Abstract
Background
Acute-on-chronic liver failure (ACLF) is a distinct syndrome associated with high short-term mortality. Early identification of patients at high risk is essential to determine emergency for transplantation and decide and prioritize the need for intensive care unit (ICU). We aimed to evaluate the performance of the different prognostic scores in the prediction of in-hospital mortality in patients with ACLF. A total of 249 patients with ACLF were included and followed till discharge from the hospital. Univariate and Cox regression analyses were used to assess the performance of liver-specific (Child-Pugh and MELD) and ACLF prognostic scores (CLIF-C OF, CLIF-SOFA, CLIF-C AD, CLIF-C ACLF) in the prediction of in-hospital mortality.
Results
Patients were mostly males (71.1%) with a mean age of 53.9 ± 12.8 years. The etiology of pre-existing liver disease was HCV in 57.8%. Sepsis was the most common precipitating factor (49.8%) and the mortality rate was 74.3%. In univariate analysis, all scores were significantly higher in the deceased group (P<0.0001). AUROC were 0.897, 0.884, 0.870, 0.861, 0.861, and 0.850 for CLIF-C OF, CLIF-C AD, CLIF-C ACLF, Child-Pugh, CLIF-SOFA, and MELD scores, respectively. In multivariate analysis, 2 independent predictors of mortality were identified: CLIF-C ACLF score (OR 3.25, 95% CI 1.03–10.25, P<0.0001) and Child-Pugh class C (OR 1.04, 95% CI 1.02–1.06, P=0.044).
Conclusions
All the studied scores could predict in-hospital mortality of patients with ACLF. However, CLIF-C ACLF and Child-Pugh class performed better as they could significantly and independently predict mortality.
Similar content being viewed by others
Background
Acute-on-chronic liver failure (ACLF) is a distinct syndrome that occurs in patients with chronic liver disease, with or without cirrhosis, characterized by acute decompensation of the liver (ascites, encephalopathy, gastrointestinal bleeding, and/or bacterial infection) and one or more extrahepatic organ dysfunction (kidney, brain, coagulation, circulation, and/or lung), with a high short-term mortality of 33% at 28 days and 51% at 90 days [1].
Even though many definitions for ACLF have been evolved, the most important definitions in clinical practice are from the Asian Pacific Association for the Study of Liver (APASL), American Association for the Study of Liver (AASLD), European Association for the Study of Liver (EASL), and World Gastroenterology Organization (WGO) [2].
In the majority of patients, ACLF is precipitated by an acute event, which provides an inflammatory burst to the background chronic inflammation that is present in patients with cirrhosis and decompensation. The resulting surge in inflammatory mediators leads to organ failure through many mechanisms including organ hypoperfusion [3]. However, in up 40% of patients with ACLF, no acute event can be identified prior to the development of ACLF [1]. ACLF is associated with features of systemic inflammation. Excessive release of pro-inflammatory cytokines and chemokines “cytokine storm” by the patient’s immune system seems to be the key operating mechanism responsible for tissue damage and organ failure [4, 5].
Compared to ACLF, decompensated cirrhosis without ACLF lacks organ failure. Organ failure could be defined by the significantly impaired function of the liver, kidneys, brain, coagulation, and circulatory and respiratory systems which could predict mortality [6].
In spite of this catastrophic presentation and outcome, there is a component of potential reversibility with adequate support and management of the precipitating factor [7].
A universally accepted prognostic model for ACLF is lacking due to discrepancies and unevenness in the definition of ACLF. Many already widely used prognostic models for cirrhosis have been applied for the evaluation of this syndrome [8,9,10]. In this regard, prognosis scores can be categorized in two: first that evaluates the severity of liver dysfunction (Child-Pugh, Model of end-stage liver disease “MELD”) [11] and second, global prognostic scores (Acute Physiology and Chronic Health Evaluation “APACHE II” [12, 13] and sequential organ failure assessment “SOFA”) [14, 15]. Although several lines of evidence demonstrate that global prognostic scores are superior to liver-specific scores for estimation of prognosis in these patients, the optimum scores with the highest performance have not been enough explored yet.
