Abstract
Background
Liver cirrhosis (LC) is the most common cause of portal hypertension. In chronic hepatitis C patients who are treated with direct-acting antiviral therapy (DAAS), the progression of cirrhosis can be reversed with treatment. Portal hypertension is also expected to improve with a virological response.
Aim
To evaluate the effect of direct-acting antiviral therapy on portal circulation hemodynamics in cirrhotic patients infected with HCV.
Methods
This study included 78 consecutive patients with chronic HCV-related liver disease. They were treated by a sofosbuvir-based regimen in combination with daclatasavir. All patients were subjected to routine investigations (complete blood count, liver and renal function tests), hepatitis B surface antigen, α feto protein, PCR of HCV RNA, imaging (abdominal ultrasound and colored Doppler and duplex examination for the assessment portal hypertension) before starting treatment and after 1 year.
Results
There was a significant improvement in Doppler parameters such as portal vein (PV) diameter, PV velocity, PV cross-sectional area, portal congestive index, splenic vein diameter, and spleen span; the decrease in portal pressure occur in about 55% of the patients; several factors are associated with non-response as a history of bilharziasis, patients from a rural area, presence of splenomegaly and varices, low HB level, low platelet count, and high level of fibrosis.
Conclusion
Sustained virological response to direct-acting antiviral therapy is associated with a reduction in portal pressure in patients with liver cirrhosis and clinically significant portal hypertension.
Similar content being viewed by others
Introduction
There are a lot of reviews published on the prevalence of hepatitis C virus (HCV) all over the world. It is estimated that 13–14% of all HCV infections are genotype4 (G4) [1]; the majority of G4 presents in Egypt, Northern Africa, the Middle East, and Sub-Saharan Africa [2]. Egypt has one of the highest burdens of chronic HCV infections globally; it is estimated that the prevalence of HCV in our country is around 4.5 to 6.7% [3, 4]. Chronic HCV is a leading cause of mortality and morbidity worldwide due to complications of liver cirrhosis (LC), hepatocellular carcinoma (HCC), and portal hypertension [5].
LC is the most common cause of portal hypertension, and variceal bleeding is considered the most serious complication of portal hypertension [6]. Initially, portal pressure increases as a consequence of increased resistance to blood flow due to the formation of regenerative nodules and fibrous tissue [7]. Also, there is intrahepatic vasoconstriction which accounts for 25% of the increased intrahepatic resistance, and mostly, that is due to a decrease in the intrahepatic production of vasodilators substance as nitric oxide [7].
Portal hypertension in LC can be treated with many drugs such as beta blockers but without any regression in the underlying pathology, and portal hypertension may be increased with time [8, 9]. Direct antiviral agents (DAAS) are very effective, safe, and changing the burden and prognosis of the disease [3]. Sustained virological response (SVR) is achieved in more than 95% of the chronic HCV patients and is associated with regression in the underlying pathology, improvement in liver function, fibrosis, and overall survival [9]. Also, portal hypertension is predicted to be improved with virological response, according to the improvement in liver fibrosis and inflammation [10]. In chronic hepatitis C patients treated with DAAS, the progression of cirrhosis can be reversed with treatment [11, 12]. Elimination of the infection allows the liver to start a slow process of renewal but within limits, as this process may not happen in patients with advanced stages of liver cirrhosis [13, 14].
Doppler ultrasonography (US) helps us to examine the hemodynamics of the abdominal vessels including portal circulation. Thus, many investigators have confirmed the usefulness of abdominal Doppler US in assessing portal pressure in cirrhotic patients. It would be highly preferred to have any Doppler parameter to be a suitable alternative for the current invasive gold standard of measuring hepatic venous pressure gradient (HVPG) for assessing portal hypertension [15].
Doppler ultrasonography has an advantage of being non-invasive; so, many parameters have been made to evaluate the hemodynamic changes in patients with liver cirrhosis and the response to medical treatment of portal hypertension [16]. Doppler parameters, which have been commonly used for assessing portal hypertension, include the measurement of portal and splenic venous blood flows and velocity and the pulsatility and resistive index at hepatic, superior mesenteric, splenic, and renal artery [15, 16]. Doppler ultrasonography has been widely used to measure the portal blood flow velocity before and after DAAS administration in cirrhotic patients infected with HCV as a non-invasive, and easy-to-perform diagnostic imaging tool and to identify the impact of treatment on the portal circulation [16, 17].
We aim to evaluate the effect of direct-acting antiviral therapy on portal circulation hemodynamics in cirrhotic patients infected with HCV
Subjects and methods
This prospective observational study involved 78 consecutive patients with chronic HCV-related liver disease among those attending virology clinics, Tropical Medicine Department in El-Minia University Hospital, Minia, Egypt.
