatocellular carcinogenesis in rats [44]. Also, administration of API caused significant decreased in WBC count and increased in RBC count, platelet count, HBG contents, and HCT value that may be due to anti-oxidant properties of apigenin on liver and bone marrow. These results were in line with those obtained with previous researches [9, 45]. The decrease in WBC count could be due to anti-inflammatory effect of API [9]. Also, serum ALT, AST, GGT, and LDH levels in API-treated groups were decreased, while serum values of total proteins, globulin, and albumin were increased especially in therapeutic group, suggesting that apigenin may treat TAA-induced hepatotoxicity. Liver damage caused by acetaminophen, furan, N-nitrosodiethylamine (NDEA), alcohol, high-fat diet, and carbon tetrachloride was decreased by API, according to earlier studies due to its anti-inflammatory and antioxidant characteristics [46,47,48,49]. Ali et al. found that API intake in dose of 1–4% solution orally administered to Wistar rats for 21 days protect against NDEA-induced hepatotoxicity [47]. Wang et al. reported that API administration in dose of 5–20 mg/kg, orally for 7 days to BALB/c mice led to protection of furan-induced hepatotoxicity [10]. This study investigated effects of oxidative stress markers, including SOD, CAT, GST, and MDA to understand potential mechanisms of apigenin in mitigating TAA-induced hepatotoxicity. According to this study findings, apigenin can elevate antioxidant markers as SOD, CAT, and GST levels in serum while lowering oxidative stress marker as MDA levels. These results align with a number of earlier researches [50,51,52]. Apigenin administration at low dosages (10, 20, and 40 mg/kg) protected rat livers from ROS-induced oxidative destruction by lowering lipid peroxidation and membrane protein destruction and release of blood serum enzymes indicators like ALP, LDH, ALT, and AST [47]. Yang et al. found that API administration to mice in dose of 100–200 mg/kg, orally for 7 days, led to elevated hepatic glutathione reductase activity [46]. Tsalkidou et al. reported that API intake in dose of 15 mg/kg, i.p. once to Wistar rats led to decline in ischemia/ reperfusion-induced hepatic necrosis by Fas/FasL pathway [53]. Paredes-Gonzalez et al. reported that API administration in dose of 1.56–6.25 μΜ, in vitro into HepG2 cell for 6–12 h, led to induction of Nrf2-mediated antioxidant gene expression [54]. Previous researches revealed that apigenin had antioxidant actions by enhancing nuclear factor erythroid 2-related factor 2 signaling pathway, suppresses expression of transcription factors regulating proliferation, differentiation, and immune cells activation, and had anti-inflammatory role by suppressing NF-κB signaling pathway [55]. Zheng et al. revealed that apigenin increased anti-oxidative capacity both in vitro and in vivo and suppressing TLR4/NF-κB/TNFα inflammation pathway in the liver, indicating that apigenin performs anti-inflammation and anti-oxidative stress effects [56]. In contrast, Singh et al. reported that apigenin therapy with 100 or 200 mg/kg once by i.p. intake in Swiss mice led to increase of serum AST, ALT, ALP, and final hepatic damage via enhancement of oxidative stress markers [57].
Based upon histological findings of this investigation, apigenin shields the liver from TAA-induced fibrosis. Our results were in agreement with Ji et al., who demonstrated that apigenin might reduce liver fibrosis in a dose-dependent way [58]. Sahindokuyucu-Kocasari et al. found that pretreatment with apigenin reduces renal and hepatic toxicity by reducing oxidative stress and tissue damage indicators, histological abnormalities, apoptosis, and inflammation [59]. Abdel-Rahman et al. reported that apigenin hinders TAA-triggered liver fibrosis in rats by, at least partly, suppressing oxidative stress and profibrogenic and pro-inflammatory biomarkers. Their results established a recent approach associating the anti-fibrotic mechanism of apigenin through inhibiting hypoxia-inducible factor 1-alpha and focal adhesion kinase pathways [60].
