Abstract
Background
Chronic hepatitis C (HCV) and B viruses (HBV) represent the commonest global causes of liver cirrhosis. Other etiologies of non-viral cirrhosis such as autoimmune, metabolic, vascular, or biliary diseases are underestimated. The study aimed to identify causes, clinicoepidemiological characteristics, and outcome of non-B non-C liver cirrhosis. This Egyptian multicenter study recruited patients with liver cirrhosis excluding HCV and HBV. Clinical evaluation and the mortality were recorded. Laboratory, radiological, and histopathological assessment to diagnose the etiology was performed.
Results
One hundred eighty-eight patients were included: 54.3% were males. Autoimmune hepatitis (AIH) was the most common cause of cirrhosis (28.2%), followed by Budd-Chiari syndrome (BCS) in 25%, and cryptogenic in 23.9%. Metabolic causes such as Wilson’s disease, non-alcoholic steatohepatitis (NASH), and hemochromatosis were reported in 7.4%, 3.2%, and 1.1%, respectively. Biliary and cardiac cirrhosis were less frequent. Older age was prevalent in hemochromatosis (67.5 ± 17.7 years) and NASH (60.7 ± 11), while young age in Wilson’s disease (29.5 ± 14.8) and secondary biliary cirrhosis (14.8 ± 4.8). Rural residence was common (60.6%). Mortality was reported in BCS (40.4%), cryptogenic (28.9%), cardiac (25%), Wilson’s disease (21.4%), AIH (17%), and NASH (16.7%). Hepatocellular carcinoma complicated 10.6% of cases. A significantly high percentage of patients had decompensated cirrhosis. Child–Pugh class and rural residence were significant predictors of mortality.
Conclusion
This first report on non-B non-C cirrhosis in Egypt revealed a high prevalence of AIH, BCS, and cryptogenic cirrhosis. Advanced Child class and rural residence were the predictors of mortality.
Similar content being viewed by others
Background
Liver cirrhosis is a significant health problem of a high global burden, resulting in 1.16 million annual deaths [1]. Chronic liver disease or liver cirrhosis results from a wide range of etiologies, and hepatitis C virus (HCV) and hepatitis B virus (HBV) infections represent the main causes. Other etiologies of non-B non-C cirrhosis include autoimmune, metabolic, vascular, biliary diseases, and others.
The autoimmune causes encompass autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC). Metabolic causes include alcoholic hepatitis, non-alcoholic steatohepatitis (NASH), hemochromatosis, alpha one antitrypsin deficiency, and Wilson’s disease. Chronic biliary diseases caused by recurrent bacterial cholangitis due to bile duct stenosis, biliary atresia, and Caroli syndrome represent other causes [2]. Some of these causes had also genetic risk factors or familial transmission such as progressive familial intra hepatic cholestasis [3].
The non-viral etiology of liver cirrhosis has significantly increased over the last decade changing the map of etiologies of liver cirrhosis. Moreover, lower incidence of development of liver cancer was reported in non-viral than in viral-related cirrhosis [4]. In a Korean study, hepatocellular carcinoma (HCC) in non-B non-C cirrhosis represented one-fourth the cases of HCC [5].
Cryptogenic cirrhosis (CC) is another term applied when there is no other known etiology could be identified and is considered a diagnosis by exclusion [6]. Globally, it represents less than 10% of liver cirrhosis and the diagnosis is considered after a detailed clinical, laboratory, and pathological investigations [7]. Cryptogenic cirrhosis has a short and long-term survival similar to other causes of non-B non-C cirrhosis [8].
Hence, identification and treatment of different pathological conditions of liver cirrhosis will ultimately halt progression of liver disease and reduce incidence of complications and mortality [4]. Moreover, the etiology of liver cirrhosis showed a wide variation in the geographical distribution. For example, alcoholic cirrhosis and NASH became an increasing etiology of cirrhosis in Japan [9]. This could be attributed to change in the geographical distribution of the risk factors such as lowered HBV infection by the nationwide vaccination or increase in alcohol consumption, obesity, and the metabolic syndrome. The evolution of these risk factors will help in understanding the future burden of these chronic liver diseases [10].