One of the inclusion criteria is patients with chronic HCV infection and liver cirrhosis.
The exclusion criteria are patients with hepatocellular carcinoma and any autoimmune diseases and very advanced patients (Child-Pugh score= 8–15).
All patients were treated by a sofosbuvir-based regimen in combination with daclatasavir with or without ribavirin, in the period from March 2018 to September 2019. Ten patients were excluded from the study due to lost to follow-up, and eight patients had not completed the treatment period.
All patients were subjected to the following (before starting the treatment and after 1 year of the end of treatment):
-
1.
Full history taking stressing on alcohol intake, smoking, chronic drug use, diabetes mellitus, rheumatological diseases, history of chronic liver disease, and history of bilharziasis
-
2.
Clinical and local examination
-
3.
Laboratory tests:
-
(a)
Routine investigations: complete blood count, liver function tests {serum bilirubin, serum albumin, transaminases (ALT, AST), serum alkaline phosphatase, prothrombine time and concentration}, kidney function tests, and fasting and postprandial blood glucose.
-
(b)
Hepatitis B surface antigen (HBs Ag)
-
(c)
α Feto protein
-
(d)
Serum albumin and ascitic gradient (SAAG) in ascitic patients only
-
(e)
Anti-bilharizial antibodies.
-
(f)
PCR of HCV RNA
-
(a)
-
4.
Imaging: abdominal ultrasound for assessment of liver, spleen, and any focal lesions and degree of ascites
-
(a)
Colored Doppler and duplex examination for the assessment of portal hypertension; the following parameters were evaluated: portal vein (PV) diameter (mm), portal vein velocity (cm/s), PV cross-sectional area (cm2), portal congestive index as the ratio between PV cross-sectional area (cm2) to portal vein velocity (cm/s.), splenic vein diameter (mm), and spleen span (cm)
-
(a)
Written and informed consent was obtained from all subjects. The Local Ethics Committee for human subject research reviewed and approved the study protocol and consent forms. The collected data were inserted, tabulated, and statistically anatomized using the Statistical Package for Social Sciences program (SPSS) software version 24. Quantitative data were expressed as mean ± standard deviation (SD), while qualitative data were expressed as proportions. Comparisons between the groups for normally distributed quantitative data were performed by the Mann-Whitney test between the two groups. Qualitative data were analyzed by the chi-square (χ2) test. Statistical significance was defined as P values less than 0.05.
Results
Demographic and laboratory data for the studied group were shown in Table 1. The age of patients is ranging from 44 to 67 years, its mean is 57, and male to female ratio was 80% versus 20%, about 50% of patients are from rural areas versus 50% from urban areas; in the studied group, 12 of the patients had ascites (20%), 21 patients had splenomegaly (35%), 15 of patients had a history of bilharziasis (35%), and 21 patients had a history of varices and done follow-up upper endoscopy (35%).
Laboratory changes before and after treatment for the studied group were shown in Table 2; there was a statistically significant improvement in laboratory parameters of patients regarding ALT, AST, albumin, INR, prothrombin concentration, and platelet count (P = 0.001); also, there was a statistically significant improvement in the fibrosis parameters of the patients such as the AST to platelet ratio index (APRI) test and FIB4 (fibrosis 4 score) (P = 0.001).
Response to treatment is shown in Table 3. The rate of sustained virological response was 95%; only 3 patients were non-responsive to DAAS therapy, and their PCR is still positive, and reduction in the portal pressure occurred in about 33 patients (55%).
Doppler changes before and after treatment for the studied group are shown in Table 4. There was a statistically significant difference in Doppler parameters of the patients before and after treatment regarding portal vein (PV) diameter, PV cross-sectional area (CSA), portal congestive index (PCI), spleen span, and splenic vein (SV) diameter (P = 0.001, P = 0.004, P = 0.006, P = 0.004, P = 0.001).
Logistic regression analysis of factors predicting the non-changes in the portal circulation hemodynamics after antiviral therapy is shown in Table 5; there were different factors associated with non-improvement in the portal parameters such as a history of Bilharzias, patients from rural areas, presence of splenomegaly and varices, low HB level, low platelet count, impaired INR, high level of FIB4 and APRI test, and Doppler indices (CSA, PV diameter, PV velocity) (P < 0.05).
Discussion
The current study was designed to evaluate the effect of direct-acting antiviral therapy on portal circulation hemodynamics in cirrhotic patients infected with HCV. We used colored Doppler and duplex examination for the assessment of portal pressure, before starting the DAAs and after 1 year from the end of treatment.
