Abstract
Background
Sarcopenia, characterised by a loss of muscle strength, quantity/quality, and physical performance, is associated with increased mortality and poor clinical outcomes in patients with liver cirrhosis. The use of the currently accepted methods for estimating muscle mass, such as computed tomography, dual-energy X-ray absorptiometry, and bioelectrical impedance analysis, in routine clinical practice is restricted because of limited availability, radiation exposure, time consumption, or high cost. Therefore, an alternative, simple, safe, reproducible, and financially accessible method for the routine assessment of sarcopenia is needed. Hence, we aim to assess the utility of handgrip strength (HGS) in diagnosing sarcopenia in patients with HCV-related cirrhosis compared to appendicular skeletal muscle index assessed by dual-energy X-ray absorptiometry (DEXA-ASMI). A total of 64 participants older than 18 years were consecutively recruited. The subjects were divided into the following groups: Control group included 32 healthy control subjects, and the HCV-related liver cirrhosis group included 32 patients who were subdivided equally into two subgroups (Child A and Child C) with 16 patients each. All participants were subjected to dominant hand dynamometer and DEXA scan.
Results
The prevalence of sarcopenia was significantly higher in the cirrhosis group than in the control group (7.75 ± 1.35 vs. 8.29 ± 1.25 kg/m2, P < 0.001), with increasing prevalence in the Child C class group (P < 0.001). HGS was significantly lower in the Child C group compared to other groups (P < 0.001). Regarding the differentiation of sarcopenic patients, defining HGS using a cutoff of ≤ 28.6 kg has an AUC of 0.879, sensitivity of 100%, specificity of 66.7%, PPV of 61.1%, and NPV of 100% (95% CI = 0.715 to 0.967; P < 0.0001).
Conclusion
Given the low cost, reproducibility, and safety of handgrip strength dynamometry, this is a promising method for both the diagnosis of sarcopenia as well as serial monitoring of muscle function in patients with HCV-related cirrhosis.
Similar content being viewed by others
Background
Sarcopenia is a malnutrition-associated syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength [1, 2]. The liver is the primary organ of nutrient metabolism of the human body; therefore, liver cirrhosis causes malnutrition and consequently results in secondary sarcopenia in 30–70% of cirrhotic patients, especially those with advanced disease [3, 4].
Cirrhotic patients with sarcopenia have impaired protein synthesis and accelerated skeletal muscle proteolysis through several mechanisms, including malnutrition, ethanol consumption, decreased hepatic protein synthesis, altered skeletal muscle proteostasis, gut microbiome dysbiosis, raised myostatin levels, hyperammonemia, and low testosterone and growth hormone levels [3, 5,6,7,8,9,10]. Furthermore, altered hepatic immune responses result in endotoxemia and systemic inflammation, which induce sarcopenia progression through mechanisms including IGF-1 and insulin suppression [11]. Finally, bile acid altered metabolism may incur excessive growth of intestinal bacteria, as well as reduce nutrient absorption, particularly fat-soluble vitamins such as vitamin D [12].
Sarcopenia represents a significant economic and social burden in patients with cirrhosis [13]. It is associated with higher rates of morbidity, especially falls, fractures, disability, hepatic encephalopathy, infections, and hospital admissions [1, 14,15,16]. Additionally, sarcopenia was found to be an independent predictor of impaired quality of life [17] and mortality [16, 18, 19]. Sarcopenia is also associated with poorer clinical outcomes after liver transplantation, such as ventilator support, higher incidence of postoperative sepsis, organ rejection, neurological complications, length of ICU and hospital stay, mortality, and lack of functional independence [20]. This highlights the importance of early diagnosis and prompt evaluation of underlying etiologic risk factors for developing a personalised management plan, as stated in the recent AASLD guidelines [1]. However, there is currently no standardised definition of sarcopenia related to liver disease [21]. Additionally, patients with cirrhosis have alterations in the hydration fraction and density of fat-free mass even in the early stages of disease. These fluid imbalances impair the performance of available methods for skeletal muscle evaluation [22, 23]. Under current conditions, the accurate diagnosis of sarcopenia in patients with cirrhosis often remains elusive, and a suitable method for skeletal muscle assessment that is not influenced by body fluid changes is clearly needed [24].
Generally, there are several definitions for the diagnosis of sarcopenia, but all include low skeletal muscle mass alone or combined with low muscle strength [2]. However, muscle strength tends to decline prior to muscle wasting and has been associated with adverse outcomes more consistently than low muscle mass [2]. Hence, more recent studies tend to focus on muscle strength rather than muscle mass to diagnose sarcopenia and predict clinical outcomes [25, 26]. In addition, the use of the currently accepted methods for estimating muscle mass, such as computed tomography, dual-energy X-ray absorptiometry (DEXA), and bioelectrical impedance analysis, in routine clinical practice is restricted because of limited availability, radiation exposure, time consumption, or high cost. Therefore, an alternative, simple, safe, reproducible, and financially accessible method for the routine assessment of sarcopenia is needed [2].
The measurement of handgrip strength (HGS) is a method to quantify muscle function and quality. It measures mainly the hand and forearm muscle strength, but it has significant correlations with the muscle strength in the lower limbs and many other parts. Consequently, HGS reflects the degree of muscle strength of the whole body [27]. Previous studies have demonstrated that HGS is an independent predictor for malnutrition and cirrhosis-related complications, such as refractory ascites, spontaneous bacterial peritonitis, variceal bleeding, hepatorenal syndrome, and mortality [26, 28,29,30].
Using HGS may be of great utility since it is simple, easily reproducible, non-invasive, low cost, sensitive to nutritional changes, and is performed at the patient’s bedside or during outpatient clinics without additional risk [2]. Therefore, we aim to assess the utility of HGS in diagnosing sarcopenia in HCV-related chronic liver disease patients compared to appendicular skeletal muscle index assessed by dual-energy X-ray absorptiometry (DEXA-ASMI).
Methods
For this observational study, a total of 64 participants older than 18 years were consecutively recruited from the outpatient clinics of the Department of Internal Medicine, Ain Shams University Hospitals, Cairo, Egypt, from June 2020 to August 2022. The subjects were divided into the following groups:
-
i.