Therefore, understanding the geographical distribution of different causes of non-B non-C cirrhosis provides the potential for disease prevention, identifies the patterns of behavior associated with the disease, or the epidemiological risk factors, considers genetic testing and preventive counselling for relatives of patients with genetic diseases such as hemochromatosis or Wilson’s disease, and provides the appropriate treatment. Although liver transplantation remains the only curative treatment for liver cirrhosis, pharmacological therapies of certain etiologies such as AIH or Wilson’s disease can halt the progression to decompensated cirrhosis or even reverse cirrhosis.
The prevalence of these causes of non-B non-C cirrhosis is still not clearly identified in large scale studies. Their characteristics and outcomes are underestimated particularly in Egypt, which had the highest prevalence of chronic HCV infection before implementing the nationwide campaigns of therapy by direct-acting antiviral drugs (DAAS).
The aim of this multicenter study was to identify different causes, clinical, epidemiological, and laboratory characteristics and to assess the outcome of non-B non-C liver cirrhosis among different Egyptian liver hospitals. Consecutively, this could draw a map of different causes of non-B non-C liver cirrhosis in Egypt.
Patients and Methods
This cross-sectional study was conducted in the departments of Tropical Medicine and Hepatogastroenterology at multiple Egyptian university hospitals representing Upper Egypt included Al Rajhi Liver University Hospital, Sohag University Hospital, and South Valley University Hospital, and those representing Lower Egypt included Al-Azhar University Hospital in Cairo and Damietta, Tanta University Hospital, and Helwan University Hospital.
All patients admitted with liver cirrhosis between October 2020 and October 2022 were evaluated. Patients with liver cirrhosis and negative viral markers for HBV (HBsAg and HBc IgG) and HCV (anti-HCV) were enrolled. The diagnosis of cirrhosis was based on imaging criteria showing irregular liver border, nodular liver surface or caudate lobe hypertrophy, presence of ascites, endoscopic detection of varices, FIB-4 score > 3.25, fibro scan with stiffness > 13 kilopascals, and liver biopsy if indicated.
Detailed medical history, clinical examination, and the outcome were recorded for all patients. Laboratory tests included complete blood picture, liver profile, prothrombin time, INR, and serum creatinine. Investigations for the etiology were done by assessing the titers of anti-nuclear antibody (ANA), anti-mitochondrial antibody (AMA), anti-smooth muscle antibody (ASMA), liver-kidney microsomal antibody type 1 (LKM 1), soluble liver antigen/liver-pancreas (SLA/LP), and immunoglobulin G (IgG) levels to diagnose autoimmune liver diseases. Testing for serum copper, serum ceruloplasmin, and 24-h urinary copper was used to confirm Wilson’s disease. Measuring lipid profile, HbA1C, and/or fasting blood glucose were performed which represent risk factors associated with NASH. In addition, measuring alpha one anti-trypsin was done to diagnose alpha one anti-trypsin deficiency, and measuring serum iron, ferritin, and transferrin saturation with iron study of liver biopsy to diagnose hemochromatosis. Electrocardiography (ECG) and echocardiography were performed if the suspected cause was cardiac cirrhosis.
Imaging assessment by abdominal ultrasound, Doppler ultrasound, and triphasic computed tomography (CT) and/or magnetic resonance imaging (MRI) was performed to aid the suspected diagnosis accordingly. Diagnosis of causes of biliary cirrhosis was conducted by magnetic resonance cholangiopancreatography (MRCP). Cases suspected to have Budd-Chiari syndrome (BCS) were further evaluated by measuring factor V; proteins C and S; antithrombin III; and JAK II mutation. Liver biopsy, if possible, in the absence of coagulopathy, was obtained if all the previous assessments were negative. If all these laboratory investigations including liver biopsy and imaging evaluation were negative, then the case was diagnosed as cryptogenic cirrhosis. Evaluation of the severity of liver cirrhosis was done using Child–Pugh classification [11].
The study was approved by the Research Ethics Committee, Faculty of Medicine, Assiut University. It was conducted in accordance with the provisions of the Declaration of Helsinki. Written informed consent was obtained from each patient before participation.
