Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is proved to be related to insulin resistance and type 2 diabetes, and it is also not rare in individuals without diabetes. The present study attempts to identify the metabolic risk factors of NAFLD among those individuals.
Results
ALT and HbA1c levels were independently associated with NAFLD development in individuals without diabetes. Receiver operating characteristic (ROC) analysis identified the optimal cutoff point of ALT (> 19 IU/ml) with AUC = 0.731, 95% CI 0.653–0.809. On the other hand, the optimal cutoff point of HbA1c was identified to be > 5.1% with AUC = 0.665, 95% CI 0.581–0.750.
Conclusions
Early identification of NAFLD among subjects without diabetes is crucial. In this study, ALT and HbA1c cutoff values had been identified, so we suggest that inclusion of both HbA1c and ALT levels may have significant implications for prediction of NAFLD among individuals without diabetes.
Similar content being viewed by others
Background
Nonalcoholic fatty liver disease (NAFLD) became a major epidemiological burden worldwide with a predictable global occurrence of 20–30% [1]. It includes a considerable variety of illnesses ranging from hepatic inflammation to fibrosis, cirrhosis, and/or liver cancer. Moreover, NAFLD is at present the most common cause of elevated liver enzymes [2]. The association between NAFLD, diabetes, and obesity owing to insulin resistance (IR) has been well proven [3, 4]. Glycosylated hemoglobin (HbA1c) is one of the advanced glycation end-products (AGEs), resulting from non-enzymatic glycation of hemoglobin. The level of HbA1c reflects the duration and severity of hyperglycemia in diabetic and non-diabetic individuals [5]. Although earlier studies showed a link between HbA1c level and the prevalence of NAFLD [6, 7], their relationship is not yet well-established especially among individuals without diabetes. Dyslipidemia is a well-known influence factor of NAFLD [8, 9]. It has been reported in 20 to 80% of NAFLD cases [10]. Recent study showed the link between NAFLD and premature cardiovascular disease through atherogenic dyslipidemia which comes from IR [11]. Triglyceride to high-density lipoprotein ratio (TG/HDL-C) has been validated as a predictive indicator for IR, type 2 diabetes, cardiovascular disease, and hypertension [12,13,14]. TG/HDL-C is supposed to be associated with incident NAFLD [15]. To the best of our knowledge, studies focusing specifically on NAFLD among individuals without diabetes in the general population are limited [7, 16, 17]. Therefore, we aimed to evaluate their unique metabolic characteristics and to reveal the independent factors associated with NAFLD among those individuals.
Methods
Study design and participant’s selection
This is a case control study included 178 participants without diabetes recruited from Hepatology outpatient clinic in Alexandria Main University Hospital. We excluded patients with alcohol consumption, viral, autoimmune, neoplastic, or hereditary liver diseases, thyrotoxicosis or hypothyroidism, fasting plasma glucose (FPG) concentration ≥ 126 mg/dl or HbA1c ≥ 6.5%, and hemoglobin less than 12 g/dl.
Sample size
Sample size was calculated based on previous literature [18]; an expected difference of average HbA1c between NAFLD and the control group is 0.2, and the standard deviation is 0.3. Using the level of confidence of 95% (alpha = 0.05) and power of 90 (beta = 0.10) and allocation of 1:2, the minimal sample size required to reject the null hypothesis is 50 in the NAFLD group and 100 in the control group. We targeted 53 participants with NAFLD and 125 age and sex matched control subjects to compensate the missing data.
Clinical and biochemical data
All participants were subjected to clinical examination including blood pressure, weight (Wt), standing height (Ht), and waist circumference (WC) measurements. WC was measured according to the WHO recommendation at the end of normal expiration from the midpoint between highest point of the iliac crest and lowest point of the costal margin. Body mass index (BMI) was calculated as body Wt (kg) divided by body Ht squared (m2). Overnight fasting blood samples were obtained from the antecubital vein for complete blood count, ALT, AST, total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), fasting insulin, plasma glucose, and HbA1c analysis. TG/HDL-C ratio was calculated by dividing serum TG level by serum HDL-C level. The Homeostasis Model Assessment 2 (HOMA2) calculator was used to estimate insulin resistance (HOMA-IR) according to the updated computer based HOMA2 mode [19].