Control group: The control group included 32 healthy control subjects who were matched with cirrhotic patients with regard to sex, age, and body mass index (BMI).
-
ii.
The HCV-related liver cirrhosis group: This group included 32 patients who were subdivided equally into two subgroups comprising 16 subjects each (Child-Turcotte-Pugh A and C cirrhotic patients).
Patients were excluded if they had other causes of liver disease, alcohol abuse, coinfection with HIV or HBV, hepatic encephalopathy, hepatocellular carcinoma or other malignant tumours, active antiviral treatment, transjugular intrahepatic portosystemic shunt insertion, or liver transplantation. Other causes of exclusion include pregnancy; the presence of severe comorbidities such as respiratory failure, heart failure, and renal failure requiring dialysis; history of recent use of drugs affecting psychometric performances; and existence of neurological, psychiatric disorders, or musculoskeletal disease or deformity that could interfere with the muscle mass or muscle strength evaluation [31].
Demographic and clinical data collection
All participants were subjected to clinical, laboratory, HGS, and DEXA exams. Anti-HCV and HCV-RNA were assessed using third-generation enzyme immunoassay (EIA; AxSYM HCV 3.0, Abbott Laboratories, Chicago, IL, USA) and an in-house direct reverse transcriptase polymerase chain reaction (RT-PCR) assay, respectively.
Cirrhosis diagnosis was based on clinical characteristics, histological criteria, laboratory data, or imaging exams [32]. Severity of liver disease was evaluated using the Child–Pugh score and the model for end-stage liver disease (MELD) score [33, 34]. Ascites grade was evaluated according to the principles of the International Club of Ascites [35].
Included individuals were subjected to the following evaluations:
Anthropometric assessment
-
Body weight was assessed with each participant standing barefoot and wearing light clothes in the centre of a single electronic scale platform with an accuracy of 0.1 kg (BOD POD; Life Measurement Instruments, Concord, CA, USA).
-
Height was measured using a single stadiometer accurate to 0.1 cm; participants stood barefoot with heels together, back straight, and arms extended at the side of the body.
-
Body mass index (BMI) was calculated as the weight divided by the squared height (kg/m2) and categorised according to the World Health Organization criteria [36].
-
The waist circumference was determined midway between the lowest rib and the top of the iliac crest at the end of a normal expiration.
-
The hip circumference was determined at a horizontal plane at the maximum extension of the buttocks.
-
The waist/hip ratio (W/H) was calculated by dividing waist measurement by hip measurement. Healthy W/H is 0.9 or less in males and 0.85 or less in females.
-
The mid-arm circumference (MAC) was measured by placing the tape at the midpoint between the tip of the acromion and the olecranon process with the subject sitting upright and their arm flexed at 90° toward the chest.
-
The mid-arm muscle circumference (MAMC) was calculated using the MAC and the triceps skinfold thickness according to standard equations as previously mentioned [37]. The threshold used to define low skeletal muscle mass was < 10th percentile of the sex- and age-specific MAMC [38]. All measurements were taken using a 150 cm non-distensible tape with an accuracy of 0.1 cm [39].
Handgrip strength assessment
HGS was assessed using a digital hand dynamometer Camry device (CAMRY Digital Hand Dynamometer, Model: EH101). The participants held an ergonomic position to perform the test, sitting upright in a chair with a backrest but no armrests. The feet were maintained on the floor with 90° knee flexion. The arm was positioned with 90° elbow flexion and neutral forearm pronosupination [40]. The subjects received explanation of the dynamometer. A single blinded instructor encouraged participants to produce their maximal HGS with their dominant hand. The best result of three attempts with a 1-min pause between was documented in kilograms (kg). Reduced HGS was defined using the cutoffs of ≤ 26 kg in males and ≤ 18 kg in females [2].
Dual-energy X-ray absorptiometry (DEXA)
All scans were performed on a single Prodigy DEXA scanner (GE Lunar, Madison, WI, USA; GE part number: LU43616EN.Jan-2010) [41]. Participants removed all metal objects and were instructed to empty their bladders. They then laid in the supine position on the scanning table centre, with palms down and arms at the side of the body [42]. Measurements of segmental muscle mass (considering the muscle limbs only) were obtained from all participants and used to calculate the ASM (the sum of the lean mass of the four limbs). We then calculated the DEXA-ASMI using the equation ASM (kg)/height2 (m) [37]. Depletion of skeletal muscle mass was identified according to the European Working Group on Sarcopenia in Older People (EWGSOP2) recommendations as ASMI < 7.0 kg/m2 in males and ASMI < 5.5 kg/m2 in females [2]. Additionally, DEXA examinations were performed on the lumbar spine and femoral neck. According to World Health Organization references, osteoporosis was described as a T score ≤ − 2.5 and osteopenia as − 2.5 < T score < − 1 [43].
Ethical approval was given from the Faculty of Medicine, Ain Shams University Ethics Committee (approval number: MD 116/2017-FWA 000017585). This study was performed in accordance with the ethics principles of the Declaration of Helsinki. Written informed consent was obtained prior to participation.
Statistical analysis
Data were evaluated using IBM© SPSS© Statistics version 23 (IBM© Corp., Armonk, NY, USA). Numerical data were presented as mean and SD, and intergroup differences were compared using an unpaired t-test. Categorical data were presented as a ratio or as number and percentage, and differences were assessed using the Pearson chi-squared test or Fisher’s exact test if appropriate. Ordinal data were assessed using the chi-squared test for trend. Correlation between numerical variables was analysed using the Pearson correlation. Associations between continuous and ordinal variables were assessed using the Kendall tau rank correlation. Associations between continuous and nominal variables were analysed using the point biserial correlation. Receiver-operating characteristic (ROC) curve analysis with estimation of Youden’s index was used to assess the diagnostic value of HGS. Two-sided P-values < 0.05 were considered statistically significant.
Results
Our study was performed on 64 subjects; HCV-related liver cirrhosis group included 32 patients (22 males and 10 females) with a mean age of 58.5 ± 11.0 years. This group was subdivided into two subgroups (Child A and Child C) with 16 patients each. The control group comprised 32 healthy volunteers (20 males and 12 females) with a mean age of 59.3 ± 12.1 years (Table 1). Mild ascites were detected in 3 patients with Child C cirrhosis.