Statistical analysis
Data analysis was performed using SPSS software version 23. Data were presented either as mean ± standard deviation or the number of cases (percentage) in case of continuous and categorical variables, respectively. In the case of 2-group comparison, numerical data were compared using independent t test, while chi square test was used for comparison between two categorical variables. Multivariate regression analysis was used to determine the predictors of mortality. Reported results significance level was set to (p < 0.05).
Results
In this study, 188 patients were included, and males represented 54.3% (n = 102). In Table 1, the etiology of cirrhosis was illustrated according to the gender. Autoimmune hepatitis (AIH) was the most common cause identified in 28.2% (n = 53), followed by Budd-Chiari syndrome (BCS) (25%) (n = 47), and cryptogenic in 23.9% (n = 45). Metabolic causes such as Wilson’s disease, NASH, and hemochromatosis were detected in 7.4%, 3.2%, and 1.1%, respectively. Biliary cirrhosis was also less frequently found; PBC in 3 patients (1.6%) and PSC in 2 patients (1.1%).
However, secondary causes of biliary cirrhosis such as progressive familial intrahepatic cholestasis (PFIC), Caroli’s disease and syndrome, and extra-hepatic biliary atresia were found in 8 patients (4.3%). Cardiac cirrhosis was reported in 2.1%, and other causes such as alcoholic, alpha one anti-trypsin deficiency, veno-occlusive diseases (VOD), or drug-induced liver injury were reported in 4 patients (2.1%). According to the gender ratio, males were more frequently affected by Wilson’s disease (2.5), secondary biliary cirrhosis (1.67), and BCS (1.14). Female predominance was significantly observed in AIH (p = 0.039), while males significantly predominated in cryptogenic cirrhosis (p = 0.005).
Table 2 shows the mean age of the included patients according to the gender in each etiology. The oldest age was observed in hemochromatosis (67.5 ± 17.7) and NASH (60.7 ± 11 years), while the youngest age was detected in Wilson’s disease (29.5 ± 14.8) and secondary biliary cirrhosis (14.8 ± 4.8 years). AIH was the only etiology showing a significant difference between males and females (p = 0.016), with younger age in females (34.9 ± 14.9 years).
Analysis of other epidemiological data regarding residence and locality is elucidated in Fig. 1a and b which showed that rural residence was reported in 114 (60.6%) and urban residence in 74 (39.4%). The majority of patients were from rural residence, while in NASH, cardiac cirrhosis, PSC, and hemochromatosis, it was equal to urban residence. Cases from Upper Egypt represented (n = 109) 58%, and from Lower Egypt represented (n = 79) 42%. The majority of patients were from Upper Egypt, while biliary cirrhosis, NASH, and cardiac cirrhosis were predominantly from Lower Egypt.
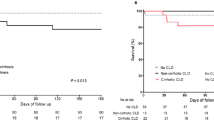
The highest percentage of diabetes mellitus was observed in patients with hemochromatosis (100%) and NASH (66.7%), other causes of cirrhosis (50%), and cardiac cirrhosis (50%). The clinical presentations are demonstrated in Table 3. Jaundice was the most common clinical presentation in AIH, BCS, metabolic, biliary, and cryptogenic cirrhosis. Hepatic encephalopathy was common in AIH, BCS, cryptogenic, and Wilson’s disease. Two patients of AIH were presented with spontaneous bacterial peritonitis. Hepatocellular carcinoma (HCC) was reported in 20 of the included patients (10.6%) and was prevalent in 50% of patients with hemochromatosis, the patient with alcoholic cirrhosis, and 16.7% of NASH, 14.9% of BCS, and 13.3% of cryptogenic cirrhosis (Fig. 2). Mortality rate was reported in BCS (40.4%), cryptogenic (28.9%), cardiac (25%), Wilson’s disease (21.4%), AIH (17%), and NASH (16.7%). The mortality was caused by uncontrolled variceal bleeding, hepatic encephalopathy, or advanced HCC (Fig. 3).
