Abstract
Background
Two chief hurdles in most cancer treatments are chemoresistance and tumor recurrence, especially counting hepatocellular carcinoma (HCC). Most conformist chemotherapy fails to completely cure HCC patients because of its susceptibility to develop multidrug resistance (MDR) through factors such as hypoxia, cancer stem cells, and drug efflux mechanism cancer stem cells (CSC) which are significant factors involved in chemoresistance. It has been exposed that targeting liver cancer stem cells and chemotherapeutic drugs have a better selected, overall survival rate for hepatocellular carcinoma patients.
Aim
This study aims to investigate the effectiveness of targeting stem cells for liver cancer using a therapy that targets EpCAM in combination with chemotherapy and how this approach can enhance the treatment outcomes in hepatocellular carcinoma, the most prevalent kind of liver cancer.
Results
The outcome was studied by flow cytometry, Western blot, RT-PCR, and cytotoxicity assays. EpCAM gene silenced and XAV939-treated cells showed decreased expression of CD133, a liver cancer stem cell (LCSC) marker in flow cytometry analysis, and reduced expression of ABCG2 gene, which is a reliable marker for chemoresistance in RT-PCR and western blot analysis; it was also unable to form colonies in colony forming assay. Similarly, in the spheroid formation assay, EpCAM gene silenced cells and XAV939-treated cells in combinations with cisplatin treatment were powerless to appear spheroid, whereas cisplatin alone-treated cells showed spheroids. In the cytotoxicity assay, cisplatin alone and combined with EpCAM silenced and XAV939-treated cells showed more lactate dehydrogenase (LDH) release than EpCAM silenced arm XAV939 treated components.
Conclusion
These findings confirm our hypothesis that conventional chemotherapy kills cancer cells but not cancer stem cells. We believe EpCAM-targeted therapy enhances chemosensitivity and decreases relapsed chances. This approach might be the best option for a better prognosis for hepatocellular carcinoma patients.
Similar content being viewed by others
Introduction
The liver is a vital organ current in vertebrates. It plays a chief role with more than 400 functions, including producing plasma protein, controlling homeostasis, glycogen, lipids storage, albumin, bilirubin production, detoxification of xenobiotics, and vital roles in metabolism. Hepatocellular carcinoma (HCC) is the world's third-largest cause of cancer mortality and the sixth most widespread cancer [1]. Cirrhosis of the liver is a significant health issue in India as well. According to the most recent WHO data from 2017, 259,749 individuals died from liver disease in India, accounting for 2.95% of all fatalities and 18.3% of all cirrhosis deaths globally [2].
The most crucial etiologic cause of HCC is chronic viral infections such as hepatitis B and C, similar to metabolic disorders, and chronic alcoholism is also a modest concern in HCC [3]. Surgical treatment, radiotherapy, and chemotherapy are the standard management options for HCC. Sorafenib is the common food and drug administration (FDA) accepted drug for HCC. Majority of the conservative treatments, such as radiotherapy and chemotherapy, fail to treat HCC patients because of numerous factors, including tumor microenvironment, DNA damage repair, ATP overexpression, compulsory cartridge drug efflux [4, 5], the hypoxia-epithelial-mesenchymal transition (E.M.T.), inducible factor1-α(Hif1-α) [6] calcium signaling, autophagy induction [7, 8], cancer stem cell, epigenetic regulation [9, 10], miRNAs, and immunosuppressive microenvironment [11, 12]; moreover, many of these facilitate resistance in multidrug use. Among all the above factors, CSC (tumor-initiating cells) plays a significant role in self-renewal, metastasis, chemoresistance, and radioresistance, leading to a high chance of tumor relapse. Since the concept of CSCs came into prominence in the belatedly 1990s, it has regularly gained universal reception and prejudiced all approaches to cancer investigation and therapy [13]. Researchers have found that chemotherapy kills cancer cells but does not affect the cancer stem cells. A minimum viable portion within the tumor, cancer stem cells, show chemoresistance features to some chemotherapy. Dallas et al. [14] showed that 5-fluorouracil and oxaliplatin drugs show chemoresistance to the colorectal cancer cells and enhance the CSC population almost 5–22-fold. Hermann et al. [15] claimed that human pancreatic cells show chemoresistance to the gemcitabine treatment and a 50-fold increase of cancer stem cell marker CD133 expression. Our previous research demonstrated that cisplatin drugs ultimately face difficulties in killing Huh7 cells. These cisplatin-resistant cells showed higher expression of liver cancer stem cell markers such as CD133 and EpCAM. In addition, these cells strongly expressed stem cell transduction factors like Oct4 and Nanog.