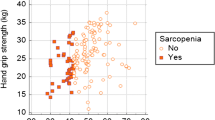
The prevalence of sarcopenia was significantly higher in the cirrhosis group than in the control group, with increasing prevalence in the Child C class group (Tables 1, 2, Fig. 1). A significant difference in the presence of bone disease between the control and HCV-related cirrhosis groups was detected. Additionally, bone disease was more prevalent in the Child C patients (Table 2, Fig. 2).
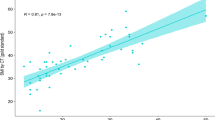
HGS was significantly lower in the Child C group compared to other groups (Table 3, Fig. 3). By analysing patients’ characteristics, we determined that sarcopenic patients are older, with higher Child class and lower HGS (Table 4, Fig. 4). Additionally, HGS was an independent predictor of sarcopenia in patients with HCV-related cirrhosis (P = 0.021) after adjusting for age and Child class by multivariable binary logistic regression analysis (Table 5). HGS significantly correlated with DEXA-ASMI, sex, Child class, MAMC, MAC, haemoglobin, serum albumin, conjugated bilirubin, prothrombin time, prothrombin concentration, INR, and bone disease by DEXA scan (Table 6).
Regarding the differentiation of sarcopenic patients, defining HGS using a cutoff of ≤ 28.6 kg has an AUC of 0.879, sensitivity of 100%, specificity of 66.7%, PPV of 61.1%, and NPV of 100% (95% CI = 0.715 to 0.967; P < 0.0001) (Fig. 5).
Discussion
Detecting sarcopenia in patients with cirrhosis can be challenging because the clinically available methods for skeletal muscle mass assessment are affected strongly by the presence of ascites and peripheral oedema [44, 45]. This challenge can be partly overcome using a DEXA scan by focusing only on the ASMI [46]. Additionally, cirrhotic osteodystrophy is a common complication in cirrhotic patients; therefore, DEXA is frequently performed in these patients to assess BMD [47]. Therefore, DEXA body composition scans can be performed simultaneously with no added time or radiation. Hence, DEXA-ASMI was recommended as a diagnostic tool for sarcopenia by the EWGSOP2 [2]. The DEXA scan is simple and safe but costly, which makes it unsuitable for serial assessment of sarcopenia in cirrhotic patients. Therefore, HGS represent an interesting and suitable method for this purpose.
In the present study, the prevalence of sarcopenia in cirrhotic patients equals 34.4%. Of note, the frequency of sarcopenia in patients with cirrhosis has varied widely among studies (ranging from 17 to 70%), fluctuating according to patient sex, severity of disease, and the criteria and method applied to diagnose this condition [41, 48].
Our result is similar to the studies of Sinclair et al. (31%) [49], Belarmino et al. (24%) [46], and Lindqvist et al. (39%) [50]. However, it was lower than previously reported, as Giusto et al. [41] published that HGS and the DEXA-ASMI detected sarcopenia in 46% of patients, and Eriksen et al. [51] reported low numbers as well (men: 49%, women: 43%). This may be attributed to the use of different cutoff values for sarcopenia [52]. Another likely explanation is the high percentage of patients with alcoholic liver disease in previous studies [46, 49, 51], since alcohol abuse causes skeletal muscle wasting independent of cirrhosis [53].
In accordance with the current study, Belarmino et al. obtained DEXA-ASMI and non-dependant HGS in 144 men with cirrhosis and found that muscle mass and strength were significantly lower in the cirrhosis group than in healthy participants (HGS, patients 25 ± 8.40 vs. control 39.69 ± 6.27 kg, P < 0.001, and DEXA-ASMI, 7.76 ± 1.45 vs. 9.29 ± 0.95 kg/m2, P < 0.001) [46]. Subsequently, they further examined 124 male patients with cirrhosis, and 30% of them were diagnosed with low DEXA-ASMI (5.5 ± 2.1 kg/m2) [54].
Another study included 231 males and 84 females with cirrhosis and 315 healthy matched controls. The aetiology of cirrhosis was alcohol in 76% of the patients and post-viral hepatitis in 9%. Low DEXA-ASMI was defined as DEXA-ASMI < 7.0 kg/m2 in males and < 5.5 kg/m2 in females. Low DEXA-ASMI was more prevalent in both males (49%) and females (43%) with cirrhosis compared with healthy males (8%) and females (5%; P < 0.001). The higher incidence of low DEXA-ASMI in cirrhosis corresponded to an odds ratio of 10.9 (3.2–37.3) in males and 15 (1.2–190) in females. In addition, DEXA-ASMI was lower in Child C compared with Child A and controls [51].
Other authors used a cutoff value of < 7.26 kg/m2, and 30.9% of 420 cirrhotic men patients were classified as sarcopenic. Total DEXA-ASMI (kg/m2) was 7.81 (7.06–8.59), sarcopenic = 6.43 (5.95–7.05), and non-sarcopenic = 8.09 (7.46–8.75), P < 0.001 [49].
In agreement with the current results, Santos et al. examined 129 subjects. More than half of the patients had hepatitis C. Mean lumbar spine T score was − 1.51 ± 1.57, and mean femoral neck T score was − 0.97 ± 1.13. Mean HGS was 25.97 ± 10.18 kg. For the lumbar spine, only low HGS was related to low T scores (P = 0.0003, 95% CI = 0.024–0.077). Of note, the variables related to liver function did not remain as significant. For the femoral neck, only age was correlated with low T scores. Once again, the other variables related to the liver disease severity did not remain significant. This result indicates that even in compensated cirrhosis, bone disease can already be present in the femoral neck of these patients [55].
Another study enrolled 300 subjects. The researchers excluded all patients with Child C cirrhosis and included patients of all aetiologies. Total HGS was 31 (22–39) kg. Compared to males, females had significantly lower HGS (22.8 ± 7.3 vs. 38.5 ± 12.4 kg, P < 0.0001) [56]. Other authors defined declined HGS as ≤ 26 kg in males and ≤ 18 kg in females. Of the 270 patients, decreased HGS was detected in 102 (38%). The median HGS was 29 kg in males and 17 kg in females [15].