In the present study, treatment with silymarin improves final body weight, liver weight, and liver index compared to TAA positive control group. Also, silymarin improve hematological and liver functions alteration caused by TAA. Silymarin’s pharmacological properties include controlling leukotriene levels, maintaining the integrity of cell membranes, and scavenging ROS. Abdel-Salam et al. reported that silymarin (22 mg/kg) suppressed leakage of hepatocellular enzymes (AST and ALT) to plasma, declined serum values of ALP, and decreased hepatic necrosis and fibrosis development induced by carbon tetrachloride [61]. Results of this study also revealed that, in comparison to TAA positive control group, silymarin considerably boosted SOD, CAT, and GST serum levels while significantly decreasing serum MDA levels. This is because silymarin has antioxidant and ROS-scavenging properties that guard versus oxidative stress. It strengthens cells’ non-enzymatic and enzymatic antioxidant defense mechanisms, which include GSH, SOD, and CAT. Due to its capacity to stop lipid peroxidation and restore depleted glutathione levels, it can shield crucial organs from oxidative damage. Additionally, silibinin has membrane-protective characteristics and might shield blood components from oxidative destruction [62, 63].
The present results revealed that silymarin-fed groups displayed significant protection of the hepatic cells from apoptotic cell death versus widespread liver damage initiated in TAA-treated group. Silymarin alleviate edema, inflammation, leucocyte cell infiltration, necrotizing hepatocytes, and connective tissue fiber propagation caused by TAA. Furthermore, liver tissue maintained its nearly normal hepatic lobular structure. These finding in line with findings of Abdelaal et al. [64] and Emam et al. [42] reported that in silymarin-treated group, the portal area appeared almost normal apart from minimal cellular infiltration and central vein congestion, decline in fibrous deposition that found only in portal area. In this study, therapeutic group appeared more improved in histological structure compared to SL+TAA protective experimental group where silymarin was given to rats before treatment with TAA. Silymarin showed hepatoprotection and ameliorated TAA-induced liver damage when given after treatment with TAA.
This study had some limitations as usage of male rats only in order to exclude hormonal effects on observed changes. Also, electron microscopic examination of liver was not made that needed further experiments to search about molecular mechanism of action of apigenin in preventing and treatment of liver injury.
Conclusions
The current research revealed that apigenin may had beneficial protective and therapeutic actions on TAA-induced hepatic fibrosis in rats, and its mechanisms may be due to enhancing antioxidant agents and decreased oxidative stress and inflammatory markers. The therapeutic effect is better than protective effect on the liver functions and improvement of oxidative stress markers. These results indicate that apigenin is antioxidant, highlighting its potential use as a food supplement to guard versus hepatotoxicity. More investigations are required to completely understand the precise molecular pathways and targets by which API exerts its hepatoprotective and therapeutic effects.
References
Luangmonkong T, Suriguga S, Mutsaers HAM, Groothuis GMM, Olinga P, Boersema M (2018) Targeting oxidative stress for the treatment of liver fibrosis. Rev Physiol Biochem Pharmacol 175:71–102. https://doi.org/10.1007/112_2018_10
Ghanim AM, Younis NS, Metwaly HA (2021) Vanillin augments liver regeneration effectively in Thioacetamide induced liver fibrosis rat model. Life Sci 286:120036. https://doi.org/10.1016/j.lfs.2021.120036