In the present study, there is a significant improvement in the liver functions and fibrosis parameters of the patients after treatment, the rate of SVR in our study was about 95%, and a similar result was obtained from Wyles et al. [18] who demonstrated high SVR more than 90% in chronic HCV patients receiving DAAS; also, Elkhayat et al. reported that in cirrhotic patients who received sofosbuvir-based regimen in combination with daclatasavir, the rates of SVR were high in both naive cirrhotic and previously treated patients (94% and 90.4%) [19]. The same results were obtained from Elnadry et al. [20] who showed that cirrhotic patients Child A and B who received sofosbuvir-based regimen in combination with daclatasavir with ribavirin had SVR more than 97%; our results are in agreement with those of Abdel-Razek and Waked, who reported that the combination of sofosbuvir and daclatasavir in a single oral dose resulted in an SVR in more than 97% after 12 weeks of treatment [21].
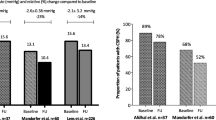
As regards portal hypertension parameters and Doppler changes before and after treatment, there was a significant improvement in Doppler parameters such as PV diameter, CSA, PCI, spleen span, and SV diameter; the decrease in portal pressure occurs in about 33 patients (55%), and several factors are associated with non-improvement in the portal parameters such as a history of bilharzias, patients from rural areas, presence of splenomegaly and varices, low HB level, low platelet count, and high level of fibrosis. Similar results were obtained from Sabela et al. [22] who assessed hepatic venous pressure gradient in large number of patients with liver cirrhosis, most of them with esophageal varices with or without previous clinical decompensation; the result shows that HVPG is significantly reduced when evaluated 24 weeks after achieving SVR with the use of interferon-free regimens more than 13% from baseline in 140 patients (62%). The presence of esophageal varices, low albumin and platelet levels, greater baseline HVPG, and a less marked reduction in fibrosis parameters after treatment were independent factors associated with non-reduction in HVPG in spite of successful antiviral therapy; also, in a previous study from those group [23], HVPG measurements performed in a small number of chronic HCV patients a median of 5 years after achieving SVR (with interferon-based antiviral treatment) showed further decreases of HVPG and reported that cirrhotic patients with clinically significant portal hypertension at baseline remain at risk for liver decompensation after 5 years, despite successful antiviral therapy. A similar reduction in HVPG was reported in a recent study by Mandorfer and collaborators [12]; however, patients included in this retrospective study presented less severe liver disease (only 41 of 60 patients had compensated liver cirrhosis at baseline and only 9 were Child B), and the time between the end of treatment and follow-up was not fixed, making the evaluation of the impact of treatment therapy on HVPG not true. The same results were obtained from Shigeo et al. [10] who assessed the usefulness of portal blood flow velocity analysis using pulse wave Doppler ultrasonography in evaluating the effectiveness of interferon-based antiviral treatment; the results of these study demonstrated that patients who had SVR showed a significantly higher portal blood flow velocity at the end of antiviral treatment than before treatment. A similar observation was made in a recent study including 33 cirrhotic patients treated with sofosbuvir with ribavirin, where 24% of the patients had a 20% reduction in HVPG at 48 weeks of follow-up [11].
Conclusion
Sustained virological response to direct-acting antiviral regimens is associated with a reduction in portal pressure in patients with liver cirrhosis and clinically significant portal hypertension. The main limitations of our study arise from a relatively small number of patients, and the long-term effects of direct-acting antiviral regimens on portal hypertension are not completely established, and further studies with longer follow-up may be needed.
Availability of data and materials
This published article contains all the knowledge produced or analyzed during this research.
References
Aly Abd Elrazek AE, Bilasy SE, Elbanna AE, et al (2014) Prior to the oral therapy, what do we know about HCV-4 in Egypt: a randomized survey of prevalence and risks using data mining computed analysis. Medicine (Baltimore) 93(28):e204.
Waked I, Doss W, El-Sayed MH et al (2014) The current and future disease burden of chronic hepatitis C virus infection in Egypt. Arab J Gastroenterol 15:45–52
World Health Organization (2016) Global health sector strategy on viral hepatitis 2016–2021. World Health Organization, Geneva. http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-eng.pdf;jsessionid=10F21056DC7293B9C437F10FF7465E60?sequence=1
World Bank (2017) Eliminating hepatitis C from Egypt: 2017 update on current trends and policy recommendations. The World Bank, Washington DC. http://documents.worldbank.org/curated/en/164381517333701631/pdf/123068 WP-P157533-PUBLIC-Eliminating-Hepatitis-C-from-Egypt-2017-Update.pdf
Gower E, Estes C, Blach S et al (2014) Global epidemiology and genotype the hepatitis C virus infection. J Hepatol 61(1 Suppl):S45–S57. https://doi.org/10.1016/j.jhep.2014.07.027
European Association for Study of Liver (2016) EASL recommendations on treatment of hepatitis C 2016. J Hepatol 64:S188.