In the current study, we aimed to compare the currently available prognostic scores to predict short-term mortality to early identify patients at high risk who will require specific treatments, intensive management, or emergency liver transplantation.
Methods
Study setting and inclusion
The current study was carried out on all patients who met the definition of ACLF according to the EASL-Chronic Liver Failure consortium (EASL-CLIF) and who were admitted to the National Liver Institute hospital in the period between April 1, 2018, and March 31, 2019. Inclusion criteria were patients with stable pre-existing liver disease who developed acute hepatic decompensation (hepatic encephalopathy, variceal hemorrhage, large ascites, bacterial infections, or any combination of these) after exposure to an identifiable or non-identifiable acute insult and associated with organ(s) failure (liver, kidney, brain, circulatory, coagulation, or respiratory failure).
Definitions
Like CANONIC study, organ failure was defined as the following: liver failure: hyperbilirubinemia of ≥ 12.0 mg/dl; renal failure: serum creatinine level of ≥ 2 mg/dl; brain failure: hepatic encephalopathy grade III/IV as per West Haven criteria; coagulation failure: international normalized ratio (INR) > 2.5 and/or a platelet count ≤ 20 × 109 /L; circulatory failure: the use of dopamine, dobutamine, or terlipressin; and respiratory failure: partial pressure of arterial oxygen (PaO2) to fraction of inspired oxygen (FiO2) ratio ≤ 200 or pulse oximetric saturation (SpO2) to FiO2 ratio ≤ 200 [1].
According to the CLIF-OF score, ACLF was graded into 3 grades: ACLF grade 1: patients with single kidney failure or non-renal single organ failure (liver, lung, coagulation, or circulatory) associated with renal dysfunction (creatinine 1.5–1.9 mg/dl) and/or brain dysfunction (grade 1 or 2 hepatic encephalopathy), ACLF grade 2: patients with two organ failures, and ACLF grade 3: patients with three or more organ failures.
Exclusion criteria
Patients with one or more of the following were excluded: prior organ transplantation, hepatocellular carcinoma (HCC), extrahepatic malignancies, and severe chronic extrahepatic diseases.
Workup
Included patients were subjected to thorough history taking and clinical examination, abdominal ultrasonography, routine laboratory investigations (CBC, liver and renal biochemical tests). Workup to identify the etiology of the acute liver insult causing ACLF was done. This panel included the routine markers for viral hepatitis and polymerase chain reactions (PCRs) for hepatitis viruses (HCV RNA, HEV RNA, HDV RNA, and HBV DNA) when the routine markers were negative. Patients who were negative for viral hepatitis were next tested for hepatitis auto-antibodies, including antinuclear antibody (ANA), anti-smooth muscle antibody (ASMA), anti-liver kidney microsomal antibody (LKM), and total IgG. Wilson’s disease workup was done if both virology and autoimmune profiles were negative, including ceruloplasmin and slit-lamp examination for Kayser-Fleisher ring. Roussel Uclaf Causality Assessment Method “RUCAM” scale was used when drug-induced liver injury “DILI” was suspected as a precipitating insult [16]. On admission, hepatic encephalopathy was diagnosed and graded using the West Haven criteria [17]. Data were collected and the following scores were calculated: Child-Pugh [18], MELD [19], CLIF-consortium organ failure (CLIF-C OF) [1], CLIF-C ACLF [20], CLIF-SOFA [15], and CLIF-C acute decompensation (CLIF-C AD) [21]. Patients were prospectively followed up for in-hospital survival outcome and were categorized into two groups: deceased and improved. Predictors of mortality were statistically analyzed.
A written informed consent was obtained from each eligible patient or his relatives before inclusion. The study protocol was consistent with the ethical principles of the Declaration of Helsinki (1975) and has been approved by the local Institutional Review Board of the National Liver Institute, Menoufia University.