Abdominal ultrasound examination
Abdominal ultrasonography was carried out by an expert radiologist. NAFLD was defined by the presence of at least two of four sonographic criteria: diffuse hyper-echoic echo-texture (bright liver), increased echo-texture compared with the kidneys, vascular blurring, and deep attenuation [20].
Ethical statement
The study was performed in alignment with revised Declaration of Helsinki (2013) and with Good Clinical Practice guidelines. Our study was approved by the Ethical Committee of Faculty of Medicine, Alexandria University (IRB No. 0304901). Informed consent was obtained from all subjects included in the study.
Statistical analysis
Analysis was done using SPSS 20.0. Chi-square test was used to compare between groups for categorical variables. Student t test was used to compare two groups for normally distributed quantitative variables, while Mann-Whitney test was used to compare between two groups for not normally distributed quantitative variables. Logistic regression (univariate and multivariate) was used to analyze factors associated with NAFLD. Receiver operating characteristic curve (ROC) is plotted to predict the role of HbA1c in identifying NAFLD among subjects without diabetes. It showed the performance of the cutoff point in terms of sensitivity versus 1-specificity. The area under the curve (AUC) is an estimate of the accurateness of cutoff point. Area more than 50% gives acceptable performance. P value less than 5% level is considered significant.
Results
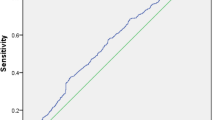
Baseline characteristics of participants are shown in Table 1. The NAFLD group has significantly higher BMI, WC, HbA1c, ALT, and GGT than the control group. Regarding lipid profile, total cholesterol, LDL-C, and TG/HDL-C ratio were significantly higher in NAFLD group than the control group (Table 1). Logistic regression analysis was used to evaluate the risk factors for NAFLD in subjects without diabetes. Eighteen variables were included into the analysis (Table 2). Our results showed that HOMA-IR, ALT, BMI, HbA1c, total cholesterol, LDL-C, and TG/HDL-C ratio were significantly associated with the risk of NAFLD. Upon them, ALT and HbA1c levels were independently associated with NAFLD development after performing the multivariate analysis. Receiver operating characteristic (ROC) analysis was plotted to study the ability of ALT level and HbA1c level for discrimination between those with and without NAFLD among non-diabetic individuals. The analysis identified the optimal cutoff point of ALT to be > 19 IU/ml. The corresponding sensitivity was 73.58%, specificity was 60.80%, and area under the curve was 0.731 (95% CI 0.653–0.809) (Fig. 1). On the other hand, the optimal cutoff point of HbA1c was identified to be > 5.1% with corresponding sensitivity and specificity being 79.25% and 41.60%, respectively. The area under the curve was 0.665 (95% CI 0.581–0.750) (Fig. 2).
Discussion
Due to the high occurrence and important medical consequences of NAFLD, early diagnosis and intervention are crucial for termination of progression and even reversal of the disease [21]. Most NAFLD patients are asymptomatic and usually identified when abnormal liver studies particularly liver enzymes are noted. However, these enzymes may not be elevated in all NAFLD cases [22]. The present study showed that ALT is significantly higher in NAFLD patients than non NAFLD. In addition, our study revealed that the cutoff point of ALT > 19 IU/ml can independently predict NAFLD development among individuals without diabetes. Similarly, Wong et al. [23] revealed that raising liver enzyme levels, chiefly ALT, may predict incident diabetes and NAFLD. Similar observations were also obtained by a study among Montenegrin population, found that ALT was independent predictor for NAFLD but with a higher cutoff point of 22 IU/L [24]. These results are also consistent with that of Al Humayed et al. [25] who identified that ALT is a predictor for NAFLD with a threshold cutoff value of 22.1 nmol/L. Our lower ALT cutoff point might be explained by that the present study was done among individuals without diabetes while the participants of other studies had diabetes.