Wójcik M, Ramadori P, Blaschke M, Sultan S, Khan S, Malik IA et al (2012) Immunodetection of cyclooxygenase-2 (COX-2) is restricted to tissue macrophages in normal rat liver and to recruited mononuclear phagocytes in liver injury and cholangiocarcinoma. Histochem Cell Biol 137(2):217–233. https://doi.org/10.1007/s00418-011-0889-9
Mi X-j, Hou J-g, Jiang S, Liu Z, Tang S, Liu X-x et al (2019) Maltol mitigates thioacetamide-induced liver fibrosis through TGF-β1-mediated activation of PI3K/Akt signaling pathway. J Agric Food Chem 67(5):1392–1401. https://doi.org/10.1021/acs.jafc.8b05943
Abdel-Rahman RF, Fayed HM, Asaad GF, Ogaly HA, Hessin AF, Salama AA et al (2021) The involvement of TGF-β1/FAK/α-SMA pathway in the antifibrotic impact of rice bran oil on thioacetamide-induced liver fibrosis in rats. PLoS One 16(12):e0260130. https://doi.org/10.1371/journal.pone.0260130
Fuloria S, Subramaniyan V, Karupiah S, Kumari U, Sathasivam K, Meenakshi DU et al (2020) A comprehensive review on source, types, effects, nanotechnology, detection, and therapeutic management of reactive carbonyl species associated with various chronic diseases. Antioxidants (Basel, Switzerland) 9(11):1075. https://doi.org/10.3390/antiox9111075
Rašković A, Pavlović N, Kvrgić M, Sudji J, Mitić G, Čapo I et al (2015) Effects of pharmaceutical formulations containing thyme on carbon tetrachloride-induced liver injury in rats. BMC Complement Altern Med 15(1):1–11. https://doi.org/10.1186/s12906-015-0966-z
Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A et al (2019) The therapeutic potential of apigenin. Int J Mol Sci 20(6):1305. https://doi.org/10.3390/ijms20061305
Ali F, Rahul NF, Jyoti S, Siddique YH (2017) Health functionality of apigenin: a review. Int J Food Prop 20(6):1197–1238. https://doi.org/10.1080/10942912.2016.1207188
Wang E, Chen F, Hu X, Yuan Y (2014) Protective effects of apigenin against furan-induced toxicity in mice. Food Funct 5(8):1804–1812. https://doi.org/10.1039/C4FO00038B
Zhou X, Wang F, Zhou R, Song X, Xie M (2017) Apigenin: a current review on its beneficial biological activities. J Food Biochem 41(4):e12376. https://doi.org/10.1111/jfbc.12376
Tsaroucha AK, Tsiaousidou A, Ouzounidis N, Tsalkidou E, Lambropoulou M, Giakoustidis D et al (2016) Intraperitoneal administration of apigenin in liver ischemia/reperfusion injury protective effects. Saudi J Gastroenterol: Off J Saudi Gastroenterol Assoc 22(6):415–422. https://doi.org/10.4103/1319-3767.195556
Goudarzi M, Kalantar M, Sadeghi E, Karamallah MH, Kalantar H (2021) Protective effects of apigenin on altered lipid peroxidation, inflammation, and antioxidant factors in methotrexate-induced hepatotoxicity. Naunyn Schmiedeberg’s Arch Pharmacol 394(3):523–531. https://doi.org/10.1007/s00210-020-01991-2
Zhao L, Zhang J, Hu C, Wang T, Lu J, Wu C et al (2020) Apigenin prevents acetaminophen-induced liver injury by activating the SIRT1 pathway. Front Pharmacol 11:514. https://doi.org/10.3389/fphar.2020.00514
Koltai T, Fliegel L (2022) Role of silymarin in cancer treatment: facts, hypotheses, and questions. J Evid-Based Integr Med 27:2515690X211068826. https://doi.org/10.1177/2515690X211068
Haddad Y, Vallerand D, Brault A, Haddad PS (2011) Antioxidant and hepatoprotective effects of silibinin in a rat model of nonalcoholic steatohepatitis. Evid-Based Complement Alternat Med : eCAM:nep164. https://doi.org/10.1093/ecam/nep164
Haddadi R, Shahidi Z, Eyvari-Brooshghalan S (2020) Silymarin and neurodegenerative diseases: therapeutic potential and basic molecular mechanisms. Phytomedicine. 79:153320. https://doi.org/10.1016/j.phymed.2020.153320
El-Marasy SA, El Awdan SA, Abd-Elsalam RM (2019) Protective role of chrysin on thioacetamide-induced hepatic encephalopathy in rats. Chem Biol Interact 299:111–119. https://doi.org/10.1016/j.cbi.2018.11.021
Cai M, Ma Y, Zhang W, Wang S, Wang Y, Tian L et al (2016) Apigenin-7-O-β-D-(-6″-p-coumaroyl)-Glucopyranoside treatment elicits neuroprotective effect against experimental ischemic stroke. Int J Biol Sci 12(1):42–52. https://doi.org/10.7150/ijbs.12275