Statistical methods
A univariate analysis of mortality was performed for baseline variables and scores using the chi-square (or if appropriate, Fisher’s exact) test and independent samples t-test for categorical and quantitative variables, respectively. A multivariate analysis of the significant factors related to mortality, from the univariate analysis, was carried out with a backward stepwise Cox regression approach to identify those variables that independently predicted mortality. The discriminative ability of the liver-specific (Child-Pugh and MELD) and ACLF prognostic scores (CLIF-C OF, CLIF-SOFA and CLIF-C AD, ACLF-C ACLF) and ACLD grades at baseline was evaluated using the area under a receiver operating characteristic (ROC) curve (AUROC). Significance was tested two-sided and set to a P-value of less than 0.05. Statistical analyses were performed using IBM SPSS Statistics for Macintosh, version 22.0 (IBM Corp, Armonk, NY, USA).
Results
Baseline characteristics of the studied patients
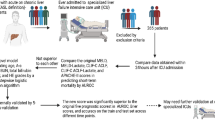
Two hundred eighty-three patients were hospitalized with ACLF in the period between 1 April 2018 and 31 March 2019. Among them, only 249 patients fulfilled the inclusion and exclusion criteria and were included in the present study. Figure 1 represents the flowchart of the study.
The baseline characteristics are presented in Tables 1 and 2. Most of the patients were males (n = 177, 71%) with a mean age of 53.9 ± 12.8 years. The main underlying etiology of chronic liver disease was HCV (n = 144, 57.8%). Sepsis was the main precipitating factor of ACLF (n=124, 49.8%), whereas the precipitating factor could not be identified in 30.9% of patients (n=77). Spontaneous bacterial peritonitis (SBP) was the most common type of infection (n=43, 34.7%). Chest infection came as the second most common infection (n=38, 30.6%) followed by urinary tract infection (n=14, 11.3%). Cellulitis was reported in 3 patients (2.4%) while the site of infection could not be determined in 26 patients (21%). On admission, the mean Child-Pugh, MELD, CLIF-C AD, CLIF-C ACLF and CLIF-C OF scores were 11.8 ± 1.5 (mostly class C, 92.8%), 17.3 ± 4.3, 70.9 ± 14.2, 52.4 ± 9.8 and 10.6 ± 2.0 respectively. ACLF grades of the studied patients were as follows: grade 1: n=89, 35.7%; grade 2: 109, 43.8%; and grade 3: 51, 20.2%. As regards ACLF grade, 89 patients (35.7%) were ACLF grade 1, 109 (43.8%) were ACLF grade 2 whereas 51 (20.2%) were ACLF grade 3. Most of the patients needed an initial admission to the ICU (n=191, 76.7%). The mean total stay in the ICU was 7.8 ± 5.6 days and in the hospital was 11.9 ± 7.7 days. At discharge, 185 (74.3%) patients were deceased.
Univariate analysis of in-hospital mortality
The results of the univariate analysis of variables associated with in-hospital mortality are presented in Tables 3 and 4. Deceased patients were significantly older (55.0 vs. 50.7 years, P=0.022) and had significantly lower baseline albumin (2.3 vs. 2.7 g/dl, P<0.0001), platelets (134.5 vs. 163.9 × 103/mm3, P=0.034), Na (123.7 vs. 132.4 mEq/l, P<0.0001), and mean arterial pressure (MAP, 70.4 vs. 79.7 mmHg, P<0.0001). They also had significantly higher baseline INR (2.2 vs. 1.7, P<0.0001), creatinine (3.2 vs. 1.4 mg/dl, P<0.0001), and peripheral leucocytes (15.2 vs. 11.3 × 103/mm3, P<0.0001). The total ICU stay was significantly shorter in patients who died at discharge (6.8 vs. 10.8 days, P<0.0001). However, the total hospital stay did not differ between both groups (P=0.937).