Similarly, only cisplatin-resistant cells formed a spheroid, not the Huh7 cells. These findings confirm that Huh7 cells are resistant to cisplatin drug, and those resistant cells express cancer stem cell-like behaviors such as chemoresistance and tumor recurrence [16]. EpCAM was initially identified as an epithelial marker; consequently, researchers found it is also a cancer stem cell marker for several epithelial cancers. EpCAM is involved in various tumor processes such as proliferation, invasion, tumorigenicity, and metastasis, mainly through the wnt-β-catenin pathway and other signaling pathways [17]. So, the combinatorial approaches targeting cancer stem cells and cancer cells could be the right choice for tumor-free survival.
Materials and method
Chemicals and reagents
NCCS (National Centre for Cell Science) provides cancer cells, so the cell lines Huh7 were purchased from NCCS, Pune, India. Antibiotic–Antimycotic (cat#15,240,062) and Fetal bovine serum (Cat#11,573,397) was purchased from Gibco (Cat#41,400,045) ITS from Invitrogen. Cisplatin (Cat#1550) from Bio vision, XAV939 from Selleckchem, ABCG2 (Cat#ab3380) from Abcam, FITC-conjugated CD133 monoclonal antibody (Cat#11–1339-41, clone #EMK08) eBioscience, PE-conjugated EpCAM monoclonal antibody (clone#EBA-1, Cat#347,198), and shRNA (EpCAM) kit from Gibco.
The generation of LCSC-enriched Huh7 cell line variants
Huh7 Cells were cultured with a low dose of 1.655 μg/ml of cisplatin in absolute RPMI 1640 medium supplemented with F.B.S. (10%), ITS, antimicrobial for 37 °C (5 days) in CO2 (5%) Incubator, and the medium was distorted every 2 days to remove the dead cells. Cisplatin was supplementary while the logarithmic enlargement phase was reached. The generation of chemo-resistant cell variants of HuH7 cells is established through the next to final confrontation to cell death through the treatment with cisplatin. The consequential phenotype resistance was confirmed constant over ≥ 6 passages devoid of cisplatin [18].
Cell culture
LCSC-enriched HuH7 cell line variants were cultured in complete RPMI 1640 medium with F.B.S. (10%), ITS, and antimicrobial up to three passages to obtain sufficient cells, after these cells were seeded in six-well plates and incubated at least for 24 h; previously reached exceeding ~ 70% convergence. These cells were cultured in six different groups, such as XAV939 (13.4 nM) alone, EpCAM gene silence using shRNA alone, cisplatin alone, XAV939 combined with cisplatin, EpCAM knockdown with cisplatin, and untreated cells as a control for 3 days at 37 oC in CO2(5%) incubator. Then these cells were harvested for subsequence analysis [18].
Silencing EpCAM gene using shRNA
RNAi mediate thump over was performed with the subsequent diminutive meddlesome RNA (shRNA): EpCAM-1: 59-UGCUCUGAGCGAGUGAGAATT-39; EpCAM-2:59 UUCUCACUCGCUCAGAGCATT-39, unconstructive control shRNA, was used in every experiment as a non-silencing control shRNA. All shRNAs (20 nM) target EpCAM was established in cells using lipofectamine 2000 reagent per the manufacturer’s procedures [19].
Flow cytometry
FITC conjugated CD133 and APC conjugated EpCAM PE-conjugated was purchased in BD Bioscience. After 3 days, XXAV939- and shRNA (EpCAM)-treated LCSC-enriched huh7 cell variants were dissociated with trypsin–EDTA (0.25%;1 mM) (Invitrogen) for 3 min and washed with Ca and Mg free Dulbecco PBS solution through spinning at 400 g for 7 min. Then these cells were watered down in FACS buffer (100 μl; PBS contains fetal calf serum 1%) and then incubated for 1 h at 4 °C in FACS buffer with the equivalent mAb: anti-CD133-FITC and anti-EpCAM-APC. The flow cytometry examination was performed with a BD FACS Canto II flow cytometer (BD Biosciences) [20].