Kober H, Tatsch E, Torbitz VD, Cargnin LP, Sangoi MB, Bochi GV et al (2016) Genoprotective and hepatoprotective effects of Guarana (Paullinia cupana Mart. var. sorbilis) on CCl4-induced liver damage in rats. Drug Chem Toxicol 39(1):48–52. https://doi.org/10.3109/01480545.2015.1020546
Nada SA, Gowifel AM, El-Denshary E, Salama A, Khalil M, Ahmed K (2015) Protective effect of grape seed extract and/or silymarin against thioacetamide-induced hepatic fibrosis in rats. J Liver 4(178):2167–0889. https://doi.org/10.4172/2167-0889.1000178
Ajiboye TO (2015) Standardized extract of Vitex doniana Sweet stalls protein oxidation, lipid peroxidation and DNA fragmention in acetaminophen-induced hepatotoxicity. J Ethnopharmacol 164:273–282. https://doi.org/10.1016/j.jep.2015.01.026
Yue S, Xue N, Li H, Huang B, Chen Z, Wang X (2020) Hepatoprotective effect of apigenin against liver injury via the non-canonical NF-κB pathway in vivo and in vitro. Inflammation. 43(5):1634–1648. https://doi.org/10.1007/s10753-020-01238-5
Bhakuni GS, Bedi O, Bariwal J, Deshmukh R, Kumar P (2016) Animal models of hepatotoxicity. Inflamm Res 65(1):13–24. https://doi.org/10.1007/s00011-015-0883-0
Al-Attar AM (2022) Hematological and biochemical investigations on the effect of curcumin and Thymoquinone in male mice exposed to Thioacetamide. Saudi J Biol Sci 29(1):660–665. https://doi.org/10.1016/j.sjbs.2021.10.037
Yuan G, Dai S, Yin Z, Lu H, Jia R, Xu J et al (2014) Toxicological assessment of combined lead and cadmium: acute and sub-chronic toxicity study in rats. Food Chem Toxicol 65:260–268. https://doi.org/10.1016/j.fct.2013.12.041
Koyuncuoğlu T, Yıldırım A, Dertsiz EK, Yüksel M, Ercan F, Yeğen BÇ (2020) Estrogen receptor agonists protect against acetaminophen-induced hepatorenal toxicity in rats. Life Sci 263:118561. https://doi.org/10.1016/j.lfs.2020.118561
Li F-F, Liu N, Liu W, Li M, Zhang F, Dong Z et al (2020) Role of dihydroceramides in the progression of acute-on-chronic liver failure in rats. Chin Med J 133(2):198–204. https://doi.org/10.1097/CM9.0000000000000601Original.Article198
Sun M, Zhang J, Liang S, Du Z, Liu J, Sun Z et al (2021) Metabolomic characteristics of hepatotoxicity in rats induced by silica nanoparticles. Ecotoxicol Environ Saf 208:111496. https://doi.org/10.1016/j.ecoenv.2020.111496
Bogahawaththa S, Kodithuwakku SP, Wijesundera KK, Siriweera EH, Jayasinghe L, Dissanayaka WL et al (2021) Anti-fibrotic and anti-angiogenic activities of Osbeckia octandra leaf extracts in thioacetamide-induced experimental liver cirrhosis. Molecules. 26(16):4836. https://doi.org/10.3390/molecules26164836
El-Gendy ZA, El-Marasy SA, Ahmed RF, El-Batran SA, Abd El-Rahman SS, Ramadan A et al (2021) Hepatoprotective effect of Saccharomyces Cervisciae Cell Wall Extract against thioacetamide-induced liver fibrosis in rats. Heliyon. 7(6):e07159. https://doi.org/10.1016/j.heliyon.2021.e07159
Younis NS, Ghanim AM, Elmorsy MA, Metwaly HA (2021) Taurine ameliorates thioacetamide induced liver fibrosis in rats via modulation of toll like receptor 4/nuclear factor kappa B signaling pathway. Sci Rep 11(1):1–17. https://doi.org/10.1038/s41598-021-91666-6
Nevzorova YA, Boyer-Diaz Z, Cubero FJ, Gracia-Sancho J (2020) Animal models for liver disease–a practical approach for translational research. J Hepatol 73(2):423–440. https://doi.org/10.1016/j.jhep.2020.04.011
Unnisa A, Khan SL, Sheikh FA, Mahefooz S, Kazi A, Siddiqui FA et al (2021) In-silico inhibitory potential of triphala constituents against cytochrome P450 2E1 for the prevention of thioacetamide-induced hepatotoxicity. J Pharm Res Int 3(43A):367–375. https://doi.org/10.9734/JPRI/2021/v33i43A32499
Bashandy SA, El Awdan SA, Mohamed SM, Omara EAA (2020) Allium porrum and Bauhinia variegata mitigate acute liver failure and nephrotoxicity induced by thioacetamide in male rats. Indian J Clin Biochem 35(2):147–157. https://doi.org/10.1007/s12291-018-0803-5