All the studied scores were significantly (P<0.0001) higher in the deceased group, including the Child-Pugh score and class, ACLF grade, and the MELD, CLIF-C AD, CLIF-C OF, CLIF-SOFA, and CLIF-C ACLF scores.
While most of the patients who improved had no ascites (58.7%) or hepatic encephalopathy (88.9%), most of the deceased patients had mild to moderate ascites (70.8%) and grade I–II hepatic encephalopathy (62.7%) (P<0.0001).
It is to be noted that the gender and etiology of chronic liver disease, total bilirubin, ALT, AST, ALP, GGT, and hemoglobin did not show a statistically significant difference between both groups.
Ability of the studied scores to predict in-hospital mortality
After plotting the ROC curves (Fig. 2), all the studied scores were found to significantly predict in-hospital mortality (P<0.0001). AUROC were 0.897, 0.884, 0.870, 0.861, 0.861, and 0.850 for CLIF-C OF, CLIF-C AD, CLIF-C ACLF, Child-Pugh, CLIF-SOFA, and MELD scores, respectively. In addition, ACLF grade as well as Child-Pugh class (C vs. B) was found to significantly predict mortality (AUROC =0.820, 0.611 and P < 0.0001, 0.009, respectively).
ROC curves of the studied scores in prediction of in-hospital mortality. Legend: ACLF, acute-on-chronic liver failure; AD, acute decompensation; CI, confidence interval; CLIF-C, chronic liver failure consortium; MELD, model for end-stage liver disease; OF, organ failure; SOFA, Sequential Organ Failure Assessment
Regression analysis for prediction of in-hospital mortality
Using the backward Cox regression, two independent variables were deduced to significantly predict in-hospital mortality (Table 5). Those significant predictors were the CLIF-C ACLF score (OR 3.25, 95% CI 1.03–10.25, P<0.0001) and the Child-Pugh class C vs. B (OR 1.04, 95% CI 1.02–1.06, P=0.044).
Discussion
ACLF is a serious condition associated with a high mortality rate which is 15 times higher as compared to patients with acute decompensation without ACLF [1].
Therefore, it is critical to stratify patients according to prognosis in order to monitor treatment responsiveness, determine emergency for transplantation, and decide allocation in the ICU.
In the present study, we have compared the performance of the conventional liver-specific scores (Child-Pugh and MELD) to the widely used international prognostic scores (CLIF-C OF, CLIF-C ACLF, CLIF-SOFA, and CLIF-C AD) in the prediction of in-hospital mortality of patients with ACLF.
Baseline characteristics of the studied patients
The age and gender of the studied patients were comparable to that of the CANONIC study, mean age of 53.9 ± 12.8 vs. 56.0 ± 11.0 years, and 71.1% males vs. 64.4%, respectively [1]. Meanwhile, our studied patients were older than those in the study by Dihman et al., who reported a mean age of 46.0 ± 13.0 years [22].
In our study, HCV was the most common cause of chronic liver disease (57.8%). This is consistent with the fact that Egypt has the highest HCV prevalence in the world, which represents the main etiology of chronic liver disease among the Egyptian population [23]. This figure is higher than that reported by Moreau et al. (13% for HCV and 9% for HCV and alcohol) [1] and Dihman et al. (10% for HCV with alcohol) [22]. In both studies, alcohol was the main etiology of chronic liver disease (60.3% and 58%, respectively). Other identifiable etiologies of the pre-existing liver disease in our study came with variable degrees of agreement with others reported in the literature. Hepatitis B was similar (5.2%) to the Dihman et al. study (6%). Autoimmune hepatitis was higher in the Dihman et al. study (6%) compared to ours (3.6%). The etiology could not be identified in 32.1% patients in our study compared to 14% in the study by Dihman et al. However, cryptogenic cirrhosis is the second most common etiology in both studies. We believe that the high rate of unidentifiable etiology of the pre-existing cirrhosis in our study could be referred considerably to nonalcoholic fatty liver disease, an important condition for which we could not stand on a confirmed diagnosis at such a late stage of cirrhosis. Conclusively, these differences in the etiologic profile of cirrhosis in ACLF reflect the etiology of cirrhosis in the respective countries. Alcoholic cirrhosis constitutes 50–70% of all the underlying liver diseases of ACLF in the western countries, whereas viral hepatitis-related cirrhosis constitutes about 10–15% of all the cases [24,25,26]. However, in most of the Asian countries, HBV constitutes 70% and alcohol only about 15% of all the etiologies [27]. In contrast, HCV-related cirrhosis constitutes the majority in Egyptian patients [23].