RT-PCR
The total RNA has been removed with the TRIzol (Invitrogen) reagent from all the drug-treated cell groups, including the control. Primary thread flattering DNA (cDNA) was manufactured, starting entirety RNA according to the manufacturer’s procedure for RNA PCR kit (Madison, Promega, WI, USA). PCR quantitatively was carried out using the ABI7300 RT-PCR system (Applied Biosystems, CA). ABCG2 appearance was enumerated, and β-actin was used as an endogenous orientation. Results were uttered as go bankrupt change in gene expression [20].
Western blot
The reagents extracted nuclear and cytoplasmic proteins; nuclear and cytoplasmic extraction (Pierce). Proteins were electrophoresed on SDS-PAGE gels and transferred to the nitrocellulose membrane (Invitrogen). Protein detection was completed using the following antibodies: anti-ABCG2 monoclonal antibody (R&D Systems, Inc.) and anti–β-actin monoclonal antibody (Sigma). Bounce antibodies were detected by chemiluminescence detection-enhanced reagents (Amersham Biosciences) [20].
Spheroid formation assay
Liver cancer stem cells enriched Huh7 cell line variants were treated with XAV939 alone, shRNA (EpCAM) alone, cisplatin alone, XAV939 combination with cisplatin, shRNA (EpCAM) combination with cisplatin, and DMSO alone as a control in several 1000 per 0.2 ml of complete stem cell medium in 96-well plates. 3D cell culture reagent Matrigel (Cat. No: 354230BD Biosciences) was used to civilization liver spheroids. The 5 mg/ml attentiveness of matrigel was equipped and used for culture spheroids. Incubate the cells under paradigm conditions at 37°C and CO2 (5%) for the most favorable spheroid size for 2 weeks. A rigid margin spheroid was observed by day 9. Culture media is replenished with freshly prepared complete stem cell medium every 2–3 days up to 9 days [20].
Colony forming assay
The colony or clonogenic configuration is an assay of in vitro cell endurance based on the capability of a solitary cell to produce a dependency. The dependency is distinct from consisting of at least fifty cells. After management, cells are seeded out in suitable dilutions to appear colonies in weeks 1–3. Colonies are permanent with glutaraldehyde (6.0%; v/v), discolored with crystal violet (0.5%; w/v), and calculated by a stereomicroscope [20].
Viability
The Huh7 cell line was grown in 96-well plates with drugs and appropriate controls. Consequently, they were incubated with reagent WST-1 (DojindoInc, Japan) for 4 h. Formosan dye fashioned was quantified with a multi-well spectrophotometer (ELISA reader). The calculated straight absorbance compared to the no of viable cells [21].
Cytotoxicity
The Huh7 cell line was grown in 96-well plates with controls and suitable standard drugs, and the supernatant was collected and incubated with LDH reagent (Caymen) for 30 min. After this incubation period, the amount of LDH release was quantified with a multi-well spectrophotometer (ELISA reader), and the absorbance measured unswervingly correlated to the no of dead cells [21].
Statistical analysis
The two-tailed Student T-test and GraphPad Prism software will be used for the statistical analysis. Results with less than a 5% chance of occurrence will be considered statistically significant since the significance level will be fixed at p < 0.05. Under the assumptions of a normal distribution and equal variance, the two-tailed Student T-test is frequently used to compare the means of two groups of continuous data. The test determines the t-value, the distinction between the two means adjusted by the difference’s standard error. A successful show for scientific data analysis and graphing is GraphPad Prism. It offers an easy-to-use interface that enables users to run different statistical tests and create graphs that are suitable for study.
Results
Absence of tumor recurrence properties in EpCAM-targeted cells
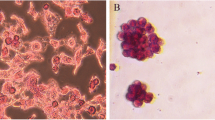
EpCAM plays a vital role in cancer stem cell activation, tumor invasion, and metastasis. EpCAM gene was silenced in LCSC-enriched Huh7 cell lines variants through shRNA technology. Further, to evaluate the tumor-initiating properties of EpCAM, these EpCAM knockout cells were cultured for colony-forming assay along with untreated cells as a control. Interestingly EpCAM knockdown cells were unable to form colonies in colony forming assay, whereas control cells were able to form colonies. It has indicated that EpCAM-positive cells are tumor-initiating cells (Fig. 1).