Đurašević S, Pejić S, Grigorov I, Nikolić G, Mitić-Ćulafić D, Dragićević M et al (2021) Effects of C60 fullerene on thioacetamide-induced rat liver toxicity and gut microbiome changes. Antioxidants 10(6):911. https://doi.org/10.3390/antiox10060911
Modesti L, Danese A, Angela Maria Vitto V, Ramaccini D, Aguiari G, Gafà R et al (2021) Mitochondrial Ca2+ signaling in health, disease and therapy. Cells 10(6):1317. https://doi.org/10.3390/cells10061317
Obeng E (2020) Apoptosis (programmed cell death) and its signals-a review. Braz J Biol 81:1133–1143. https://doi.org/10.1590/1519-6984.228437
Selim NM, Melk MM, Melek FR, Saleh DO, Sobeh M, El-Hawary SS (2022) Phytochemical profiling and anti-fibrotic activities of Plumbago indica L. and Plumbago auriculata Lam. in thioacetamide-induced liver fibrosis in rats. Sci Rep 12(1):1–12. https://doi.org/10.1038/s41598-022-13718-9
Sadasivan S, Latha PG, Sasikumar JM, Rajashekaran S, Shyamal S, Shine VJ (2006) Hepatoprotective studies on Hedyotis corymbosa (L.). Lam J Ethnopharmacol 106(2):245–249. https://doi.org/10.1016/j.jep.2006.01.002
Chen TM, Subeq YM, Lee RP, Chiou TW, Hsu BG (2008) Single dose intravenous thioacetamide administration as a model of acute liver damage in rats. Int J Exp Pathol 89(4):223–231. https://doi.org/10.1111/j.1365-2613.2008.00576.x
Emam MA, Farouk SM, Abdo M (2018) The ameliorative potential of probiotics and/or silymarin on thioacetamide induced hepatotoxicity in rats: histological and immunohistochemical study. Int J Morphol 36(2):661–669. https://doi.org/10.4067/S0717-95022018000200661
Hammam O, Hussein SAA, Magdi W (2021) Thioacetamide-induced acute liver failure and prospect of nano antioxidant based therapy; [herbal approach]. Egypt J Chem 64(11):5–6. https://doi.org/10.21608/ejchem.2021.78342.3845
Jeyabal PVS, Syed MB, Venkataraman M, Sambandham JK, Sakthisekaran D (2005) Apigenin inhibits oxidative stress-induced macromolecular damage in N-nitrosodiethylamine (NDEA)-induced hepatocellular carcinogenesis in Wistar albino rats. Mol Carcinog: Published Coop Univ Texas MD Anderson Cancer Center 44(1):11–20
Ali AA-M, Mansour AB, Attia SA (2021) The potential protective role of apigenin against oxidative damage induced by nickel oxide nanoparticles in liver and kidney of male Wistar rat, Rattus norvegicus. Environ Sci Pollut Res 28:27577–27592
Yang J, Wang X-Y, Xue J, Gu Z-L, Xie M-L (2013) Protective effect of apigenin on mouse acute liver injury induced by acetaminophen is associated with increment of hepatic glutathione reductase activity. Food Funct 4(6):939–943. https://doi.org/10.1039/C3FO60071H
Ali F, Rahul NF, Jyoti S, Siddique YH (2014) Protective effect of apigenin against N-nitrosodiethylamine (NDEA)-induced hepatotoxicity in albino rats. Mutat Res Genet Toxicol Environ Mutagen 767:13–20. https://doi.org/10.1016/j.mrgentox.2014.04.006
Lv Y, Gao X, Luo Y, Fan W, Shen T, Ding C et al (2019) Apigenin ameliorates HFD-induced NAFLD through regulation of the XO/NLRP3 pathways. J Nutr Biochem 71:110–121. https://doi.org/10.1016/j.jnutbio.2019.05.015
Qiao M, Yang J, Zhu Y, Zhao Y, Hu J (2020) Transcriptomics and proteomics analysis of system-level mechanisms in the liver of apigenin-treated fibrotic rats. Life Sci 248:117475. https://doi.org/10.1016/j.lfs.2020.117475
Vargas-Mendoza N, Madrigal-Santillán E, Morales-González Á, Esquivel-Soto J, Esquivel-Chirino C, y González-Rubio MG et al (2014) Hepatoprotective effect of silymarin. World J Hepatol 6(3):144. https://doi.org/10.4254/wjh.v6.i3.144
El-Maddawy ZK, Gad SB (2012) Hepato-renal protection of silymarin in comparison with vitamin E in rats. Glob J Pharmacol 6(3):236–244. https://doi.org/10.5829/idosi.gjp.2012.6.3.71129