In the same stream, the acute insult precipitating ACLF was variable among different studies. In our cohort, sepsis represented the most common precipitating factor (49.8%). We have no definite explanation for such a high rate. However, this might be attributed to the immune derangement commonly found in patients with advanced stages of cirrhosis, which makes them more prone to bacterial infections. Similarly, Dhiman et al. reported that bacterial infections represented 66% of the ACLF precipitating factors [22]. The CANONIC study reported a lower rate of bacterial infections (32.6%) [1]. It is to be noted that the APASL definition does not include infection/sepsis as the acute precipitating event of ACLF [28].
Acute GI bleeding represented the second most common precipitating factor in our study (9.2%). Negligence, missing variceal screening programs, and/or non-adherence to portal pressure decompressing medications represented the main factors associated with acute variceal hemorrhage in this group of patients. Moreau et al. reported a comparable rate of variceal hemorrhage (13.2%) [1]. Meanwhile, Dhiman et al. reported a lower rate of 4% [22]. It is noteworthy that both studies reported a high proportion of active alcoholism (40% and 24.5%, respectively), which was not encountered in any of our studied patients. Dihman et al. also reported a higher incidence of autoimmune hepatitis flares (8%) compared to our rate of exacerbation (3.2%) and a higher rate of HEV (2%) compared to ours (0.4%) [22]. No precipitating factor could be identified in 30.9% of the patients in our study, which is lower than the figure reported by Moreau et al. (43.6%) [1].
Risk factors for mortality
The 28-day mortality in ACLF ranged between 30 and 40% [6]. The estimated global 90-day mortality was 58%, with some relative regional variations. South America had the highest rate (73%), followed by South Asia (68%) [29]. The reported mortality rate in our study was 74.3%. These variations might be attributed to the variance in the definition of ACLF and guidelines used in these different areas, the heterogeneity of patients’ characteristics and ethnicities, and the relative variation in the reversibility of the acute precipitating insult besides the ACLF grade. Another important point that could influence the mortality rate is the availability of salvage liver transplantation for patients who develop progressive irreversible deterioration. The candidacy for liver transplantation becomes more sophisticated and perplexing when patients develop intractable sepsis, a relatively common condition that could contraindicate liver transplantation. In addition, liver transplantation would be declined for patients who develop kidney failure, the most common organ failure in ACLF, unless a combined liver-kidney transplant is available. Adding to that, most patients with advanced ACLF grade are not sufficiently stable to undergo liver transplantation. Indeed, all these factors collectively could influence the mortality rates among different studies and regions. Unfortunately, many of these factors have been reported in many of our patients, including intractable sepsis, advanced ACLF grade, donor unavailability, and improper conditions for receiving liver transplants. This could explain the higher mortality rate disclosed in our cohort.
In univariate analysis, the Child-Pugh score and class, CLIF-AD grades, and the MELD, CLIF-C AD, CLIF-C OF, CLIF-SOFA, and CLIF-ACLF scores were significantly worse in patients who were deceased at discharge.