HbA1c and HOMA-IR are considered screening tools for insulin resistance [26]. The relationship between NAFLD and insulin resistance is obscure. Previous studies declared that insulin resistance is a well-established driver for NAFLD [27, 28], while another study showed that presence of NAFLD is an important marker of multi-organ insulin resistance [29]. The present study revealed that HbA1c level was independently associated with NAFLD in individuals without diabetes. These results are consistent with that of Chen et al. [7] who confirmed an association between the levels of HbA1c and NAFLD in metabolically intact patients with HbA1c levels of 5.6% or less. Similar results were obtained by Masroor et al. [16]. Independent association of NAFLD with HbA1c was also observed by Sharma et al. [17] who showed hepatic gluconeogenesis derangement in non-diabetic and non-obese subjects with NAFLD. Moreover, hemoglobin glycation index showed its ability to identify the non-diabetic individuals at risk of developing NAFLD [30]. In addition, our study showed that insulin resistance assessed by HOMA-IR is significantly positively correlated with NAFLD. These results go hand in hand with the findings of Bae et al. [31]. The question about whether or not glycemic derangement is a spectator, a cause, or a consequence of NAFLD is still unsettled. This relationship might be explained by one of the following two mechanisms. The first one is that it impaired hepatic lipid settling and increased oxidative stress in liver cells, played a crucial role in the hepatic insulin signaling, impaired insulin inhibition of hepatic glucose production, and affected insulin sensitivity in muscle and adipose tissue resulting in insulin resistance [32]. The other possible mechanism is that the insulin resistance plays an important role in NAFLD pathogenesis by allowing storage of free fatty acids in the liver [33]. Recent study showed that the activation of receptor for advanced glycation end products pathway (AGEs/RAGE) triggers further inflammation and oxidative stress and impairs insulin signaling and thus provokes the development and progression of NAFLD [34].
Obesity represents an essential risk factor for NAFLD development and progression, and weight loss can improve lipid metabolism in the liver [35]. Our study showed that high BMI is significantly associated with NAFLD. These results go hand in hand with the findings of Masroor et al. [16]. These findings can be also explained by that obesity may lead to an imbalanced production of pro- and anti-inflammatory adipokines, which contributes to NAFLD development. The other justification is that obesity is usually associated with insulin resistance and increase in HbA1c [36].
Deranged carbohydrate metabolism affects lipid metabolism and results in increased production of TG that deposits in various tissues including liver leading to fatty liver [37]. One of main features of NAFLD is dyslipidemia including increased TG, increased LDL-C, and decreased HDL-C [38].
In agreement with the results of the present study, Fukuda et al. [39] found a significant positive correlation between TG/HDL-C ratio and NAFLD, but on the other hand, Fukuda et al. [39] and Fan et al. [15], in discordance with the results of the current study, reported that TG/HDL-C ratio was an independent predictor of NAFLD. This difference could be attributed to the difference in study population and the small sample size of the current study compared to the other studies.
Though the relationship between TG/HDL-C and NAFLD has not been fully decoded, insulin resistance is a potential mediator. Compared with other lipid parameters, TG/HDL-C was declared to be strongly correlated with insulin resistance [40]. Experimentally, insulin resistance was shown to increase the secretion of TG over-enriched VLDL particles and decrease the level of HDL-C [41]. In conclusion, our results showed that serum ALT and HbA1c levels were independently associated with NAFLD in individuals without diabetes and may be used as surrogates for NAFLD in this cohort.
