Abstract
Background
The prognostic ability of albumin-bilirubin score (ALBI) to assess the hepatic dysfunction in patients with hepatocellular carcinoma (HCC) was previously studied. Its role in the staging of liver fibrosis post chronic hepatitis C Virus (HCV) infection needs to be investigated.
Aim
to assess the diagnostic value of the ALBI score compared to other non-invasive fibrosis scores in chronic HCV patients.
Methods
This cross-sectional study included consecutive chronic HCV patients from January 2015 till December 2018. Liver stiffness measurement (LSM) by transient elastography (TE) is currently one of the most validated noninvasive methods for liver fibrosis staging and is used in daily practice as a reference for fibrosis assessment. ALBI grade as well as Fibrosis-4 (FIB-4), Aspartate aminotransferase to platelet ratio index (APRI), LOK index and Göteborg University Cirrhosis (GUCI) scores were calculated for all of the patients.
Results
A total of 781 chronic HCV patients were included. Around 54% of them had compensated cirrhosis. GUCI score was the most sensitive one to difference between early fibrosis stages, F0 vs. F1. LOK index and ALBI score did not differ significantly between F1 and F2 stages unlike the other study markers. ROC curves revealed good diagnostic capability of FIB-4 (AUROC: 0.85, 0.84), APRI (AUROC: 0.83, 0.83) and GUCI score (AUROC: 0.83, 0.83) for detecting advanced fibrosis and cirrhosis, respectively. ALBI score had a moderate diagnostic role for diagnosing advanced fibrosis and cirrhosis, AUROC of 0.73 and 0.74 respectively. At a cutoff value of -2.95, the sensitivity of ALBI score approached 79%, the specificity was 53% for advanced fibrosis.
Conclusion
ALBI score has a moderate diagnostic power score in the diagnosis of HCV-associated advanced liver fibrosis and cirrhosis; however, FIB-4, APRI and GUCI scores outperformed the ALBI score.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Introduction
Liver fibrosis is an important public health issue which represents the end result of sustained liver injury usually from multiple, simultaneous factors. Assessment of the degree of liver fibrosis is of paramount importance in the management of patients with chronic liver disease (CLD) [1] as it is important for decision making regarding the initiation of therapy (e.g., antivirals for hepatitis B [HBV] or hepatitis C [HCV]) and surveillance strategies for hepatocellular carcinoma [HCC] and esophageal varices in patients with cirrhosis as well as predicting the prognosis [2].
Although liver biopsy is the traditional method of reference for assessment of the degree of liver fibrosis in patients with CLD, it should be reserved for certain cases where more accurate fibrosis staging would impact treatment decisions due to its invasiveness and potential to cause significant complications [3, 4]. Accordingly, there was a necessity to develop non-invasive methods to assess liver fibrosis resulting in dramatically enhanced clinical decision making in patients with CLD [1]. These methods incorporated imaging-based techniques (transient elastography, shear wave elastography, magnetic resonance elastography) as well as serum-based indices which have been increasingly used in routine practice. With continuous progress in the reliability, reproducibility and feasibility of these non-invasive methods, their potential role in disease management has been expanded [1].
Transient elastography (TE), an ultrasound-based elastography, is increasingly used for the measurement of liver stiffness (LS) as an alternative to liver biopsy. TE is well validated in viral hepatitis with performance equivalent in hepatitis B, C and HIV/HCV co-infection [5]. Its main limitation is the non-feasibility of obtaining results in case of ascites or morbid obesity as well as the device is expensive and not widely available [6]. Consequently, several serum biomarkers were developed as non-invasive methods using simple and inexpensive routine laboratory tests to determine liver fibrosis stages. The fibrosis-4 (FIB-4) index and aspartate aminotransferase to platelet ratio index (APRI) are commonly used to determine liver fibrosis stages using age and serum biochemistry such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet count [7, 8]. Other biological scores including Lok index and Göteborg University Cirrhosis (GUCI) score are also used; these scores calculated from standard biological tests that are used in daily practice such as serum AST, ALT, international normalized ratio of prothrombin (INR) and platelet count [9]. Recently, the albumin–bilirubin (ALBI) score was basically developed to predict prognosis in patients suffering from liver cirrhosis with or without hepatocellular carcinoma (HCC) [10]. According to this score, which is calculated based solely on serum total bilirubin (T-Bil) and albumin (Alb) alone, patient prognosis is classified into one of three grades, called the ALBI grade. However, the diagnostic accuracy of the ALBI score when used for liver fibrosis staging in patients with chronic hepatitis has not been investigated well [11].