Wiest R, Groszmann RJ (2000) Nitric oxide and portal hypertension: its role in the regulation of intrahepatic and splanchnic vascular resistance. Seminar Liver Dis 19:411–426
Calvaruso V et al (2018) The course of oesophagogastric varices in patients with cirrhosis after DAA-induced HCVclearance. The International Liver Congress, Paris, 2018, abstract FRI-376, 2018. J Hepatol 68:S533
Mandorfer M, et al (2018) Resolution of clinically significant portal hypertension after sustained virologic response to interferon-free regimens prevents hepatitis decompensation. The International Liver Congress, Paris, 2018, abstract FRI-380, 2018. J Hepatol 68:S532.
Nakanishi S, Shiraki K, Yamamoto K et al (2005) Hemodynamics in the portal vein evaluated by pulse wave Doppler ultrasonography in patients with chronic hepatitis C treated with interferon. World J Gastroenterol 11(3):396–399 World Journal of Gastroenterology ISSN 1007-9327
Afdhal N, Everson GT, Calleja JL et al (2017) Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat 24:823–831
Mandorfer M, Kozbial K, Schwabl P et al (2016) Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol Mandorfer J Hepatol 65:692–699
De Franchis R, Baveno VI, Faculty. (2015) Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 63:743–752
Afdhal N, Zeuzem S, Kwo P et al (2014) Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370:1889–1898
Walsh KM, Leen E, MacSween RN et al (1998) Hepatic blood flow changes in chronic hepatitis C measured by duplex Doppler color sonography: relationship to histological features. Dig Dis Sci 43:2584–2590
Reddy KR, Bourlière M, Sulkowski M et al (2015) Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: an integrated safety and efficacy analysis. Hepatology 62:79–86
Kutlu R, Karaman I, Akbulut A et al (2002) Quantitative Doppler evaluation of the splenoportal venous system in various stages of cirrhosis: differences between right and left portal veins. J Clin Ultrasound 30:537–543
Wyles D, Ruane PJ, Sulkowski M et al (2015) (K Sherman presenting). Daclatasvir plus sofosbuvir for treatment of HCV genotypes 1-4 in HIV-HCV coinfection: the ALLY-2 Study. Digestive Disease Week 6-19:901d
El-Khayat H, Fouad Y, Mohamed H et al (2018) Sofosbuvir plus daclatasvir with or without ribavirin in 551 patients with hepatitis C-related cirrhosis, genotype 4. Aliment Pharmacol Ther. 47:674–679. https://doi.org/10.1111/apt.14482
Elnadry MH, Abdel-Aziz SA, Ghareb M et al (2018) Impact of direct-acting antiviral therapy in Egyptian patients with chronic hep C and liver cirrhosis. Sci J Al-Azhar Med Faculty Girls 2:181–188
Abdel-Razek W, Waked I (2015) Optimal therapy in genotype 4 chronic hepatitis C: finally cured? LiverInt 35(Suppl1):27–34
Lens S, Alvarado-Tapias E, Mariño Z et al (2017) Effects of all-oral anti-viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus-associated cirrhosis gastroenterology. Gastroenterology 153:1273–1283
Lens S, Rincón D, García-Retortillo M et al (2015) Association between severe portal hypertension and risk of liver decompensation in patients with hepatitis C, regardless of response to antiviral therapy. Clin Gastroenterol Hepatol 13:1846–1853.e1
Acknowledgements
We would like to thank all the patients who participated in this work. I hope that with this and other studies, we can alleviate their sufferings.
Funding
No financial support
Author information
Authors and Affiliations
Contributions
WS was concerned with the design of the study. EA and AE were responsible for the statistical analysis. MS performed the PCR and laboratory parameters. MM was responsible for the radiological examination. AH analyzed the data and drafted the manuscript. All authors critically revised the manuscript, approved the final version to be published, and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed and written consent was obtained from all individual participants included in the study and also for the publication of the work. The Local Research Ethics Committee for human subject research reviewed and approved the research protocol and consent forms. All procedures performed in the study were in accordance with the ethical standards of the National Research Committee and with the 1975 Helsinki Declaration.
Consent for publication
Informed and written consent was obtained from all individual participants included in the study and also for the publication of the work.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassnine, A.A., Soliman, W., Elsayed, A.M. et al. Effect of direct-acting antiviral drugs on portal circulation hemodynamics in cirrhotic patients infected with HCV. Egypt Liver Journal 12, 17 (2022). https://doi.org/10.1186/s43066-022-00181-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-022-00181-4