The wnt-β-catenin signaling passageway is an explanation regulating pathway for cell proliferation, invasion, metastasis, and maintaining stemness. XAV939, a tankyrase 1 inhibitor, promotes cell apoptosis by inhibiting the wnt-β-catenin signaling pathway. LCSC-enriched cells alone as a control, and XAV939 treated cells were cultured for the colony-forming assay. This assay helps to identify the functional assessment of tumor recurrence properties. Fortunately, XAV939-treated cells were unable to form any colonies. This data confirms that wnt-β-catenin signaling is essential in the signaling pathway for cancer stem cell activation and tumorigenesis, and EpCAM-positive cells are the tumor-initiating cells (Fig. 1).
Evaluation of chemoresistant property in EpCAM-targeted therapy
To evaluate the efficacy of cisplatin-sensitive on XAV939 (wnt β-catenin inhibitor) and EpCAM gene silenced by shRNA on LCSC-enriched Huh7 cell variants, these treated cells were studied ATP binding cassette G2 (ABCG2) gene, which is highly expressed in many chemo-resistant cancers. The expression of ABCG2 is directly correlated with resistance to chemo drugs. ABCG2 gene expression was analyzed by RT-PCR and western blot analysis in cisplatin-treated (+ ve control), ShEpCAM-treated, and XAV939-treated cells. The results observed in RT-PCR analysis showed a significant decrease of ABCG2 gene expression in sh-EpCAM- and XAV939-treated cells, whereas in cisplatin-treated cells, the aberrant expression of ABCG2 was observed. Correspondingly in western blot analysis, ABCG2 expression was drastically decreased in sh-EpCAM- and XAV939-treated cells. These findings support our hypothesis that sh-EpCAM and XAV939 treatment suppresses the expression of ABCG2, and a combination of cisplatin along with sh-EpCAM, or XAV939, might help for better progression in EpCAM-positive hepatocellular carcinoma (Fig. 2a, b).
Reduced expression of CSC-associated gene after EpCAM-targeted therapy
CD133 and EpCAM are well-known markers for liver cancer stem cells by numerous researchers. Flow cytometry analysis was carried out to evaluate the expression of LCSC in sh-EpCAM, XAV939, cisplatin alone, cisplatin with sh-EpCAM, and cisplatin with XAV939-treated LCSC-enriched Huh7 cell variants. The results showed an aberrant decrease of EpCAM and CD133 expression in single and combination with EpCAM-targeted therapy, whereas in control, the expression remained the same, and in cisplatin-treated arms, the LCSC expression was drastically elevated. These results confirm that sh-EPCAM, XAV939 as a single, or combination therapy might suppress the LCSC activation drastically in the Huh7 cell line (Fig. 3).
Inhibiting the tumor recurrence properties in a combination of chemotherapy along with EpCAM-targeted therapy
Chemoresistance and tumor deterioration are unfortunate but prevalent impediments in cancer therapy. EpCAM-targeted therapies such as shRNA (EpCAM) and XAV939 inhibit tumor initiation, colony formation, tumor invasion, and decreased expression of the chemo-resistant gene ABCG2. CSC-enriched Huh7 cells were cultured with XAV939, shEpCAM, cisplatin individual, and a combination of cisplatin with XAV939 and cisplatin with shEpCAM for spheroid formation assy. Spheroid formation assay, a novel in vitro assay, helps to study the efficacy of inhibiting tumor formation by EpCAM-targeted therapy as an individual or in combination with cisplatin. Cells without any drugs were cultured as a control. Interestingly none of the EpCAM-targeted therapy individuals and the combination with cisplatin could not form a single colony, whereas cisplatin alone and control cells show spheroids after the 9th day of culture. These findings confirm that the combinatorial approach of EpCAM-targeted therapy alongside cisplatin improves progression in EpCAM-positive hepatocellular carcinoma (Fig. 4).
EpCAM-targeted therapy promotes chemo-sensitivity to cisplatin
CSC-enriched cells were cultured without drug as a control and with cisplatin alone, XAV939 alone, shEpCAM alone, XAV939 with cisplatin, and shEpCAM with cisplatin for 3 days. Drug media were refreshed every 24 h. The culture supernatant was collected at all the time points for cytotoxicity assay in kinetic. After the third day of culture, the cell viability was measured using M.T.T. XAV939 and sh-EpCAM treatment arms noted a slight drop in viability compared to the control. In cisplatin alone, combined with sh-EpCAM and with XAV939 treated cells, viability was drastically decreased than control. The cytotoxicity assay observed an elevated LDH expression in cisplatin alone and combined with XAV939 and shEpCAM treatment than control. However, in treating XAV939 alone and sh-EpCAM alone, cells released much lesser LDH than in the combination. This data suggests that EpCAM-targeted therapies and cisplatin promote chemo-sensitivity in liver cancer (Fig. 5a, b).