This study aims to evaluate the performance of the ALBI score in comparison to other non-invasive markers in the staging of liver fibrosis in chronic HCV patients using transient elastography (TE) as a reference method.
Materials and methods
Participants
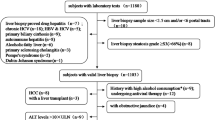
This cross-sectional study included consecutive 781 chronic HCV patients attending Kasr Al-Aini Viral Hepatitis Center, Cairo University from January 2015 till December 2018. Patients aged 18–75 years with seropositivity for HCV antibodies and detectable HCV RNA for more than 6 months were eligible for this study. Patients who had concomitant HBV or human immunodeficiency virus infection, underlying liver disease e.g. autoimmune hepatitis, decompensated liver cirrhosis, and hepatocellular carcinoma, or extra-hepatic malignancy were excluded. Additionally, patients in whom TE examinations could not be performed were excluded.
The demographic, clinical data and routine laboratory work-up were extracted from the participants’ medical records including: age, gender, body mass index [BMI], presence of cirrhosis, and laboratory tests (complete blood counts, liver biochemical profile (serum transaminases (AST, ALT), total serum bilirubin (TSB), serum albumin, the international normalized ratio [INR]). The upper limit of normal (ULN) for aminotransferase level (ALT and AST) was defined as 40 IU/L. In addition, ultrasound scan of abdomen was done to assess the general hepatic condition and to exclude ascites or focal hepatic lesions. For each patient, liver stiffness measurements (LSM) were assessed by TE using the M probe for patients with BMI < 30 kg/m2 and the XL probe for patients with BMI ≥ 30 kg/m2. Non-invasive fibrosis scores were also calculated.
Transient elastography
LS was assessed by TE using FibroScan® 502 (EchoSens, Paris, France). The same technical background and examination procedures were done according to the standards of the manufacturer’s recommendations [12]. Ten valid measurements were performed, and the median of LS was expressed in kilopascals (kPa). Results were considered reliable if the success rate was ≥ 60% and the interquartile range (IQR) was < 30% of the median value (M) of LSM (IQR/M ≤ 0.30%). In this study, TE was considered as the reference method for liver fibrosis evaluation to which the performance of the ALBI score was compared. Staging of liver fibrosis was established by TE according to predefined thresholds as follows: < 7.1 kPa for non-significant fibrosis (F < 2), ≥ 7.1 kPa for significant fibrosis (F ≥ 2), 9.5 kPa for advanced fibrosis (F ≥ 3), and ≥ 12.5 kPa for liver cirrhosis (F4) [5, 13]. The operator was blinded to the patients’ clinical and laboratory data.
Calculated scores for assessing fibrosis
These scores were selected based on the criteria of being simple and inexpensive routine laboratory tests.
-
a)
ALBI score was calculated using the formula [10]:
Log10 total bilirubin (μmol/L) × 0.66 + albumin (g/L) × (0.085).
-
b)
APRI score was calculated using Wai's formula [8]:
(AST/upper limit of normal)/ Platelet count (109/L) X 100.
-
c)
FIB-4 score was calculated using Sterling's formula [7]:
Age (years) X AST (IU/L) / platelet count (109/L) X √ALT (IU/L).
-
d)
GUCI, calculated using the formula [14]:
AST (Upper Limits of Normal) x INR × 100/ platelet count (× 109/L).
-
e)
Lok score, calculated using the formula [15]:
Log odds = -5.56—0.0089 × number of platelets (103/mm3) + 1.26 × (AST/ALT) + 5.27 × INR.
Lok = [exp (log odds)]/ [1 + exp (log odds)].
The study was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Cairo University. Written informed consent was obtained. The study was designed to respect all the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments with Good Clinical Practice (GCP) guidelines.
Statistical analysis
For descriptive statistics, mean (SD) or median (IQR) were used for numerical data and frequency (percentage) was used for categorical data. The distribution of data was tested using the Shapiro-Wilks test. The t test was used for the comparison of normally distributed numerical variables between the two independent groups; while the Mann–Whitney test was used in case of non-normal distribution. ROC curves were constructed to assess the diagnostic value of the non-invasive fibrosis markers in detecting advanced fibrosis and liver cirrhosis. P values < 0.05 are considered significant. STATA 15.1 (Copyright 1985–2017 StataCorp LLC) statistical program was used for the analysis.