Age significantly predicted mortality in our study as well as in five previous studies [30,31,32,33,34]. Albumin was significantly lower in the deceased group (2.3 vs. 2.7, P<0.0001). This is similar to the finding by Sun et al. (2.8 vs. 3.1 P<0.001) [34]. In our study, the white blood cell count was significantly higher in the deceased group (11.3 vs. 15.2, P<0.0001). The same was reported in the study by Sun et al.; survivors had a significantly lower WBCs count (6.7 vs. 8.1, P=0.036) [34]. We noted that platelets were significantly higher in the group of survivors. This finding was reported in four previous studies [34,35,36,37]. In the current study, INR was significantly lower in the group of survivors. This was also reported in four previous studies [34, 37,38,39]. It is noteworthy that although bilirubin is an important component of these scores, it did not show statistical significance between both groups. This finding was replicated in six previous studies [34,35,36,37,38, 40].
It is also to be noted that the total stay in ICU was significantly shorter in the deceased group (6.8 vs. 10.8, P<0.0001) and the total hospital stay was not statistically significant regarding mortality.
Multivariate analysis, using Cox regression, revealed that Child-Pugh (class C vs. B) and CLIF-C ACLF scores significantly and independently predict mortality. In the study by Jalan et al., CLIF-C ACLF was superior to MELD and Child-Pugh scores in predicting mortality in patients with ACLF in the validation database, with a higher c-statistics (0.744 vs. 0.645 vs. 0.653, respectively) [20]. We also found that the AUROC for the CLIF-C ACLF was larger than that of Child-Pugh and MELD scores (0.870 vs. 0.850 vs. 0.861, respectively). The Child-Pugh class had a smaller AUROC (0.611). When using Cox regression and including time to mortality, which adds to the accuracy of testing the discriminating ability, the CLIF-C ACLF had a higher odds ratio as compared to the Child-Pugh class C vs. B (3.25 vs. 1.04).
The limitations of the current study include that it was a single-center study. The high proportion of patients with HCV-related liver cirrhosis could hinder the generalization of the results. However, it adds to the spectrum of ACLF studies with other etiologies of chronic liver disease and strengthens the concept that cirrhosis is one of the baseline hallmarks of ACLF regardless of its etiology. In addition, the large sample size represents an important strength point.
Conclusions
In conclusion, all the liver-specific and ACLF-specific scores could significantly predict in-hospital mortality of patients with ACLF. However, CLIF-C ACLF and Child-Pugh class C were superior to other scores as they could significantly and independently predict in-hospital mortality.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AASLD:
-
American Association for the Study of Liver
- ACLF:
-
Acute-on-chronic liver failure
- AD:
-
Acute decompensation
- AIH:
-
Autoimmune hepatitis
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- ANA:
-
Antinuclear antibody
- APASL:
-
Asian Pacific Association for the Study of Liver
- ASMA:
-
Anti-smooth muscle antibody
- AST:
-
Aspartate aminotransferase
- AUC:
-
Area under the ROC curve
- BP:
-
Blood pressure
- CLD:
-
Chronic liver disease
- CLIF-C:
-
Chronic liver failure consortium
- CP:
-
Child-Pugh
- DILI:
-
Drug-induced liver injury
- EASL:
-
European Association for the Study of Liver
- FiO2:
-
Fraction of inspired oxygen
- GGT:
-
Gamma-glutamyl transferase
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HDV:
-
Hepatitis D virus
- HEV:
-
Hepatitis E virus
- ICU:
-
Intensive care unit
- INR:
-
International normalized ratio
- LKM:
-
Anti-liver kidney microsomal antibody
- MAP:
-
Mean arterial pressure
- MELD:
-
Model for end-stage liver disease
- Na:
-
Sodium
- OF:
-
Organ failure
- PaO2:
-
Partial pressure of arterial oxygen
- ROC:
-