Conclusions
Our study had some limitations that should be taken into account. First, the diagnosis of NAFLD was performed by ultrasonography. Although ultrasonography is non-invasive, reasonably accurate, and widely used in clinical practice and epidemiological studies of NAFLD, it is not sensitive enough to identify mild steatosis. Second, the detection of the degree of steatosis and fibrosis in NAFLD patients by fibroscan is highly recommended nowadays. Unfortunately, it was not performed in the current study since it is expensive, needs special experts, and is not available in our institute. Third, there were no follow-up to confirm the results. Despite these study limitations, our study was unique in describing the predictors connected to NAFLD among individuals without diabetes in Alexandria, Egypt. Furthermore, this study highlights that the inclusion of both HbA1c and ALT levels may have significant clinical implications for prediction of NAFLD in individuals without diabetes. Thus, we suggest that individuals without diabetes with ALT values of > 19 IU/ml and HbA1c > 5.1% should be referred for ultrasound examination for the possibility of NAFLD.
Availability of data and materials
The datasets used and/or analyzed during current study are available from corresponding author on reasonable request.
Abbreviations
- NAFLD:
-
Nonalcoholic fatty liver disease
- IR:
-
Insulin resistance
- HbA1c:
-
Glycosylated hemoglobin
- AGEs:
-
Advanced glycation end-products
- TG/HDL-C:
-
Triglyceride to high-density lipoprotein ratio
- FPG:
-
Fasting plasma glucose
- Wt:
-
Weight
- Ht:
-
Height
- WC:
-
Waist circumference
- BMI:
-
Body mass index
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- TC:
-
Total cholesterol
- TG:
-
Triglycerides
- HDL-C :
-
High-density lipoprotein
- LDL-C:
-
Low-density lipoprotein
- HOMA:
-
Homeostasis Model Assessment
- GGT:
-
Gamma-glutamyltransferase
- ROC:
-
Receiver operating characteristic
- VLDL:
-
Very low-density lipoprotein
References
Marchesini G, Day CP, Dufour JF et al (2016) EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 64(6):1388–1402. https://doi.org/10.1016/J.JHEP.2015.11.004
Vernon G, Baranova A, Younossi ZM (2011) Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 34(3):274–285. https://doi.org/10.1111/J.1365-2036.2011.04724.X
Chalasani N, Younossi Z, Lavine JE et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 67(1):328–357. https://doi.org/10.1002/HEP.29367
Association AD (2020) 4. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes—2020. Diabetes Care. 43(Supplement 1):S37–S47. https://doi.org/10.2337/DC20-S004
Chamberlain JJ, Rhinehart AS, Shaefer CF, Neuman A (2016) Diagnosis and management of diabetes: synopsis of the 2016 American diabetes association standards of medical care in diabetes. Ann Intern Med. 164(8):542–552. https://doi.org/10.7326/M15-3016
Tanaka K, Takahashi H, Hyogo H et al (2019) Epidemiological survey of hemoglobin A1c and liver fibrosis in a general population with non-alcoholic fatty liver disease. Hepatol Res. 49(3):296–303. https://doi.org/10.1111/HEPR.13282
Chen C, Zhu Z, Mao Y et al (2020) HbA1c may contribute to the development of non-alcoholic fatty liver disease even at normal-range levels. Biosci Rep. 40(1). https://doi.org/10.1042/BSR20193996
Yang SH, Du Y, Li XL et al (2017) Triglyceride to high-density lipoprotein cholesterol ratio and cardiovascular events in diabetics with coronary artery disease. Am J Med Sci. 354(2):117–124. https://doi.org/10.1016/J.AMJMS.2017.03.032
Wu L, Parhofer KG (2014) Diabetic dyslipidemia. Metabolism. 63(12):1469–1479. https://doi.org/10.1016/J.METABOL.2014.08.010
MR de A S, M de FF de M D, de JEM M-F, de MST A (2012) Metabolic syndrome and risk factors for non-alcoholic fatty liver disease. Arq Gastroenterol. 49(1):89–96. https://doi.org/10.1590/S0004-28032012000100015