In accordance with the present study, Sinclair et al. assessed 145 men using the sarcopenia cutoff for DEXA-ASMI of < 7.26 kg/m2; finding the incidence of sarcopenia was 38.7% (46/119). The median DEXA-ASMI was 7.52 (6.87–8.36) kg/m2. Using a cutoff value for HGS of < 30 kg, the incidence of sarcopenia was 45.9% (50/109). The median HGS was 30.9 (25–28) kg. Additionally, HGS was associated with DEXA-ASMI (P < 0.001) [26].
Ye et al. corroborated our results with a study that determined that HGS and DEXA-ASMI of patients were reduced compared to the control group (P < 0.05). Moreover, HGS was associated with the Child–Pugh score (P < 0.05). In addition, in comparing Child–Pugh A, B, and C groups with the control group, the investigators found that HGS decreased significantly along advanced Child class (Child A = 25.86 ± 9.39 kg, Child B = 23.36 ± 8.54 kg, Child C = 19.78 ± 9.57 kg, compared to control = 36.27 ± 11 kg, P < 0.001) [57].
In partial agreement with the present study, a previous cross-sectional study that investigated 58 non-cirrhotic patients with chronic HCV hepatitis who were not under active pharmacological therapy. For nondominant HGS, 15 (57.7%) males and 22 (68.8%) females had HGS values below the 50th percentile. Additionally, HGS did not correlate with other clinical characteristics, such as age, gender, and liver fibrosis grade [58].
Another cross-sectional study included 80 patients with HCV-related cirrhosis and 80 control subjects. The authors compared HGS in patients against the control group using both the right hand (18.9 ± 4.8 vs. 41.9 ± 4.8 kg) and left hand (22.6 ± 3.7 vs. 29.6 ± 3.7 kg), P < 0.001. They also compared the HGS in individuals with F0–F3 fibrosis against those with cirrhosis using the right (17.8 ± 3.7 vs. 16.8 ± 1.6 kg) and left hand (15.8 ± 2.4 vs. 14.2 ± 3.8 kg), P < 0.001. Compared to control participants, patients with cirrhosis had significantly lower values of MAC (23.3 ± 2.6 vs. 28.1 ± 4.7 cm, P < 0.001) and MAMC (18.9 ± 5.7 vs. 22.9 ± 4.2 mm, P < 0.001). Additionally, HGS was significantly negatively correlated with the degree of liver fibrosis [59].
Similar to the present study, other investigators found that 15 of the 122 male patients with cirrhosis had lower HGS (19.57 vs. 30.55 kg, P < 0.001) and albumin (3.10 vs. 3.75, P = 0.037) than non-sarcopenic patients. Sarcopenia diagnosis was determined by considering DEXA-ASMI < 7.0 kg/m2 and nondominant HGS < 27 kg [60].
Elucidating risk factors for the HGS loss in patients with cirrhosis seems clinically meaningful. Similar to our findings, researchers revealed significant associations between HGS loss with age, serum albumin, total bilirubin, prothrombin time-INR, and platelets count [61]. In agreement with the current findings, Hiraoka et al. described a significant correlation between serum albumin level and the HGS loss [62]. Additionally, Sung et al. reported that advanced age and sarcopenia were independent adverse predictors for skeletal muscle mass loss in 166 patients with cirrhosis [63]. In contrast, sarcopenia and advanced age were not independent factors linked to the HGS loss in another study [61].
In agreement with the current results, in Luengpradidgun et al. study, 30 (16.5%) of the 146 patients had low HGS. The total mean HGS was 21.7 kg, in patients without sarcopenia = 31.3 kg, and in patients with sarcopenia = 16.7 kg (P < 0.001). Among 30 patients with sarcopenia, the median age was older, although not statistically significant, than non-sarcopenic patients. Likewise, platelets count and serum albumin in patients with sarcopenia were significantly lower than those without [64].
There are no standard cutoff values for diagnosing decreased HGS in patients with cirrhosis [50]. Of note, published cutoffs are based on heterogenous patient populations like those with different stages and aetiologies of liver disease [65] and those with cancer [66]. Therefore, a validated cutoff for sarcopenia diagnosis in HCV-related cirrhosis is of clinical interest. In the current study, we found that using a cutoff for HGS decline of ≤ 28.6 kg has a good predictive ability for sarcopenia. In an earlier report, HGS had an excellent diagnostic performance for detecting sarcopenia by using the Japan Society of Hepatology criteria (HGS < 26 kg for male, < 18 for female), where the sensitivity, specificity, NPV, and PPV were 88.2%, 100%, 98.7%, and 100%, respectively. Similarly, applying the European Working Group on Sarcopenia in Older People criteria (HGS < 30 kg for male, < 20 kg for female), the sensitivity, specificity, NPV, and PPV were 94.1%, 81.2%, 99.2%, and 82.5%, respectively [64].
This study is limited by small sample size. To the best of our knowledge, this is the first study to assess sarcopenia and HGS and its related factors among patients with HCV-related cirrhosis with the inclusion of Child C cirrhosis class. Early identification of sarcopenia would allow for prompt interventions to increase muscle mass and function, including nutritional supplementation [67], physical training [68], or medical therapy [69, 70], which will improve patients’ prognosis [71].
Conclusions
Given the low cost, reproducibility, and safety of handgrip strength dynamometry, this is a promising method for both the diagnosis of sarcopenia as well as serial monitoring of muscle function in patients with HCV-related cirrhosis.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- DEXA-ASMI:
-
Dual-energy X-ray absorptiometry appendicular skeletal muscle index
- HCV:
-
Hepatitis C virus
- HSG:
-
Handgrip strength
- INR:
-
International normalized ratio
- MAC:
-
Mid-arm circumference
- MAMC:
-
Mid-arm muscle circumference
- MELD:
-
Model for end-stage liver disease
References
Lai JC, Tandon P, Bernal W, Tapper EB, Ekong U, Dasarathy S, Carey EJ (2021) Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 74(3):1611–1644. https://doi.org/10.1002/hep.32049. Erratum in: Hepatology.2021Dec;74(6):3563.PMID:34233031;PMCID:PMC9134787.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), Extended Group for EWGSOP2 (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31. https://doi.org/10.1093/ageing/afy169. Erratum in: Age Ageing. 2019 Jul 1;48(4):601. PMID: 30312372; PMCID: PMC6322506.