a In cytotoxicity assay, cisplatin alone and combination with XAV939 and (EpCAM)-treated cells shown higher cytotoxicity level than in XAV939 and shRNA (EpCAM) alone-treated cells. b Similarly, in cell viability assay, cisplatin alone and combination with EpCAM-targeted therapies shown decreased viability compared to the XAV939 alone and shRNA (EpCAM) alone treatments
Discussion
Chemoresistance and tumor recurrence is the major obstacle in cancer therapy. To understand the molecular mechanism of tumor recurrence, we should know the molecular difference between primary and relapsed tumors. Adam et al. and his group reported that the primary tumor showed low expression of CD44, ALDH1A1, and CD133, whereas the recurrence tumor after the chemotherapy showed an increased expression of CD44, ALDH1A1, and CD133. CD133 expression was significantly increased by about 14% in platinum-resistant patients compared to the matched most important tumors [22]. Tsunaki Yamashina et al. [23] and colleagues found that cancer stem-like cells develop chemo-resistant tumors by producing an assortment of pro-inflammatory cytokines and generating M2-like immunoregulatory myeloid cells from CD14 + monocytes [23]. FundaMeric-Bernstamet al. [24] and his team reported that by understanding the genomic alteration between the most critical and persistent breast tumors, they observed the increased frequency of CDK4/MDM2 amplifications in recurrences than the primary tumor.
Cheng-Wei Lin et al. [25] demonstrated how EpCAM plays a significant role in the tumor; they have found that EpCAM and stem cell transcription factors (Sox2, Nanog, Oct4, and c-Myc) be simultaneously prominent in TICs, which lead to more personality regeneration, invasiveness, enhanced tumorsphere, and tumor initiate ability. In further, EpCAM knockdown inhibited the reprogramming expressions feature and epithelial-mesenchymal transition (EMT) genes, in so doing tumor suppress initiation, invasiveness, and self-renewal [25]. According to their research, Yi Chen et al. [26] concludes that EpCAM + CD133 + cells have the characteristic of tumor initiate cells compared to CD133-EpCAM + , CD133 + EpCAM − , and CD133-EpCAM − cells. In addition to that, CD133 + EpCAM + cells showed elevated differentiation capability, increased formation capability, higher expression of stem cell transduction factors, drug-resistant to some chemo-drugs, better spheroid formation and spheroid solid formation, and strong tumorigenicity in NOD/SCID mice [26].
The signaling of Wnt/ β-catenin necessary pathway for upholding stemness in normal livers and liver carcinomas, particularly in Wnt/β-catenin signaling HCC, acting as a chief responsibility in the spheroid configuration as well as the continuation of acinous. Atsushi Takai et al. [27] reported that positive EpCAM cells are supplementary responsive for TGF/β encouraged epithelial-mesenchymal transition with extremely metastatic and tumorigenic impending in vivo. Curtis et al. [28] and his colleagues confirmed through flow cytometry that wnt-β-catenin signaling embarrassment could decrease EpCAM expression, wnt-β-catenin signaling pathway inhibitor (XAV939), and EpCAM gene silencing through shRNA inhibited the liver cancer stem cells expression. Grippingly in the colony formation assay, there was no colony formation in XAV939-treated cells compared to the control. So, sh-EpCAM and XAV939 treatment inhibits tumor initiation and invasion properties by suppressing the cancer stem cell.
Partha Krishnamurthy et al. [29] and his colleague reveal that the marker and A.B.C. transporter progenitor and stem cells are recognized as the breast cancer resistance protein (ABCG2 or BCRP), conferring a brawny survival advantage under hypoxic conditions. Guang Zhang et al. [30] and Wu et al. [31] and his team studied the characteristic of ABCG2-positive cells in HCC. They found that ABCG2-positive cells expressed high tumorigenicity, enhanced cell proliferation, improved migration, and were doxorubicin-resistant, whereas, in ABCG2, downregulation led to inhibition of tumorigenicity, suppressed tumor invasion, and sensitivity to doxorubicin chemo drugs [32, 33].