Receiver operating characteristic curve
- RUCAM:
-
Roussel Uclaf Causality Assessment Method
- SOFA:
-
Sequential Organ Failure Assessment
- WBCs:
-
White blood cells
- WGO:
-
World Gastroenterology Organization
References
Moreau R, Jalan R, Gines P et al (2013) Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144(7):1426–1437
Jalan R, Yurdaydin C, Bajaj JS et al (2014) Toward an improved definition of acute-on-chronic liver failure. Gastroenterology 147:4–10
Moreau R (2016) The pathogenesis of ACLF: the inflammatory response and immune function. Semin Liver Dis 36(2):133–140
Solé C, Solà E, Morales-Ruiz M et al (2016) Characterization of inflammatory response in acute-on-chronic liver failure and relationship with prognosis. Sci Rep 6:32341
Choudhury A, Kumar M, Sharma BC et al (2017) Systemic inflammatory response syndrome in acute on chronic liver failure - relevance of ‘Golden Window’—a prospective study. J Gastroenterol Hepatol 32(12):1989–1997
Arroyo V, Moreau R, Jalan R, Ginès P (2015) Acute-on-chronic liver failure: a new syndrome that will re-classify cirrhosis. J Hepatol 62(1 Suppl):S131–S143
Gustot T, Fernandez J, García E et al (2014) Short-term (28-day) clinical course and transplant-free mortality in acute-on-chronic liver failure (ACLF): evidence for reversibility of ACLF (a study from the CANONIC database). J Hepatol 60:S228
Silva P, Fayad L, Lazzarotto C et al (2015) Single-centre validation of the EASL-CLIF Consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int 35:1516–1523
Gao F, Sun L, Ye X et al (2017) Development and validation of a prognostic model for acute-on-chronic hepatitis B liver failure. Eur J Gastroenterol Hepatol 29(6):669–678
Yi ZQ, Lu MH, Xu XW et al (2015) A novel prognostic score for acute-on-chronic hepatitis B liver failure. J Huazhong Univ Sci Technol 35:87–92
Mookerjee RP (2016) Prognosis and biomarkers in acute-on-chronic liver failure. Semin Liver Dis 36(02):127–132
Karvellas CJ, Pink F, McPhail M et al (2010) Bacteremia, acute physiology and chronic health evaluation II and modified end stage liver disease are independent predictors of mortality in critically ill non transplanted patients with acute on chronic liver failure. Crit Care Med 38:121–126
Duseja A, Choudhary NS, Gupta S, Dhiman RK, Chawla Y (2013) APACHE II score is superior to SOFA, CTP and MELD in predicting the short-term mortality in patients with acute-on-chronic liver failure (ACLF). J Dig Dis 14(9):484–490
Zhang Y, Nie Y, Liu L, Zhu X (2020) Assessing the prognostic scores for the prediction of the mortality of patients with acute-on-chronic liver failure: a retrospective study. PeerJ 8:e9857
Grochot RM, Luz LB, Garcia R et al (2019) CLIF-SOFA is superior to other liver-specific scores for predicting mortality in acute-on-chronic liver failure and decompensated cirrhosis. Austin J Gastroenterol 6(2):1105
Rockey DC, Seeff LB, Rochon J et al (2010) Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. US Drug-Induced Liver Injury Network. Hepatology 51(6):2117–2126
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT (2002) Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 35(3):716–721
Pugh RN, Murray-Lyon IM, Dawson JL et al (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649
Kamath PS, Kim WR (2007) The model for end-stage liver disease (MELD). Hepatology 45(3):797–805
Jalan R, Saliba F, Pavesi M et al (2014) Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 61:1038–1047
Jalan R, Pavesi M, Saliba F et al (2015) The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol 62:831–840
Dhiman RK, Agrawal S, Gupta T et al (2014) Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol 20:14934–14941
Gomaa A, Allam N, Elsharkawy A, El Kassas M, Waked I (2017) Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med 9:17–25
Mann RE, Smart RG, Govoni R (2003) The epidemiology of alcoholic liver disease. Alcohol Res Health 27(3):209–219