Bunchorntavakul C, Reddy KR (2020) Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther 51(1):64–77. https://doi.org/10.1111/apt.15571. Epub 2019 Nov 8 PMID: 31701570.
Dhaliwal A, Armstrong MJ (2020) Sarcopenia in cirrhosis: a practical overview. Clin Med (Lond) 20(5):489–492. https://doi.org/10.7861/clinmed.2020-0089. PMID:32934043;PMCID:PMC7539699.
Aby ES, Saab S (2019) Frailty, sarcopenia, and malnutrition in cirrhotic patients. Clin Liver Dis 23(4):589–605. https://doi.org/10.1016/j.cld.2019.06.001. Epub 2019 Aug 21 PMID: 31563213.
Adamek A, Kasprzak A (2018) Insulin-like growth factor (IGF) system in liver diseases. Int J Mol Sci 19(5):1308. https://doi.org/10.3390/ijms19051308. PMID:29702590;PMCID:PMC5983723.
Moctezuma-Velázquez C, Low G, Mourtzakis M, Ma M, Burak KW, Tandon P, Montano-Loza AJ (2018) Association between low testosterone levels and sarcopenia in cirrhosis: a cross-sectional study. Ann Hepatol 17(4):615–623. https://doi.org/10.5604/01.3001.0012.0930. PMID: 29893704.
Kant S, Davuluri G, Alchirazi KA, Welch N, Heit C, Kumar A, Gangadhariah M, Kim A, McMullen MR, Willard B, Luse DS, Nagy LE, Vasiliou V, Marini AM, Weiner ID, Dasarathy S (2019) Ethanol sensitizes skeletal muscle to ammonia-induced molecular perturbations. J Biol Chem 294(18):7231–7244. https://doi.org/10.1074/jbc.RA118.005411. Epub 2019 Mar 14. PMID: 30872403; PMCID: PMC6509515.
Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, Schulze JM, Barnes D, McCullough AJ, Engelen MP, Deutz NE, Dasarathy S (2015) Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology 61(6):2018–29. https://doi.org/10.1002/hep.27717. Epub 2015 Feb 27. PMID: 25613922; PMCID: PMC4441611.
Siriett V, Salerno MS, Berry C, Nicholas G, Bower R, Kambadur R, Sharma M (2007) Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol Ther 15(8):1463–1470. https://doi.org/10.1038/sj.mt.6300182. Epub 2007 Jun 5 PMID: 17551508.
Bhanji RA, Montano-Loza AJ, Watt KD (2019) Sarcopenia in cirrhosis: looking beyond the skeletal muscle loss to see the systemic disease. Hepatology 70(6):2193–2203. https://doi.org/10.1002/hep.30686. PMID: 31034656.
Saeki C, Kanai T, Nakano M, Oikawa T, Torisu Y, Saruta M, Tsubota A (2020) Low serum 25-hydroxyvitamin D levels are related to frailty and sarcopenia in patients with chronic liver disease. Nutrients 12(12):3810. https://doi.org/10.3390/nu12123810. PMID:33322706;PMCID:PMC7764249.
Beaudart C, Reginster JY, Geerinck A, Locquet M, Bruyère O (2017) Current review of the SarQoL®: a health-related quality of life questionnaire specific to sarcopenia. Expert Rev Pharmacoecon Outcomes Res 17(4):335–341. https://doi.org/10.1080/14737167.2017.1360768. PMID: 28749192.
Traub J, Bergheim I, Eibisberger M, Stadlbauer V (2020) Sarcopenia and liver cirrhosis-comparison of the European Working Group on Sarcopenia Criteria 2010 and 2019. Nutrients 12(2):547. https://doi.org/10.3390/nu12020547. PMID:32093198;PMCID:PMC7071440.
Miwa T, Hanai T, Nishimura K, Maeda T, Ogiso Y, Imai K, Suetsugu A, Takai K, Shiraki M, Shimizu M (2022) Handgrip strength stratifies the risk of covert and overt hepatic encephalopathy in patients with cirrhosis. JPEN J Parenter Enteral Nutr 46(4):858–866. https://doi.org/10.1002/jpen.2222. Epub 2021 Aug 23 PMID: 34287991.
Wang S, Whitlock R, Xu C, Taneja S, Singh S, Abraldes JG, Burak KW, Bailey RJ, Lai JC, Tandon P (2022) Frailty is associated with increased risk of cirrhosis disease progression and death. Hepatology 75(3):600–609. https://doi.org/10.1002/hep.32157. Epub 2021 Dec 5 PMID: 34528267.
Ohashi K, Ishikawa T, Imai M, Suzuki M, Hoshii A, Abe H, Koyama F, Nakano T, Ueki A, Noguchi H, Hasegawa E, Hirosawa S, Kobayashi M, Hirosawa H, Sato K, Munakata M, Yoshida T (2019) Relationship between pre-sarcopenia and quality of life in patients with chronic liver disease: a cross-sectional study. Eur J Gastroenterol Hepatol 31(11):1408–1413. https://doi.org/10.1097/MEG.0000000000001415. PMID: 30964810.
Tandon P, Ismond KP, Riess K, Duarte-Rojo A, Al-Judaibi B, Dunn MA, Holman J, Howes N, Haykowsky MJF, Josbeno DA, McNeely M (2018) Exercise in cirrhosis: translating evidence and experience to practice. J Hepatol 69(5):1164–1177. https://doi.org/10.1016/j.jhep.2018.06.017. Epub 2018 Jun 30 PMID: 29964066.
Hiraoka A, Kitahata S, Izumoto H, Ueki H, Aibiki T, Okudaira T, Miyamoto Y, Yamago H, Iwasaki R, Tomida H, Mori K, Kishida M, Tsubouchi E, Miyata H, Ninomiya T, Hirooka M, Tokumoto Y, Abe M, Matsuura B, Hiasa Y, Michitaka K (2018) Muscle volume loss a prognostic factor for death in liver cirrhosis patients and special relationship to portal hypertension. Hepatol Res 48(3):E354–E359. https://doi.org/10.1111/hepr.12984. Epub 2017 Oct 18 PMID: 28940597.