Results
Patients’ characteristics
The main characteristics of the studied patients are outlined in Table 1. The median age of the study patients was 55 years (range 23–72) with male predominance (62.48%). Stages of hepatic fibrosis based on LSM using TE as the reference method were as follows; 108 (13.8%) patients had F0, 56 (7.17%) had F1, 101 (12.9%) had F2, 95 (12.16%) had F3 and 421 (53.9%) had F4. The median values of the ALBI score, FIB-4 score, APRI score, LOK index, and GUCI score were -2.77, 2.27, 0.78, 0.51, and 0.88, respectively.
Performance of the ALBI score and the conventional non-invasive fibrosis indices for hepatic fibrosis staging
The median values of the ALBI score, FIB-4, APRI score, LOK index, and GUCI score for different fibrosis stages are presented in Fig. 1. There was a significant difference in the ALBI score between F4 vs F3 (P = < 0.0001) but no significant difference in its value was observed when comparing between no to moderate degree of fibrosis (F0-F2) with advanced fibrosis and cirrhosis (F3-F4) and between F3 and F2, F2 and F1, and F1 vs F0 (P = 0.1, 0.7, and 0.3, respectively). The other non-invasive fibrosis indices were more sensitive to difference in earlier fibrosis stages e.g. FIB-4 score, APRI score, LOK index, and GUCI score significantly differed between F3 and F2 and between F4 and F3 as shown in Fig. 1.
Receiver operating characteristics (ROC) curves were performed to determine the best cut-off values for the ALBI score in discriminating advanced fibrosis (F3) and cirrhosis (F4). As shown in Table 2, the AUROC of ALBI score was 0.73 and 0.74 in identifying F3 and F4 respectively. At Cut-off value of -2.95, the sensitivity was 77.78%, specificity was 52.83% and accuracy was 70.04% for predicting advanced fibrosis (F3). At a cut-off of -2.91, the sensitivity was 80.05%, the specificity was 53.3% and accuracy 67.73% for predicting cirrhosis (F4).
The AUROC for FIB-4, APRI, and GUCI scores showed good accuracy in the detection of advanced fibrosis (F3) and liver cirrhosis. The AUROC values were 0.85(0.82–0.88) and 0.84 (0.82–0.87) for FIB-4, 0.83 (0.79–0.85) and 0.83 (0.80–0.86) for APRI score, and 0.83(0.80–0.85) and 0.83 (0.80–0.86) for GUCI score for advanced fibrosis and cirrhosis respectively. LOK index showed fair accuracy in advanced fibrosis and cirrhosis detection, AUROC value of 0.77and 0.76 as shown in (Table 2 and Figs. 2 and 3).
Discussion
Several non-invasive tests have been developed and have revolutionized the way we diagnose liver fibrosis for their safety, easy-to-perform, and good reliability and feasibility. This leads the researchers to expand their role in disease management and has driven much attention in evaluation of novel innovative markers [1].
This study aimed at evaluating the performance of the ALBI score as a non-invasive marker for assessment of advanced hepatic fibrosis and liver cirrhosis and comparing its value to other non-invasive fibrosis markers; FIB-4 and APRI scores, using TE as a reference method. Additionally, the GUCI score and LOK index were investigated.
The ALBI score has been initially reported by Johnson et al. to estimate liver function status in patients with HCC and to stratify them into categories at different risk stages [10]. Since ALBI-based score has been shown to be an independent prognostic factor for survival and have a similar or a better prognostic performance to Child–Pugh (CP) and MELD scores in patients with HCC; [16, 17] therefore, researchers are encouraged to assess the accuracy of the ALBI score in predicting outcomes in liver diseases other than HCC such as primary biliary cholangitis [18], hepatitis B [19], acute upper gastrointestinal bleeding [20] and acute-on-chronic liver failure [21]. However, to date only a few studies evaluated the role of ALBI score as a novel marker of non-invasive assessment of hepatic fibrosis in chronic hepatitis C [11].
In the present study, we found that the ALBI score was significantly higher in patients with liver cirrhosis compared to those with F3 stage (P = < 0.0001); nonetheless, it did not show significant difference between each two of the earlier fibrosis stages. We could also demonstrate that the ALBI score exhibited a fair diagnostic ability in detecting advanced fibrosis (F3) and liver cirrhosis (F4) with an AUROC values of 0.73 (0.69–0.77) and 0.74 (0.71–0.78) respectively.