The laboratory data in each etiology as highlighted in Table 4 showed that albumin was markedly reduced in AIH and Wilson’s disease (2.7 ± 0.8 and 2.7 ± 0.6, respectively), INR was markedly prolonged in BCS (2.7 ± 8.3), and bilirubin level was markedly elevated in PBC (16.3 ± 7.4). Patients diagnosed as AIH in this study according to the simplified IAIHG scoring system [12]. Positive ANA with a cutoff ≥ 1/40 was detected in 38 patients and elevated IgG level of mean 2249.7 ± 835.9 mg/dl in all patients confirmed by the characteristic pathological pattern in liver biopsy. No patient had a positive LKM-1 or SLA/LP. Analysis of the hypercoagulation status showed that 19 cases had low protein C, 8 low protein S, 4 had lowered both proteins C and S, 6 with low antithrombin III, 6 low proteins C and S and antithrombin III, 3 had factor V Leiden mutation, and 1 case had JAK II mutation.
Subsequently, as represented in Table 5, the classification of patients according to Child–Pugh class in each etiology revealed a significantly higher percentage of patients in AIH and BCS had Child class B and C. Similarly, a high percentage of patients in the other etiologies had advanced Child classes. The predictors of mortality as illustrated in Table 6 show that Child class (OR 5.169) and rural residence (OR 2.346) were the significant predictors of mortality (p = < 0.05). Notably, the cause of liver cirrhosis was not a significant predictor.
Discussion
Identifying the cause of liver cirrhosis is important since it can predict complications and helps in deciding the treatment strategy. Early studies reported that in the Western countries, alcoholic liver disease and HCV are the most common causes, while HBV is the predominant cause in most parts of Asia and sub-Saharan Africa [13].
In the present study, AIH was the etiology in 28.2% of non-B non-C cirrhosis representing the most commonly reported etiology, followed by BCS in 25%. Suzuki et al. nationwide study in Japan reported that alcoholic cirrhosis was found in 55% as the most common cause of non-B non-C cirrhosis in their study followed by NASH which represented 14.5%, while AIH occurred in 6.8% [14]. Similarly, in the nationwide Brazilian study by de Carvalho et al., estimating the burden of chronic viral hepatitis and liver cirrhosis in the country, alcohol had the major contribution to non-viral cirrhosis [15].
This is different from our study, because the rate of alcohol consumption is very low in Egypt due to religious factors, with a high prevalence of HCV across the country. In a previous report, the mortality of alcohol-related liver disease was lowest in north Africa and the Middle East (5.3%) and in Egypt specifically (4.8%) [16].
AIH in the current study was the most frequently reported cause of non-B non-C cirrhosis, which is in concordance with the recent rise in the incidence of AIH in other countries [17]. AIH affects any age from children to the very elderly, but it is most commonly identified in middle-aged women in all ethnicity [18, 19], and similar results were reported in this study, as AIH was common in the middle-aged females.
BCS affects all races and is relatively common in females during the third or fourth decade of life [20]. In the present work, BCS was the second most identified cause of non-B non-C cirrhosis with higher frequency among middle-aged males, and the mortality was reported in 40.4% of the affected patients. Cryptogenic cirrhosis in this study represented 23.9% of non-B non-C cirrhosis, which is higher than the prevalence of 10.5% reported by previous studies [14]. The histological examination of liver tissue in patients with cryptogenic cirrhosis can be useful in identifying a possible cause[21]. Notably, other causes of non-B non-C cirrhosis in the current study such as metabolic, vascular, or biliary causes represented less than 10% of causes. Similarly, a study published from Egypt aimed to estimate the burden of non-viral causes of liver diseases reported that NASH was frequent in 3.2%, other metabolic diseases in 1.2%, vascular diseases in 0.7%, autoimmune diseases in 0.6%, and alcoholic liver diseases in 0.2% of patients [22].
However, these causes of non-B non-C cirrhosis have low incidence worldwide. The prevalence of Wilson’s disease is estimated to range between 1/10.000 and 1/30.000 worldwide [23,24,25]. Autoimmune biliary cirrhosis also shows a significant global variation in the incidence rates: PBC ranges from 0.84 to 2.75per 100 000 and PSC from 0.1 to 4.39 per 100 000 [26].