Kumar V, Benjamin J, Shasthry V, Subramanya Bharathy KG, Sinha PK, Kumar G, Pamecha V (2020) Sarcopenia in cirrhosis: fallout on liver transplantation. J Clin Exp Hepatol 10(5):467–476. https://doi.org/10.1016/j.jceh.2019.12.003. Epub 2019 Dec 31. PMID: 33029056; PMCID: PMC7527849.
Carey EJ, Lai JC, Sonnenday C, Tapper EB, Tandon P, Duarte-Rojo A, Dunn MA, Tsien C, Kallwitz ER, Ng V, Dasarathy S, Kappus M, Bashir MR, Montano-Loza AJ (2019) A North American Expert Opinion Statement on Sarcopenia in Liver Transplantation. Hepatology 70(5):1816–1829. https://doi.org/10.1002/hep.30828. Epub 2019 Aug 19. PMID: 31220351; PMCID: PMC6819202.
Molfino A, Johnson S, Medici V (2017) The challenges of nutritional assessment in cirrhosis. Curr Nutr Rep 6(3):274–280. https://doi.org/10.1007/s13668-017-0216-8. Epub 2017 Jul 18. PMID: 29201536; PMCID: PMC5703218.
Cederholm T, Barazzoni R, Austin P, Ballmer P, Biolo G, Bischoff SC, Compher C, Correia I, Higashiguchi T, Holst M, Jensen GL, Malone A, Muscaritoli M, Nyulasi I, Pirlich M, Rothenberg E, Schindler K, Schneider SM, de van der Schueren MA, Sieber C, Valentini L, Yu JC, Van Gossum A, Singer P (2017) ESPEN guidelines on definitions and terminology of clinical nutrition. Clin Nutr. 36(1):49–64. https://doi.org/10.1016/j.clnu.2016.09.004. Epub 2016 Sep 14. PMID: 27642056.
Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H (2015) Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 31(1):193–199. https://doi.org/10.1016/j.nut.2014.07.005. Epub 2014 Jul 30 PMID: 25441595.
Hanai T, Shiraki M, Watanabe S, Imai K, Suetsugu A, Takai K, Moriwaki H, Shimizu M (2019) Prognostic significance of minimal hepatic encephalopathy in patients with liver cirrhosis in Japan: a propensity score-matching analysis. J Gastroenterol Hepatol 34(10):1809–1816. https://doi.org/10.1111/jgh.14635. Epub 2019 Mar 14 PMID: 30779213.
Sinclair M, Chapman B, Hoermann R, Angus PW, Testro A, Scodellaro T, Gow PJ (2019) Handgrip strength adds more prognostic value to the model for end-stage liver disease score than imaging-based measures of muscle mass in men with cirrhosis. Liver Transpl 25(10):1480–1487. https://doi.org/10.1002/lt.25598. Epub 2019 Aug 18 PMID: 31282126.
Porto JM, Nakaishi APM, Cangussu-Oliveira LM, Freire Júnior RC, Spilla SB, Abreu DCC (2019) Relationship between grip strength and global muscle strength in community-dwelling older people. Arch Gerontol Geriatr. 82:273–278. https://doi.org/10.1016/j.archger.2019.03.005. Epub 2019 Mar 6. PMID: 30889410.
Hanai T, Shiraki M, Imai K, Suetsugu A, Takai K, Moriwaki H, Shimizu M (2019) Reduced handgrip strength is predictive of poor survival among patients with liver cirrhosis: a sex-stratified analysis. Hepatol Res 49(12):1414–1426. https://doi.org/10.1111/hepr.13420. Epub 2019 Oct 9 PMID: 31408558.
Yoh K, Nishikawa H, Enomoto H, Iwata Y, Ikeda N, Aizawa N, Nishimura T, Iijima H, Nishiguchi S (2020) Grip strength: a useful marker for composite hepatic events in patients with chronic liver diseases. Diagnostics (Basel) 10(4):238. https://doi.org/10.3390/diagnostics10040238. PMID:32325995;PMCID:PMC7236004.
Tapper EB, Zhang P, Garg R, Nault T, Leary K, Krishnamurthy V, Su GL (2019) Body composition predicts mortality and decompensation in compensated cirrhosis patients: a prospective cohort study. JHEP Rep 2(1):100061. https://doi.org/10.1016/j.jhepr.2019.11.005. PMID: 32039402; PMCID: PMC7005567.
Kokudo N, Takemura N, Hasegawa K, Takayama T, Kubo S, Shimada M, Nagano H, Hatano E, Izumi N, Kaneko S, Kudo M, Iijima H, Genda T, Tateishi R, Torimura T, Igaki H, Kobayashi S, Sakurai H, Murakami T, Watadani T, Matsuyama Y (2019) Clinical practice guidelines for hepatocellular carcinoma: the Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol Res 49(10):1109–1113. https://doi.org/10.1111/hepr.13411. Epub 2019 Sep 6 PMID: 31336394.
Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR (2020) Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 71(1):306–333. https://doi.org/10.1002/hep.30866. PMID: 31314133.
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60(8):646–649. https://doi.org/10.1002/bjs.1800600817. PMID: 4541913.
Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR (2001) A model to predict survival in patients with end-stage liver disease. Hepatology 33(2):464–470. https://doi.org/10.1053/jhep.2001.22172. PMID: 11172350.
Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V (2003) The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 38(1):258–266. https://doi.org/10.1053/jhep.2003.50315. PMID: 12830009.
Shepherd JA, Baim S, Bilezikian JP, Schousboe JT (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on Body Composition. J Clin Densitom 16(4):489–95. https://doi.org/10.1016/j.jocd.2013.08.005. PMID: 24183639.
Carnevale V, Castriotta V, Piscitelli PA, Nieddu L, Mattera M, Guglielmi G, Scillitani A (2018) Assessment of skeletal muscle mass in older people: comparison between 2 anthropometry-based methods and dual-energy X-ray absorptiometry. J Am Med Dir Assoc 19(9):793–796. https://doi.org/10.1016/j.jamda.2018.05.016. Epub 2018 Jul 5 PMID: 29983360.
Frisancho AR (1981) New norms of upper limb fat and muscle areas for assessment of nutritional status. Am J Clin Nutr 34(11):2540–2545. https://doi.org/10.1093/ajcn/34.11.2540. PMID: 6975564.