Our data demonstrate the aberrant wnt-β-catenin activation in LCSC-enriched Huh7 cell variants. The aberrant wnt-β-catenin activation was suppressed by targeting XAV939 and enhanced the cisplatin sensitivity. To support this data, ABCG2 expression was drastically decreased in both shEpCAM- and XAV939-treated cells, but in contrast, the expression was increased in cisplatin-treated LCSC-enriched Huh7 cell variants. The viability of XAV939 and sh-EpCAM treatment arms was slightly dropped compared to the control, but cisplatin alone and combined with sh-EpCAM and with XAV939 treated cells viability drastically decreased than the control. The cytotoxicity assay observed an elevated LDH expression in cisplatin alone and combined with XAV939 and shEpCAM treatment than control. However, in XAV939 alone and shEpCAM alone, treatment cells have much lesser LDH release than the combination. Interestingly, in the spheroid formation assay, none of the EpCAM-targeted therapy, neither individual nor combination with cisplatin, could form a spheroid, whereas, in cisplatin alone and control, cells showed spheroids. Due to the study’s limitations, the drug dose can only be assessed indirectly through gene expression, which can substitute for XAV939 or EpCAM knockdowns. In addition to cellular absorption, drug metabolism, and protein stability, other factors may be affecting knockdown effectiveness. Even though conventional therapies may have some promising results against tumors, they are fraught with limitations that result in local recurrences, metastases, and poor long-term survival. Alternative treatment approaches for liver cancer may target CSCs instead of conventional therapies.
Conclusion
EpCAM-targeted therapy such as sh-EpCAM and XAV939 were proven to be potential inhibitors of liver cancer stem cells by inhibiting colony formation, invasion properties, and decreased expression of ABCG2, the gene responsible for chemoresistance. The LCSC expression and spheroid formation were drastically reduced in single and combination cisplatin, and EpCAM-targeted therapy. Overall, targeting EpCAM with therapies such as sh-EpCAM and XAV939 shows promise as a potential treatment for liver cancer, although further exploration is needed to regulate their efficacy and safety in clinical trials. These studies imply that combining cisplatin and EpCAM-targeted therapy may benefit both and improve cancer treatment outcomes. More research is required to determine the ideal dose, schedule, and patient selection for this combination therapy approach. It would be necessary to conduct further research to determine whether combining XAV939, EpCAM knockdown, and cisplatin could synergistically affect stem cell populations. A well-designed controlled study with appropriate sample size, randomisation, and statistical analysis is typically required to determine whether the combination of cisplatin and a specific drug has a synergistic, additive, or antagonistic effect. Research should be conducted to validate any claims regarding drug interactions to ensure that the results are accurate and reliable. These assays may provide functional evidence of the effects of the treatments on stem cell properties.
Availability of data and materials
Applicable.
References
Katherine AMG, London WT (2014) The global epidemiology of hepatocellular carcinoma, present and future. Clin Liver Dis 15(2):223–x
Mokdad AA, Lopez AD, Shahraz S et al (2014) Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med 12(1):145
Kimura O, Yasuteru K, Takayuki K et al (2014) Expression of EpCAM increases in the hepatitis B related and the treatment-resistant hepatocellular carcinoma. BioMed Res Int (3):172913
Gottesman MM, Pastan I (1993) Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 62:385–427
Wang L, Mosel AJ, Oakley GG et al (2012) Deficient DNA damage signaling leads to chemoresistance to Cisplatin in oral cancer. Mol Cancer Ther 11:2401–2409
Comerford KM, Timothy JW, Jorn K et al (2002) Hypoxia-inducible factor-1- dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62:3387–3394
VanZijl F, Sabine M, Georg M et al (2011) A human model of epithelial to mesenchymal transition to monitor drug efficacy in hepatocellular carcinoma progression. Mol Cancer Ther 10:850–860
Wen L, Liang C, Chen E et al (2016) Regulation of multidrug resistance in hepatocellular carcinoma cells is TRPC6/calcium dependent. Sci Rep 6:23269
Yang ZJ, Chee CE, Huang S et al (2011) The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther 10:1533–1541