Akhtar DH, Iqbal U, Vazquez-Montesino LM, Dennis BB, Ahmed A (2019) Pathogenesis of insulin resistance and atherogenic dyslipidemia in nonalcoholic fatty liver disease. J Clin Transl Hepatol. 7(4):362–370. https://doi.org/10.14218/JCTH.2019.00028
Murguía-Romero M, Jiménez-Flores JR, Sigrist-Flores SC et al (2013) Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. J Lipid Res. 54(10):2795–2799. https://doi.org/10.1194/JLR.M040584
Turak O, Afşar B, Ozcan F et al (2016) The role of plasma triglyceride/high-density lipoprotein cholesterol ratio to predict new cardiovascular events in essential hypertensive patients. J Clin Hypertens. 18(8):772–777. https://doi.org/10.1111/JCH.12758
Onat A, Can G, Kaya H, Hergenç G (2010) “Atherogenic index of plasma” (log10 triglyceride/high-density lipoprotein−cholesterol) predicts high blood pressure, diabetes, and vascular events. J Clin Lipidol. 4(2):89–98. https://doi.org/10.1016/J.JACL.2010.02.005
Fan N, Peng L, Xia Z et al (2019) Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: a cross-sectional study. Lipids Heal Dis 181(1):1–6. https://doi.org/10.1186/S12944-019-0986-7
Masroor M, Haque Z (2021) HbA1C as a biomarker of non-alcoholic fatty liver disease: comparison with anthropometric parameters. J Clin Transl Hepatol. 9(1):15. https://doi.org/10.14218/JCTH.2019.00046
Sharma R, Sinha S, Danishad KA et al (2009) Investigation of hepatic gluconeogenesis pathway in non-diabetic Asian Indians with non-alcoholic fatty liver disease using in vivo (31P) phosphorus magnetic resonance spectroscopy. Atherosclerosis. 203(1):291–297. https://doi.org/10.1016/J.ATHEROSCLEROSIS.2008.06.016
Lin TC, Lee HM, Seo HN et al (2018) Correlation between non-alcoholic fatty liver disease and hemoglobin A1c level in adult males without diabetes. Korean J Fam Pract. 8(1):131–135. https://doi.org/10.21215/KJFP.2018.8.1.131
Diabetes Trials Unit: HOMA Calculator News archive. Accessed July 26, 2021. http://www.dtu.ox.ac.uk/generic/newsarchive.php?Study=91
Gore RM, Levine MS. Textbook of Gastrointestinal Radiology (NEED PHYSICAL COPY). Published online 2008. Accessed 26 Jul 2021. http://ucs.cirqahosting.com/HeritageScripts/Hapi.dll/retrieve2?SearchTerm0=001x000157079&Dispfmt=F
Cai J, Zhang X-J, Li H (2019) Progress and challenges in the prevention and control of nonalcoholic fatty liver disease. Med Res Rev. 39(1):328–348. https://doi.org/10.1002/MED.21515
Paschos P, Paletas K (2009) Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia 13(1):9 Accessed 26 Jul 2021. /pmc/articles/PMC2633261/
Wong CA, Araneta MRG, Barrett-Connor E, Alcaraz J, Castañeda D, Macera C (2008) Probable NAFLD, by ALT levels, and diabetes among Filipino-American Women. Diabetes Res Clin Pract. 79(1):133–140. https://doi.org/10.1016/J.DIABRES.2007.07.012
Klisic A, Kavaric N, Ninic A (2019) Predictive values of serum uric acid and alanine-aminotransferase for fatty liver index in Montenegrin population. J Med Biochem. 38(4):407. https://doi.org/10.2478/JOMB-2019-0001
SM Al H, AA Al S, Mahfouz AA, Awadalla NJ, Musa MJ, Patel A (2020) Clinical and biochemical predictors of nonalcoholic fatty liver disease among type 2 diabetes mellitus patients at primary health care level in South Western Saudi Arabia. Diagnostics 10:809. https://doi.org/10.3390/DIAGNOSTICS10100809
Önal Z, Atasayan V, Gürbüz T, Hepkaya E, Nuhoğlu C (2014) Association of glycosylated hemoglobin (HbA1c) levels with Iinsulin resistance in obese children. Afr Health Sci. 14(3):533–538. https://doi.org/10.4314/ahs.v14i3.6
Kitade H, Chen G, Ni Y, Ota T (2017) Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutr 9:387. https://doi.org/10.3390/NU9040387
Stephen S, Baranova A, Younossi ZM et al (2014) Nat Rev Dis Primers 6(2):163–171. https://doi.org/10.1586/EGH.11.97