World Health Organization (1995) Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 854:1–452. PMID: 8594834.
Su CY, Lin JH, Chien TH, Cheng KF, Sung YT (1994) Grip strength in different positions of elbow and shoulder. Arch Phys Med Rehabil 75(7):812–815. PMID: 8024431.
Giusto M, Lattanzi B, Albanese C, Galtieri A, Farcomeni A, Giannelli V, Lucidi C, Di Martino M, Catalano C, Merli M (2015) Sarcopenia in liver cirrhosis: the role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur J Gastroenterol Hepatol 27(3):328–334. https://doi.org/10.1097/MEG.0000000000000274. PMID: 25569567.
Vulcano DS, Carvalhaes MA, Bakonyi NA (2013) Evaluation of nutritional indicators and body composition in patients with advanced liver disease enrolled for liver transplantation. Acta Cir Bras 28(10):733–739. https://doi.org/10.1590/s0102-86502013001000008. PMID: 24114303.
Loria I, Albanese C, Giusto M, Galtieri PA, Giannelli V, Lucidi C, Di Menna S, Pirazzi C, Corradini SG, Mennini G, Rossi M, Berloco P, Merli M (2010) Bone disorders in patients with chronic liver disease awaiting liver transplantation. Transplant Proc 42(4):1191–1193. https://doi.org/10.1016/j.transproceed.2010.03.096. PMID: 20534258.
Woodward AJ, Wallen MP, Ryan J, Ward LC, Coombes JS, Macdonald GA (2021) Evaluation of techniques used to assess skeletal muscle quantity in patients with cirrhosis. Clin Nutr ESPEN 44:287–296. https://doi.org/10.1016/j.clnesp.2021.05.029. Epub 2021 Jun 11 PMID: 34330481.
Santos LAA, Lima TB, Ietsugu MDV, Nunes HRC, Qi X, Romeiro FG (2019) Anthropometric measures associated with sarcopenia in outpatients with liver cirrhosis. Nutr Diet 76(5):613–619. https://doi.org/10.1111/1747-0080.12523. Epub 2019 Mar 14 PMID: 30869199.
Belarmino G, Gonzalez MC, Sala P, Torrinhas RS, Andraus W, D’Albuquerque LAC, Pereira RMR, Caparbo VF, Ferrioli E, Pfrimer K, Damiani L, Heymsfield SB, Waitzberg DL (2018) Diagnosing sarcopenia in male patients with cirrhosis by dual-energy x-ray absorptiometry estimates of appendicular skeletal muscle mass. JPEN J Parenter Enteral Nutr 42(1):24–36. https://doi.org/10.1177/0148607117701400. Epub 2017 Dec 6 PMID: 28402708.
Guañabens N, Parés A (2018) Osteoporosis in chronic liver disease. Liver Int 38(5):776–785. https://doi.org/10.1111/liv.13730. Epub 2018 Mar 25 PMID: 29479832.
Peterson SJ, Braunschweig CA (2016) Prevalence of sarcopenia and associated outcomes in the clinical setting. Nutr Clin Pract 31(1):40–48. https://doi.org/10.1177/0884533615622537. Epub 2015 Dec 24 PMID: 26703961.
Sinclair M, Hoermann R, Peterson A, Testro A, Angus PW, Hey P, Chapman B, Gow PJ (2019) Use of dual X-ray absorptiometry in men with advanced cirrhosis to predict sarcopenia-associated mortality risk. Liver Int 39(6):1089–1097. https://doi.org/10.1111/liv.14071. Epub 2019 Feb 25 PMID: 30746903.
Lindqvist C, Brismar TB, Majeed A, Wahlin S (2019) Assessment of muscle mass depletion in chronic liver disease: dual-energy x-ray absorptiometry compared with computed tomography. Nutrition 61:93–98. https://doi.org/10.1016/j.nut.2018.10.031. Epub 2018 Nov 14 PMID: 30703575.
Eriksen CS, Kimer N, Suetta C, Møller S (2021) Arm lean mass determined by dual-energy x-ray absorptiometry is superior to characterize skeletal muscle and predict sarcopenia-related mortality in cirrhosis. Am J Physiol Gastrointest Liver Physiol 320(5):G729–G740. https://doi.org/10.1152/ajpgi.00478.2020. Epub 2021 Mar 17 PMID: 33729006.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39(4):412–23. https://doi.org/10.1093/ageing/afq034. Epub 2010 Apr 13. PMID: 20392703; PMCID: PMC2886201.
Bhanji RA, Narayanan P, Moynagh MR, Takahashi N, Angirekula M, Kennedy CC, Mara KC, Dierkhising RA, Watt KD (2019) Differing impact of sarcopenia and frailty in nonalcoholic steatohepatitis and alcoholic liver disease. Liver Transpl 25(1):14–24. https://doi.org/10.1002/lt.25346. PMID:30257063;PMCID:PMC7187989.
Belarmino G, Torrinhas RS, Magalhães NV, Heymsfield SB, Waitzberg DL (2021) New anthropometric and biochemical models for estimating appendicular skeletal muscle mass in male patients with cirrhosis. Nutrition 84:111083. https://doi.org/10.1016/j.nut.2020.111083. Epub 2020 Nov 21. PMID: 33418229.
Santos LA, Lima TB, Augusti L, Franzoni Lde C, Yamashiro Fda S, Bolfi F, Nunes Vdos S, Dorna Mde S, de Oliveira CV, Caramori CA, Silva GF, Romeiro FG (2016) Handgrip strength as a predictor of bone mineral density in outpatients with cirrhosis. J Gastroenterol Hepatol 31(1):229–234. https://doi.org/10.1111/jgh.13062. PMID: 26212461.
Tapper EB, Baki J, Parikh ND, Lok AS (2019) Frailty, psychoactive medications, and cognitive dysfunction are associated with poor patient-reported outcomes in cirrhosis. Hepatology 69(4):1676–1685. https://doi.org/10.1002/hep.30336. Epub 2019 Feb 19. PMID: 30382584; PMCID: PMC6438757.