Martín V, Sanchez-Sanchez A, Herrera F et al (2013) Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br J Cancer 108:2005–2012
BC, (2006) Baguley Tumor stem cell niches: a new functional framework for the action of anticancer drugs. Recent Pat Anticancer Drug Discov 1:121–127
Teicher BA (2009) Acute and chronic in vivo therapeutic resistance. BiochemPharmacol 77:1665–1673
Jerry MA, S. Andreas A, (2008) Is tumor growth sustained by rare cancer stem cells or dominant clones. Cancer Res 68:4018–402
Dallas NA, Ling X, Fan F et al (2009) Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer Res 69:1951–1957
Hermann PC, Huber SL, Herrler T et al (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1:313–323
Gires O, Pan M, Schinke H et al (2020) Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years?. Cancer Metastasis Rev 39:969-87
Zhan T, Rindtorff N, Boutros M (2017) Wntsignaling in cancer. Oncogene 36(11):1461–1473
George S, Wilson ZH et al (2013) Efficacy of using cancer stem cell markers in isolating and characterizing liver cancer stem cells. Stem Cells Dev 22(19):2655–2664
Tao W, Michael PG, Dongxi X et al (2015) EpCAM aptamer-mediated survivin silencing sensitized cancer stem cells to doxorubicin in a breast cancer model. Wei DuanTheranostics 5(12):1456–1472
Granato G, Ruocco M, Iaccarino A et al (2017) Generation and analysis of spheroids from human primary skin myofibroblasts: an experimental system to study myofibroblasts deactivation. Cell Death Discov 3:17038
Antonella C, Roberto P, Natale D et al (2015) Cytotoxic activity of the novel small molecule A.K.T. inhibitor SC66 in hepatocellular carcinoma cells. Oncotarget 6(3):1707–1722
Adam DS, Kerri SB, Ashwini AK et al (2012) Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res 18(3):869–881
Tsunaki Y, Muhammad B, Akihiro Y et al (2014) Cancer stem-like cells derived from chemoresistant tumors have a unique capacity to prime tumorigenic myeloid cells. Cancer Res 74(10):2698–709
Funda M.B., Garrett M.F., Jaime FL. et al (2014) oncordance of genomic alterations between primary and recurrent breast cancer. Mol Cancer Ther 13(5):1382–9
Lin CW, Liao MY, Lin WW et al (2012) Epithelial cell adhesion molecule regulates tumor initiation and tumorigenesis via activating reprogramming factors and epithelia-mesenchymal transition gene expression in colon cancer. J Biol Chem 287(47):39449–59
Yi C, Dongke Y, Hao Z et al (2012) CD133+EpCAM+ phenotype possesses more characteristics of tumor initiating cells in hepatocellular carcinoma Huh7 cells. Int J Biol Sci 8(7):992–1004
Atsushi T., Hien TD, Xin WW (2016) Identification of drivers from cancer genome diversity in hepatocellular carcinoma. Int J MolSci 15(6):11142–60
Curtis J, Budhu A, Yu Z (2013) High-throughput screening for identification of inhibitors of EpCAM-dependent growth of hepatocellular carcinoma cells. Chem Biol Drug Des 82(2):131–9
Partha Krishnamurthy DD, Ross TN et al (2004) The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem 279:24218–24225
Guang Z, Zhongxia W, Weihuan L et al (2013) Expression of potential ancer stem cell marker ABCG2 is associated with malignant behaviors of hepatocellular carcinoma. Gastroenterol Res Pract 2013:782581
Wu Q, Yang Z, Nie Y et al (2014) Multidrug resistance in cancer chemotherapeutics: mechanisms and lab approaches. Cancer Lett 347:159–166
Vasanthakumar S, Dhandapani M, Balakrishnan B et al (2018) Understanding the mechanism of drug-resistant and tumor recurrence in liver cancer. J Drug Deliv Therapeut 8(5):224–229
Vasanthakumar S, Sasikala P, Padma M et al (2017) EpCAM as a novel therapeutic target for hepatocellular carcinoma. J Oncol Sci 3:71–76
Acknowledgements
Authors acknowledge P.G. & Research Department of Zoology & Biotechnology, AV.V.M. Sri Pushpam College (Autonomous Institution Affiliated to Bharathidasan University), Poondi, for for providing the necessary facility to carry out research works.
Author information
Authors and Affiliations
Contributions
VS has written the contents. RV and AP corrected the data and edited the figures. GS designed the study, corrected, revised, and approved the manuscript for submission. All the authors approved the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sekar, V., Veerabathiran, R., Pandian, A. et al. Targeting liver cancer stem cell through EpCAM therapy targeted with chemotherapy endorse enhanced progression in hepatocellular carcinoma. Egypt Liver Journal 13, 29 (2023). https://doi.org/10.1186/s43066-023-00263-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00263-x