The clinicopathologic analysis of patients with cryptogenic cirrhosis indicates some leading causes such as unrecognized NASH, silent AIH, occult viral hepatitis, or occult alcohol ingestion [27]. Moreover, clinicopathological and genomic correlations will be crucial in making a diagnosis, or even, discovery of a new entity [7]. Similarly, in the current study, liver biopsy played an essential role to diagnose different etiologies such as AIH, NASH, hemochromatosis, Caroli syndrome, progressive familial intrahepatic cholestasis, drug-induced injury, and VOD.
Regarding the clinicoepidemiological characters in this study, males were predominantly affected by non-B non-C cirrhosis relative to females, similar to the Brazilian study by de Carvalho et al., which reported high male/female ratio in non-viral cirrhosis compared to HCV or HBV-related cirrhosis [15]. However, residence was a significant predictor of mortality in the current study as higher prevalence was reported in Upper Egypt in the majority of causes. Meanwhile, biliary cirrhosis either primary or secondary, NASH, and cardiac cirrhosis were predominant in Lower Egypt. This could be explained by alterations of environmental factors or changes in lifestyle or diet. These factors were also found to trigger the development of AIH and biliary cirrhosis [28].Similarly, obesity and metabolic syndrome are growing epidemics which are considered risk factors for global increase in the incidence of NASH. This is evident by the high percentage of patients with NASH and cardiac cirrhosis in this study who had diabetes mellitus.
One of the complications of liver cirrhosis is the development of HCC with an annual incidence between 2 and 5% in HBV or HCV infections [29]. In Africa, the overall survival of patients with HCC in Egypt is longer than in other sub-Saharan and East African countries [30]. In the current work, HCC occurred in 10.6% of non-B non-C cirrhosis without a significant difference regarding the gender. This is in concordance with the Egyptian study by El Azm et al., which reported 13.87% of HCC occurrence in non-B non-C cirrhosis without a significant difference detected between non-B non-C HCC, and those of viral association as regard to age or gender [31].
HCC in our study was reported in cirrhosis caused by hemochromatosis, alcohol, NASH, and BCS. This is in concordance with previous reports of HCC in non-viral cirrhosis. HCC develops in about 6% of hemochromatosis-related cirrhosis which represents a 20-fold increased lifetime risk compared to the general population [32].The mechanism for increased risk of HCC in these patients is due to excessive iron which promotes oxidative DNA damage and free radical activity [33]. Similarly, alcohol is an independent risk factor for HCC, with a relative risk of 2.07 for heavy drinkers compared to non-drinkers with a high annual incidence of HCC(2.9%) [34, 35]. Additionally, excessive alcohol consumption may act in synergy with iron overload [36], diabetes mellitus, or viral hepatitis to promote liver carcinogenesis [37].
Between 10 and 20% of HCC cases in the US are attributed to NAFLD [38, 39]. Mitochondrial dysfunction in hepatic steatosis leads to free radical production and oxidative stress, which allows progression to steatohepatitis, liver cirrhosis, and HCC [40]. Hepatic venous congestion caused by obstruction of hepatic venous outflow as in BCS or cardiac cirrhosis can lead to HCC. In this study, HCC developed in 14.9% of BCS. However, the prevalence of HCC in BCS shows a wide geographical variation ranging from 2 to 51.6% due to variation in the characteristics of the studied populations and difference in the time of follow up. A previous meta-analysis revealed that the prevalence of HCC in BCS was 2.0–46.2% in Asia, 40.0–51.6% in Africa, 11.3% in Europe, and 11.1% in America [41].
HCC developed in 13.3% of cryptogenic cirrhosis which was lower compared to other etiologies. This is in line with Abe et al. Japanese study, which showed that 20% of HCC had non-B non-C cirrhosis, 55 patients were habitual alcohol drinkers, 10 had NASH, and 7 had cryptogenic cirrhosis [42].
On the other hand, Suzuki et al. study on a survey of non-B non-C liver cirrhosis in Japan revealed that patients with cryptogenic cirrhosis were more likely to have HCC similar to NASH patients [14]. Recently, the Korean study by Kim et al. reported that alcoholic cirrhosis was the most common etiology developing HCC in 59.7%, followed by AIH, while cryptogenic cirrhosis represented 38% [43]. Ikeda et al. suggested that occult HBV infection could be a reason for increase the risk of HCC in non-B non-C cirrhosis by 8 times [44].