Bugianesi E, Gastaldelli A, Vanni E et al (2005) Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetol 48((4):634–642. https://doi.org/10.1007/S00125-005-1682-X
Hu DS, Zhu SH, Li X et al (2019) Association between hemoglobin glycation index and NAFLD in Chinese nondiabetic individuals. Can. J Gastroenterol Hepatol. 2019. https://doi.org/10.1155/2019/8748459
Bae JC, Cho YK, Lee WY et al (2010) Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol. 105(11):2389–2395. https://doi.org/10.1038/AJG.2010.275
D’Adamo E, Giannini C, Chiavaroli V et al (2011) What is the significance of soluble and endogenous secretory receptor for advanced glycation end products in liver steatosis in obese prepubertal children? Antioxid Redox Signal 14(6):1167–1172. https://doi.org/10.1089/ARS.2010.3719https://home.liebertpub.com/ars
Fracanzani AL, Valenti L, Bugianesi E et al (2008) Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 48(3):792–798. https://doi.org/10.1002/HEP.22429
Palma-Duran SA, Kontogianni MD, Vlassopoulos A et al (2018) Serum levels of advanced glycation end-products (AGEs) and the decoy soluble receptor for AGEs (sRAGE) can identify non-alcoholic fatty liver disease in age-, sex- and BMI-matched normo-glycemic adults. Metabolism. 83:120–127. https://doi.org/10.1016/J.METABOL.2018.01.023
Cantero I, Abete I, JM del B et al (2018) Changes in lysophospholipids and liver status after weight loss: the RESMENA study. Nutr Metab 15(1):1–11. https://doi.org/10.1186/S12986-018-0288-5
Polsky S, Ellis SL (2015) Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 22(4):277–282. https://doi.org/10.1097/MED.0000000000000170
Speliotes EK, Massaro JM, Hoffmann U et al (2010) Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham heart study. Hepatology. 51(6):1979–1987. https://doi.org/10.1002/HEP.23593
Foster T, Anania FA, Li D, Ronit Katz, Budoff M. The prevalence and clinical correlates of nonalcoholic fatty liver disease (NAFLD) in African Americans: the multiethnic study of atherosclerosis (MESA). doi:https://doi.org/10.1007/s10620-013-2652-7
Fukuda Y, Hashimoto Y, Hamaguchi M et al (2016) Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; a population-based cohort study. Liver Int. 36(5):713–720. https://doi.org/10.1111/LIV.12977
Zhou M, Zhu L, Cui X et al (2016) The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of β cell function in a Chinese population with different glucose tolerance status. Lipids Heal Dis 15(1):1–9. https://doi.org/10.1186/S12944-016-0270-Z
Lucero D, Miksztowicz V, Macri V et al (2015) Overproduction of altered VLDL in an insulin-resistance rat model: Influence of SREBP-1c and PPAR-α. Clín Invest Arterioscler. 27(4):167–174. https://doi.org/10.1016/J.ARTERI.2014.11.002
Acknowledgements
Not applicable
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
HN and HK have conceived and designed the study, participated in researching and analyzing data, wrote the manuscript, and approved the final version to be published.
Corresponding author
Ethics declarations
Competing of interests
The authors declare that they have no potential competing interests.
Ethics approval and consent to participate
The study was performed in alignment with revised Declaration of Helsinki (2013) and with Good Clinical Practice guidelines. Our study was approved by the Ethical Committee of Faculty of Medicine, Alexandria University (IRB No. 0304901). Informed consent was obtained from all subjects included in the study.
Consent for publication
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Naguib, H., Kassab, H. Potential relation between non-alcoholic fatty liver disease and glycemic and metabolic parameters in subjects without diabetes. Egypt Liver Journal 11, 85 (2021). https://doi.org/10.1186/s43066-021-00154-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-021-00154-z