Compared to FIB-4 and APRI scores, ALBI score has lower AUROC in distinguishing advanced fibrosis as well as cirrhosis. The AUROC for detecting cirrhosis were 0.84 (0.82–0.87) for FIB-4 and 0.83 (0.80–0.86) for the APRI score.
Our results agreed with Fujita et al., who demonstrated fair diagnostic ability for the ALBI score in distinguishing advanced fibrosis with an AUROC between 0.7 and 0.8, which was smaller than those of the FIB-4 and APRI scores. On the contrary, they showed a good performance in distinguishing cirrhosis from non-cirrhosis (AUROC between 0.8 and 0.9) which was comparable to FIB-4 and APRI scores [11], unlike what was found in our study. Moreover, another study showed that the ALBI score has a moderate diagnostic ability in HBV patients, distinguishing cirrhotic from non-cirrhotic status with an AUROC of between 0.8 and 0.9 [22].
ALBI score was also assessed in another study which showed its good ability in detection of advanced fibrosis and cirrhosis in Egyptian patients with HCV. They identified a cut off value of -2.781 as a predictor of advanced liver fibrosis and cirrhosis with an AUROC of 0.832 showing a sensitivity of 74.8%, specificity of 80.2%, PPV 86.8%, NPV 64.6% and positive likelihood ratio = 3.77 [23].
The best cut-off values for ALBI score in our study were -2.95 (sensitivity 78.9% and specificity 52.8%) and-2.91 (sensitivity 80.1% and specificity 53.3%) for diagnosing advanced fibrosis and cirrhosis respectively.
On the other hand, there was no significant difference in ALBI score in the current study between moderate and mild fibrosis which was contradictory to what was shown by Fujita and his colleagues who stated that there was a significant difference in ALBI levels between patients with a fibrosis stage of F3 compared to those with a stage of F2 [11].
The ALBI score differs from other fibrosis indices that it does not include platelets, hepatic transaminases or age. The diagnostic accuracy of the ALBI score for liver fibrosis staging is preserved in thrombocytopenic patients caused by diseases not related to liver fibrosis, such as idiopathic thrombocytopenia and drug-induced thrombocytopenia. A potential weakness of the ALBI score is that it depends on serum albumin in its equation which its level could be influenced by other disease such as glomerulonephritis.
The limitations of the study include its retrospective nature, lack of assessment of the prognostic role of the ALBI score in this cohort added to its limitation to patients with chronic HCV infection disallowing its application in liver diseases of other etiologies. In addition, liver biopsy which remains the gold standard for assessment of liver fibrosis and cirrhosis was not performed which would have been more accurate as a reference method.
Conclusion
Evaluation of different serum fibrosis biomarkers is necessary for non-invasive assessment of liver status. ALBI score despite being a tool estimating the degree of liver fibrosis, it did not appear to add more value to the other indices currently used in practice.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Chin JL, Pavlides M, Moolla A, Ryan JD (2016) Non-invasive markers of liver fibrosis: adjuncts or alternatives to liver biopsy? Front Pharmacol 7:159. https://doi.org/10.3389/fphar.2016.00159
Myers RP, Elkashab M, Ma M, Crotty P, Pomier-Layrargues G (2010) Transient elastography for the noninvasive assessment of liver fibrosis: A multicentre Canadian study. Can J Gastroenterol 24(11):661–670. https://doi.org/10.1155/2010/153986
Cadranel JF, Rufat P, Degos F (2000) Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology 32:477–481
AASLD-IDSA HCV Guidance Panel (2018) Hepatitis C Guidance 2018 Update: AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Clin Infect Dis 67(10):1477–1492. https://doi.org/10.1093/cid/ciy585
Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK (2011) Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 54(4):650–659
European Association for Study of Liver (2015) EASL Recommendations on Treatment of Hepatitis C 2015. J Hepatol 63(1):199–236 Epub 2015 Apr 21
Sterling RK, Lissen E, Clumeck N et al (2006) Development of a simple noninvasive index to predict significant fibrosis inpatients with HIV/HCV co-infection. Hepatology 43:1317–1325
Wai CT, Greenson JK, Fontana RJ et al (2003) A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 38:518–526
Bota S, Sirli R, Sporea I, Focsa M, Popescu A, Danila M et al (2011) A new scoring system for prediction of fibrosis in chronic hepatitis C. Hepat Mon 11(7):548–555
Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, O’Beirne J, Fox R, Skowronska A, Palmer D, Yeo W, Mo F, Lai P, Iñarrairaegui M, Chan SL, Sangro B, Miksad R, Tada T, Kumada T, Toyoda H (2015) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 33:550–558
Fujita K, Oura K, Yoneyama H, Shi T, Takuma K, Nakahara M, Tadokoro T, Nomura T, Morishita A, Tsutsui K, Himoto T, Masaki T (2019) Albumin–bilirubin score indicates liver fibrosis staging and prognosis in patients with chronic hepatitis C. Hepatol Res 49:731–742
Sandrin L, Fourquet B, Hasquenoph JM, Yon S et al (2003) Transient elastography: a new noninvasive method for assess-ment of hepatic fibrosis. Ultrasound Med Biol 29:1705–1713
De ledinghen V, Vergniol J (2008) Transient elastography (FibroScan). Gastroenterol Clin Biol 32(6 Suppl 1):58–67
Westin J, Ydreborg M, Islam S et al (2008) A non-invasive fibrosis score predicts treatment outcome in chronic hepatitis C virus infection. Scand J Gastroenterol 43:73–80
Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK et al (2005) Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology 42(2):282–292. https://doi.org/10.1002/hep.20772
Xavier SA, Vilas-Boas R, Boal Carvalho P, Magalhães JT et al (2018) Assessment of prognostic performance of Albumin-Bilirubin, Child-Pugh, and Model for End-stage Liver Disease scores in patients with liver cirrhosis complicated with acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol 30(6):652–658
Sabrina Shafiq, Mohammad Nuruzzaman Khan, BipashaMajumder et al (2019) Correlation of Albumin-Bilirubin (ALBI) Score with Child-Turcotte-Pugh (CTP) Score in the Evaluation of Liver Cirrhosis. Am J Lab Med 4(3):60–64
Chan AW, Chan RC, Wong GL, Wong VW, Choi PC, Chan HL, To KF (2015) New simple prognostic score for primary biliary cirrhosis: albumin–bilirubin score. J Gastroenterol Hepatol 30:1391–1396
Wang J, Zhang Z, Yan X et al (2019) Albumin-bilirubin (ALBI) as an accurate and simple prognostic score for chronic hepatitis B-related liver cirrhosis. Dig Liver Dis 51(8):1172–1178
Peng Y, Qi X, Dai J, Li H, Guo X (2015) Child-Pugh versus MELD score for predicting the in-hospital mortality of acute upper gastrointestinal bleeding in liver cirrhosis. Int J Clin Exp Med 8:751–757
Chen B, Lin S (2017) Albumin-bilirubin (ALBI) score at admission predicts possible outcomes in patients with acute-on-chronic liver failure. Medicine (Baltimore) 96(24):e7142
Fujita K, Nomura T, Morishita A, Oura K, Yoneyama H et al (2019) Albumin-Bilirubin Score Differentiates Liver Fibrosis Stage and Hepatocellular Carcinoma Incidence in Chronic Hepatitis B Virus Infection: A Retrospective Cohort Study. Am J Trop Med Hyg 101(1):220–225
Ali D, AlMoslemany M, Mohamed O, Raafat K (2022) Albumin- Bilirubin Score as a Non-Invasive Serum Biomarker for Advanced Liver Fibrosis and Cirrhosis in Egyptian Patients with Chronic Hepatitis C Infection: A Case-Control Study. Afr J Gastroenterol Hepatol 5(2):32–47. https://doi.org/10.21608/ajgh.2022.173503.1019
Acknowledgements
NA
Code availability
N/A.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MB made substantial contributions to the conception and design of the work. He approved the submitted version and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. SA made substantial contributions to the conception of the work. She approved the submitted version and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. AE made substantial contributions to the conception and design of the work. She approved the submitted version and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. RF made substantial contributions to the conception of the work. She approved the submitted version and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. GE made substantial contributions to the conception of the work. He approved the submitted version and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. ZA made substantial contributions to the conception of the work. She approved the submitted version and agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval consent to participate
The study was accepted by the investigational review board (IRB) of Cairo University.
All patients signed informed consent.
Consent for publication
All authors accept publication.
Competing interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hashem, M.B., Alem, S.A., Elsharkawy, A. et al. Performance of Albumin-Bilirubin (ALBI) score in comparison to other non-invasive markers in the staging of liver fibrosis in chronic HCV patients. Egypt Liver Journal 13, 40 (2023). https://doi.org/10.1186/s43066-023-00274-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00274-8