Regarding the outcome of cryptogenic cirrhosis in this study, the mortality was reported in 28.9% lower than in BCS but relatively higher to other etiologies. In one study, longer duration of hospitalization was observed in patients with cryptogenic cirrhosis at an early stage of the disease compared to non-cryptogenic cirrhosis due to complications not related to the liver disease [45]. In the current study, longer duration of hospitalization was observed in cryptogenic cirrhosis because of both the long time required for diagnostic workup and the complications related to advanced liver disease.
In the current study, according to Child classification, more than half of the patients had advanced cirrhosis with decompensation which was the most significant risk factor of mortality. In a global systematic analysis of liver cirrhosis, the number of prevalent cases of decompensated cirrhosis increased from > 5.20 million in 1990 to > 10.6 million in 2017 [16].The development of complications such as ascites, encephalopathy, and gastrointestinal bleeding denotes the decompensated stage of liver cirrhosis with 2 years survival compared to the median survival of about 12 years in compensated cirrhosis [46]. The liver centers involved in this study are tertiary care hospitals; therefore, patients referred to these centers are usually presented with severe disease or complications, which could explain the high percentage of patients with decompensated disease.
The present study had some limitations which need to be addressed. First, the relatively small sample size; however, as mentioned, there is low global prevalence of the studied etiologies of cirrhosis. Additionally, patients are usually admitted to the tertiary hospitals of this study for the management of advanced disease or complications. Second, the lack of investigations for occult HBV or HCV in the recruited patients because this test is relatively expensive and unavailable in all hospitals. Third, the lack of pathological data of liver biopsy in many of the included patients which could reveal unidentified causes in cryptogenic cirrhosis. Nevertheless, liver decompensation and coagulopathy hindered obtaining liver biopsy in these cases.
Currently, there is proof of shifting burden of cirrhosis due to different causes. In a systematic analysis of the global burden of cirrhosis, NASH was predicted to become the leading cause of cirrhosis in the future [16]. Previously, Egypt had one of the highest burdens of HCV infections globally [47]. However, after the extended treatment program by DAAs therapy in Egypt, a change in the causes of liver cirrhosis is expected.
To the best of our knowledge, this is the first multicenter study on non-B non-C cirrhosis in Egypt. The study included patients from different centers in different areas all over Egypt, which highlights a potential change in the prevalence of non-viral cirrhosis in the near future.
Conclusions
In this first multicenter report on non-B non-C cirrhosis in Egypt, a high prevalence of AIH and BCS was reported. Cryptogenic cirrhosis showed a high prevalence as well. Advanced Child class and rural residence were significant predictors of mortality. Further, nationwide studies will be needed to assess the change in the prevalence of other causes of non-viral cirrhosis and to identify the potential risk factors of these causes.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Asrani SK, Devarbhavi H, Eaton J, Kamath PS (2019) Burden of liver diseases in the world. J Hepatol 70:151–171
Asghar MS, Ahsan MN, Rasheed U, Hassan M, Jawed R, Abbas MB et al (2020) Severity of non-B and non-C hepatitis versus hepatitis B and C associated chronic liver disease: a retrospective, observational, comparative study. Cureus 26(12):e12294
Davit-Spraul A, Gonzales E, Baussan C, Jacquemin E (2009) Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis 4:1–12
Yoshiji H, Nagoshi S, Akahane T, Asaoka Y, Ueno Y, Ogawa K et al (2021) Evidence-based clinical practice guidelines for liver cirrhosis 2020. J Gastroenterol 56:593–619