Ye Q, Yin W, Zhang L, Xiao H, Qi Y, Liu S, Qian B, Wang F, Han T (2017) The value of grip test, lysophosphatidlycholines, glycerophosphocholine, ornithine, glucuronic acid decrement in assessment of nutritional and metabolic characteristics in hepatitis B cirrhosis. PLoS One 12(4):e0175165. https://doi.org/10.1371/journal.pone.0175165. PMID: 28384211; PMCID: PMC5383249.
Bruch JP, Álvares-DA-Silva MR, Alves BC, Dall’alba V (2016) Reduced hand grip strength in overweight and obese chronic hepatitis C patients. Arq Gastroenterol 53(1):31–5. https://doi.org/10.1590/S0004-28032016000100007. PMID: 27281502.
Gabr SA, Alghadir AH (2021) Handgrip strength and vitamin D as predictors of liver fibrosis and malnutrition in chronic hepatitis C patients. Dis Markers 2(2021):6665893. https://doi.org/10.1155/2021/6665893. PMID:33884041;PMCID:PMC8041557.
Espirito Santo Silva DD, Waitzberg DL, Passos de Jesus R, Oliveira LPM, Torrinhas RS, Belarmino G (2019) Phase angle as a marker for sarcopenia in cirrhosis. Clin Nutr ESPEN 32:56–60. https://doi.org/10.1016/j.clnesp.2019.05.003. Epub 2019 May 31. PMID: 31221291.
Nishikawa H, Yoh K, Enomoto H, Ikeda N, Takashima T, Aizawa N, Nishimura T, Nishiguchi S, Iijima H (2021) Predictors for grip strength loss in patients with chronic liver diseases. In Vivo 35(1):363–371. https://doi.org/10.21873/invivo.12267. PMID: 33402485; PMCID: PMC7880795.
Hiraoka A, Michitaka K, Izumoto H, Ueki H, Kitahata S, Aibiki T, Okudaira T, Yamago H, Miyamoto Y, Iwasaki R, Tomida H, Mori K, Miyata H, Tsubouchi E, Kishida M, Hirooka M, Abe M, Matsuura B, Ninomiya T, Hiasa Y (2018) Relative changes in handgrip strength and skeletal muscle volume in patients with chronic liver disease over a 2-year observation period. Hepatol Res 48(7):502–508. https://doi.org/10.1111/hepr.13051. Epub 2018 Feb 6 PMID: 29314571.
Sung JH, Uojima H, Hidaka H, Tanaka Y, Wada N, Kubota K, Nakazawa T, Shibuya A, Fujikawa T, Yamanoue H, Kako M, Koizumi W (2019) Risk factors for loss of skeletal muscle mass in patients with cirrhosis. Hepatol Res 49(5):550–558. https://doi.org/10.1111/hepr.13308. Epub 2019 Feb 1 PMID: 30623996.
Luengpradidgun L, Chamroonkul N, Sripongpun P, Kaewdech A, Tanutit P, Ina N, Piratvisuth T (2022) Utility of handgrip strength (HGS) and bioelectrical impedance analysis (BIA) in the diagnosis of sarcopenia in cirrhotic patients. BMC Gastroenterol 22(1):159. https://doi.org/10.1186/s12876-022-02236-7. PMID:35354434;PMCID:PMC8969388.
Carey EJ, Lai JC, Wang CW, Dasarathy S, Lobach I, Montano-Loza AJ, Dunn MA (2017) Fitness, life enhancement, and exercise in liver transplantation consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl 23(5):625–633. https://doi.org/10.1002/lt.24750. PMID: 28240805; PMCID: PMC5762612.
Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE (2013) Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 31(12):1539–1547. https://doi.org/10.1200/JCO.2012.45.2722. Epub 2013 Mar 25 PMID: 23530101.
Butterworth RF (2019) L-ornithine L-aspartate for the treatment of sarcopenia in chronic liver disease: the taming of a vicious cycle. Can J Gastroenterol Hepatol 28(2019):8182195. https://doi.org/10.1155/2019/8182195. PMID:31183339;PMCID:PMC6512019.
Aamann L, Dam G, Borre M, Drljevic-Nielsen A, Overgaard K, Andersen H, Vilstrup H, Aagaard NK (2020) Resistance training increases muscle strength and muscle size in patients with liver cirrhosis. Clin Gastroenterol Hepatol 18(5):1179-1187.e6. https://doi.org/10.1016/j.cgh.2019.07.058. Epub 2019 Aug 5 PMID: 31394282.
Wagner KR, Abdel-Hamid HZ, Mah JK, Campbell C, Guglieri M, Muntoni F, Takeshima Y, McDonald CM, Kostera-Pruszczyk A, Karachunski P, Butterfield RJ, Mercuri E, Fiorillo C, Bertini ES, Tian C, Statland J, Sadosky AB, Purohit VS, Sherlock SP, Palmer JP, Binks M, Charnas L, Marraffino S, Wong BL (2020) Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy. Neuromuscul Disord 30(6):492–502. https://doi.org/10.1016/j.nmd.2020.05.002. PMID: 32522498.
Sinclair M, Grossmann M, Hoermann R, Angus PW, Gow PJ (2016) Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol 65(5):906–913. https://doi.org/10.1016/j.jhep.2016.06.007. Epub 2016 Jun 14 PMID: 27312945.
Tandon P, Raman M, Mourtzakis M, Merli M (2017) A practical approach to nutritional screening and assessment in cirrhosis. Hepatology 65(3):1044–1057. https://doi.org/10.1002/hep.29003. Epub 2017 Feb 6 PMID: 28027577.
Acknowledgements
Not applicable
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MS, EB, MS, SA, and SS designed the study; AZ participated in the acquisition of data; MS, EB, MS, SA, SS, AZ, and GM participated in the analysis and interpretation of the data; MS, EB, MS, SA, SS, AZ, and GM revised the article critically for important intellectual content; and GM wrote the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was given from the Faculty of Medicine, Ain Shams University Ethics Committee (approval number: MD 116/2017-FWA 000017585). This study was performed in accordance with the ethics principles of the Declaration of Helsinki. Written informed consent was obtained prior to participation.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salama, M.M., Bayoumi, E.M., Sayed, M.M. et al. Evaluation of handgrip strength as a predictor of sarcopenia in patients with HCV-related cirrhosis. Egypt Liver Journal 13, 24 (2023). https://doi.org/10.1186/s43066-023-00261-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-023-00261-z