Karamese M, Erol HS, Albayrak M, Findik Guvendi G, Aydin E, Aksak Karamese S (2016) Anti-oxidant and anti-inflammatory effects of apigenin in a rat model of sepsis: an immunological, biochemical, and histopathological study. Immunopharmacol Immunotoxicol 38(3):228–237. https://doi.org/10.3109/08923973.2016.1173058
Tsalkidou EG, Tsaroucha AK, Chatzaki E, Lambropoulou M, Papachristou F, Trypsianis G et al (2014) The effects of apigenin on the expression of Fas/FasL apoptotic pathway in warm liver ischemia-reperfusion injury in rats. Biomed Res Int 2014:157216. https://doi.org/10.1155/2014/157216
Paredes-Gonzalez X, Fuentes F, Jeffery S, Saw CLL, Shu L, Su ZY et al (2015) Induction of NRF2-mediated gene expression by dietary phytochemical flavones apigenin and luteolin. Biopharm Drug Dispos 36(7):440–451. https://doi.org/10.1002/bdd.1956
Che DN, Cho BO, Kim J-s, Shin JY, Kang HJ, Jang SI (2020) Effect of luteolin and apigenin on the production of IL-31 and IL-33 in lipopolysaccharides-activated microglia cells and their mechanism of action. Nutrients. 12(3):811. https://doi.org/10.3390/nu12030811
Zheng S, Cao P, Yin Z, Wang X, Chen Y, Yu M et al (2021) Apigenin protects mice against 3, 5-diethoxycarbonyl-1, 4-dihydrocollidine-induced cholestasis. Food Funct 12(5):2323–2334. https://doi.org/10.1039/D0FO02910F
Singh P, Mishra SK, Noel S, Sharma S, Rath SK (2012) Acute exposure of apigenin induces hepatotoxicity in Swiss mice. PLoS One 7(2):e31964. https://doi.org/10.1371/journal.pone.0031964
Ji J, Yu Q, Dai W, Wu L, Feng J, Zheng Y et al (2021) Apigenin alleviates liver fibrosis by inhibiting hepatic stellate cell activation and autophagy via TGF-β1/Smad3 and p38/PPARα pathways. PPAR Res 2021. https://doi.org/10.1155/2021/6651839
Sahindokuyucu-Kocasari F, Akyol Y, Ozmen O, Erdemli-Kose S, Garli S (2021) Apigenin alleviates methotrexate-induced liver and kidney injury in mice. Hum Exp Toxicol 40(10):1721–1731. https://doi.org/10.1177/096032712110099
Abdel-Rahman RF, Fayed HM, Mohamed MAE, Hessin AF, Asaad GF, AbdelRahman S et al (2023) Apigenin role against thioacetamide-triggered liver fibrosis: deciphering the PPARγ/TGF-β1/NF-κB and the HIF/FAK/AKT pathways. J Herbmed Pharmacol 12(2):202–213
Abdel-Salam OM, Sleem AA, Morsy FA (2007) Effects of biphenyldimethyl-dicarboxylate administration alone or combined with silymarin in the CCL4 model of liver fibrosis in rats. ScientificWorldJournal 7:1242–1255. https://doi.org/10.1100/tsw.2007.193
Poroch-Seriţan M, Jarcău M, Cornea NM (2022) Quantitative research on the active component of silymarin in silybum marianum seed powder from various geographical areas. Food Environ Safety J 21(1):76–81. https://doi.org/10.4316/fens.2022.008
Subramaniyan V, Chakravarthi S, Jegasothy R, Seng WY, Fuloria NK, Fuloria S et al (2021) Alcohol-associated liver disease: a review on its pathophysiology, diagnosis and drug therapy. Toxicol Rep 8:376–385. https://doi.org/10.1016/j.toxrep.2021.02.010
Abdelaal S, HS EM, Ahmed SM (2019) Effect of Silymarin versus Silymarin and green coffee extract on Thioacetamide induced liver injury in adult male albino rats (histological and Immunohistochemical study). Egypt J Histol 42(1):133–146. https://doi.org/10.21608/EJH.2018.5079.1022
Funding
This is a self-funded study.
Author information
Authors and Affiliations
Contributions
ZZA conceptualization, resources, methodology, writing—original draft, preparation—review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted after obtaining ethical approval from Ethical Committee of King Abdulaziz University, Jeddah, Saudi Arabia.
Consent for publication
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alamri, Z.Z. Protective and therapeutic effects of apigenin on thioacetamide-induced hepatotoxicity in male rats: physiological and morphological study. Egypt Liver Journal 14, 15 (2024). https://doi.org/10.1186/s43066-024-00318-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-024-00318-7