Kim BH, Lim YS, Kim EY, Kong HJ, Won YJ, Han S et al (2018) Temporal improvement in survival of patients with hepatocellular carcinoma in a hepatitis B virus-endemic population. J Gastroenterol Hepatol 33:475–483
Greeve M, Ferrell L, Kim M, Combs C, Roberts J, Ascher N et al (1993) Cirrhosis of undefined pathogenesis: absence of evidence for unknown viruses or autoimmune processes. Hepatology 17:593–598
Nalbantoglu I, Jain D (2019) Cryptogenic cirrhosis: old and new perspectives in the era of molecular and genomic medicine. Semin Diagn Pathol 36:389–394
Thuluvath PJ, Hanish S, Savva Y (2018) Liver transplantation in cryptogenic cirrhosis: outcome comparisons between NASH, alcoholic, and AIH cirrhosis. Transplantation 102:656–663
Enomoto H, Ueno Y, Hiasa Y, Nishikawa H, Hige S, Takikawa Y et al (2020) Transition in the etiology of liver cirrhosis in Japan: a nationwide survey. J Gastroenterol 55:353–362
Rowe IA (2017) Lessons from epidemiology: the burden of liver disease. Dig Dis 35:304–309
Turcotte J, Child C (1964) Surgery and portal hypertension. Major Probl Clin Surg 1:1–85
Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL et al (2008) Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 48:169–176
Schuppan D, Afdhal NH (2008) Liver cirrhosis. The Lancet 371:838–851
Suzuki Y, Ohtake T, Nishiguchi S, Hashimoto E, Aoyagi Y, Onji M et al (2013) Survey of non-B, non-C liver cirrhosis in J apan. Hepatol Res 43:1020–1031
de Carvalho JR, Villela-Nogueira CA, Perez RM, Portugal FB, Flor LS, Campos MR et al (2018) Burden of chronic viral hepatitis and liver cirrhosis in Brazil-the Brazilian Global Burden of Disease Study. Ann Hepatol 16:893–900
Collaborators G (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017
Takahashi A, Ohira H, Abe K, Zeniya M, Abe M, Arinaga-Hino T et al (2020) Increasing incidence of acute autoimmune hepatitis: a nationwide survey in Japan. Sci Rep 10:1–9
Kim BH, Choi HY, Ki M, Kim K-A, Jang ES, Jeong S-H (2017) Population-based prevalence, incidence, and disease burden of autoimmune hepatitis in South Korea. PLoS One 12:e0182391
Takahashi A, Arinaga-Hino T, Ohira H, Torimura T, Zeniya M, Abe M et al (2017) Autoimmune hepatitis in Japan: trends in a nationwide survey. J Gastroenterol 52:631–640
Valla D-C (2002) Hepatic vein thrombosis (Budd-Chiari syndrome). Semin Liver Dis 22:005–014
Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL et al (2002) Cryptogenic cirrhosis: clinicopathologic findings at and after liver transplantation. Hum Pathol 33:1098–1104
Alboraie M, Youssef N, Sherief AF, Afify S, Wifi M-N, Omran D et al (2019) Egyptian liver library: an indexed database for liver disease evidence in Egypt. Arab J Gastroenterol 20:109–113
Reilly M, Daly L, Hutchinson M (1993) An epidemiological study of Wilson’s disease in the Republic of Ireland. J Neurol Neurosurg Psychiatry 56:298–300
Coffey AJ, Durkie M, Hague S, McLay K, Emmerson J, Lo C et al (2013) A genetic study of Wilson’s disease in the United Kingdom. Brain 136:1476–1487
Gialluisi A, Incollu S, Pippucci T, Lepori MB, Zappu A, Loudianos G et al (2013) The homozygosity index (HI) approach reveals high allele frequency for Wilson disease in the Sardinian population. Eur J Hum Genet 21:1308–1311
Trivedi PJ, Hirschfield GM (2021) Recent advances in clinical practice: epidemiology of autoimmune liver diseases. Gut 70:1989–2003
Caldwell S (2010) Cryptogenic cirrhosis: what are we missing? Curr Gastroenterol Rep 12:40–48
Tanaka A, Mori M, Matsumoto K, Ohira H, Tazuma S, Takikawa H (2019) Increase trend in the prevalence and male-to-female ratio of primary biliary cholangitis, autoimmune hepatitis, and primary sclerosing cholangitis in Japan. Hepatol Res 49:881–889
Yang JD, Mohamed HA, Cvinar JL, Gores GJ, Roberts LR, Kim WR (2016) Diabetes mellitus heightens the risk of hepatocellular carcinoma except in patients with hepatitis C cirrhosis. Am J Gastroenterol 111:1573
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16:589–604