Lucey MR, Mathurin P et al (2009) Alcoholic hepatitis. N Engl J Med 360:2758–2769
Louvet A, Mathurin P (2015) Alcoholic liver disease: mechanisms of injury and targeted treatment. Nat Rev Gastroenterol Hepatol 12(4):231–242
Custer B, Sullivan SD, Hazlet TK, Iloeje U, Veenstra DL, Kowdley KV (2004) Global epidemiology of hepatitis B virus. J Clin Gastroenterol 38(10 Suppl 3):S158–S168
Sarin SK, Kedarisetty CK, Abbas Z et al (2014) Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int 8:453–471
Mezzano G, Juanola A, Cardenas A et al (2022) Global burden of disease: acute-on-chronic liver failure, a systematic review and meta-analysis. Gut 71(1):148–155
Yang S-S, Cheng K-S, Lai Y-C et al (2002) Decreasing serum alpha-fetoprotein levels in predicting poor prognosis of acute hepatic failure in patients with chronic hepatitis B. J Gastroenterol 37:626–632
Yu JW, Wang GQ, Li SC (2006) Prediction of the prognosis in patients with acute-on-chronic hepatitis using the MELD scoring system. J Gastroenterol Hepatol 21:1519–1524
Huang K, Hu JH, Wang HF et al (2011) Survival and prognostic factors in hepatitis B virus-related acute-on-chronic liver failure. World J Gastroenterol 17:3448–3452
Zhihui X, Xiaoqiang R, Yan L et al (2011) Association of hepatitis B virus mutations in basal core promoter and precore regions with severity of liver disease: an investigation of 793 Chinese patients with mild and severe chronic hepatitis B and acute-on-chronic liver failure. J Gastroenterol 46:391–400
Sun Q-F, Ding J-G, Xu D-Z et al (2009) Prediction of the prognosis of patients with acute-on-chronic hepatitis B liver failure using the model for end-stage liver disease scoring system and a novel logistic regression model. J Viral Hepat 16:464–470
Garg H, Sarin SK, Kumar M et al (2011) Tenofovir improves the outcome in patients with spontaneous reactivation of hepatitis B presenting as acute-on-chronic liver failure. Hepatology 53:774–780
Zheng MH, Shi KQ, Fan YC et al (2011) A model to determine 3-month mortality risk in patients with acute-on-chronic hepatitis B liver failure. Clin Gastroenterol Hepatol 9:351–356
Garg H, Kumar A, Garg V et al (2012) Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis 44:166–171
Katoonizadeh A, Laleman W, Verslype C et al (2010) Early features of acute-on-chronic alcoholic liver failure: a prospective cohort study. Gut 59:1561–1569
Krishna YR, Saraswat VA, Das K et al (2009) Clinical features and predictors of outcome in acute hepatitis A and hepatitis E virus hepatitis on cirrhosis. Liver Int 29:392–398
Zhai S, Zhang L, Dang S et al (2011) The ratio of Th-17 to Treg cells is associated with survival of patients with acute-on-chronic hepatitis B liver failure. Viral Immunol 24:303–310
Acknowledgements
We would like to sincerely acknowledge physicians, nurses, and all colleagues working in the emergency, ward, and ICU departments, National Liver Institute, Menoufia University, for their kind cooperation to achieve this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AW was the one who put the research idea. AM and ZT shared in the study design. SS and EM were assigned to data collection and processing. AW, AM, and ZT contributed in the data analysis and interpretation. EM and SS prepared the literature review. ZT wrote the manuscript. AW and AM revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study protocol was approved by the local Institutional Review Board of National Liver Institute, Menoufia University, Egypt. A signed written informed consent was obtained from all patients or their relatives before participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zakareya, T., Akl, M., Shibl, S. et al. Utility of prognostic scores in predicting short-term mortality in patients with acute-on-chronic liver failure. Egypt Liver Journal 12, 21 (2022). https://doi.org/10.1186/s43066-022-00183-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-022-00183-2