El Azm ARA, Yousef M, Salah R, Mayah W, Tawfeek S, Ghorabah H et al (2013) Serum anti-P53 antibodies and alpha-fetoprotein in patients with non-B non-C hepatocellular carcinoma. Springerplus 2:1–6
Harrison SA, Bacon BR (2005) Relation of hemochromatosis with hepatocellular carcinoma: epidemiology, natural history, pathophysiology, screening, treatment, and prevention. Med Clin 89:391–409
Thompson HJ, Kennedy K, Witt M, Juzefyk J (1991) Effect of dietary iron deficiency or excess on the induction of mammary carcinogenesis by 1-methyl-1-nitrosourea. Carcinogenesis 12:111–114
Ganne-Carrié N, Chaffaut C, Bourcier V, Archambeaud I, Perarnau J-M, Oberti F et al (2018) Estimate of hepatocellular carcinoma incidence in patients with alcoholic cirrhosis. J Hepatol 69:1274–1283
Ganne-Carrié N, Nahon P (2019) Hepatocellular carcinoma in the setting of alcohol-related liver disease. J Hepatol 70:284–293
Ioannou GN, Weiss NS, Kowdley KV (2007) Relationship between transferrin-iron saturation, alcohol consumption, and the incidence of cirrhosis and liver cancer. Clin Gastroenterol Hepatol 5:624–629
Hassan MM, Hwang L-Y, Hatten CJ, Swaim M, Li D, Abbruzzese JL et al (2002) Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology 36:1206–1213
Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M et al (2015) Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology 62:1723–1730
Yang JD, Mohammed HFA, Harmsen WS, Enders F, Gores GJ, Roberts LR (2017) Recent trends in the epidemiology of hepatocellular carcinoma in Olmsted County, Minnesota: a US population-based study. J Clin Gastroenterol 51:742
Angulo P (2002) Nonalcoholic fatty liver disease. N Engl J Med 346:1221–1231
Ren W, Qi X, Yang Z, Han G, Fan D (2013) Prevalence and risk factors of hepatocellular carcinoma in Budd-Chiari syndrome: a systematic review. Eur J Gastroenterol Hepatol 25:830–841
Abe H, Yoshizawa K, Kitahara T, Aizawa R, Matsuoka M, Aizawa Y (2008) Etiology of non-B non-C hepatocellular carcinoma in the eastern district of Tokyo. J Gastroenterol 43:967–974
Kim J, Kang W, Sinn DH, Gwak G-Y, Paik Y-H, Choi MS et al (2020) Potential etiology, prevalence of cirrhosis, and mode of detection among patients with non-B non-C hepatocellular carcinoma in Korea. Korean J Intern Med 35:65
Ikeda K, Kobayashi M, Someya T, Saitoh S, Hosaka T, Akuta N et al (2009) Occult hepatitis B virus infection increases hepatocellular carcinogenesis by eight times in patients with non-B, non-C liver cirrhosis: a cohort study. J Viral Hepatitis 16:437–443
Mohammed OK, Mahadeva S (2015) Clinical outcomes of cryptogenic compared with non-cryptogenic cirrhosis: A retrospective cohort study. J Gastroenterol Hepatol 30:1423–1428
D’Amico G, Garcia-Tsao G, Pagliaro L (2006) Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 44:217–231
Blach S, Zeuzem S, Manns M, Altraif I, Duberg A-S, Muljono DH et al (2017) Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. The lancet Gastroenterology & hepatology 2:161–176
Acknowledgements
Not applicable.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
H.K., S.Z, S.A., and M.Z: conception and design of the work. H.K., F.E., S.Z., A.B., R.B, S.A, E.M., S.E., M.E., and M.Z.: acquisition of the data from different centers. H.K, F.E., and S.Z.: drafting the work. M.H: analysis and interpretation of the data. The authors read and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee, Faculty of Medicine, Assiut University (IRB no: 17300194). It was conducted in accordance with the provisions of the Declaration of Helsinki. Written informed consent was obtained from each patient before participation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramadan, H.KA., El-Raey, F., Zaky, S. et al. A paradigm shift in non-viral liver cirrhosis: a multicenter study on clinicoepidemiological characteristics and outcome of non-B non-C cirrhosis. Egypt Liver Journal 13, 35 (2023). https://doi.org/10.1186/s43066-023-00270-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00270-y