Abstract
Background
Hepatitis B virus (HBV) may reactivate when treating chronic hepatitis C (CHC) with direct-acting antivirals (DAA). We aimed to investigate the risk of HBV infection and reactivation during DAA therapy by performing a prospective observational study carried on 200 patients positive for chronic HCV who were candidates for treatment by DAA therapy according to the Egyptian guidelines from February 2019 to December 2019; the patients identified to carry HBsAg at baseline or with positive HBc Abs were further assessed for other HBV markers: hepatitis B e antigen at baseline, and serum HBV DNA quantitative measurement at baseline, week 4 of treatment, end of treatment. On the other hand, recent infection by HBV among those patients was observed.
Results
Of all participants, 49% were males and 51% were females, aged above 18 years. There is a highly statistically significant difference (p-value < 0.05) between HCV RNA PCR (at the beginning, at the end of 4 weeks, and at the end of 12 weeks) in studied patients. There was a highly statistically significant difference found between the liver function tests at the beginning, at the end of 4 weeks, and at the end of 12 weeks of treatment where it shows improvement except for serum albumin. At beginning of the study, there were 34 patients who are co-infected with HCV and HBV with quantitative PCR test for HBV DNA ≥ 20 IU/ml. After 1 month of DAA therapy, reactivation was detected in 6 cases (4 occult cases show reverse seroconversion (became HBs Ag positive), and 2 co-infected cases show increased HBV DNA > 1000 IU/L above the baseline level). In addition, 3 new cases acquired recent infection with the positivity of HBc IgM and detectable levels of HBV DNA. After 3 months of study, reactivation was detected in one patient with co-infection (where increased HBV DNA > 1000 IU/L above the baseline level), and 5 new cases acquired recent infection late in the study.
Conclusion
Screening for HBV infection prior to DAA therapy is required to detect recent infection of reactivation of previous infection during or after DAA therapy.
Similar content being viewed by others
Background
Hepatitis C virus (HCV) infection is the leading cause of cirrhosis, hepatic decompensation, hepatocellular carcinoma, and liver transplantation [1,2,3]. Globally, due to the shared modes of transmission, co-infection with both hepatitis B virus (HBV) and hepatitis C virus (HCV) is not uncommon. This is especially so in high-risk populations such as intravenous drug abusers, patients on hemodialysis, patients who have received an organ transplant, human immunodeficiency virus-positive patients, and b-thalassemia patients [4]. Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV). It is a major global health problem and can cause chronic infection and puts people at high risk of death from cirrhosis and liver cancer [5,6,7]. The landscape of HCV management was dramatically changed by the recent advent of direct-acting antivirals (DAAs) for chronic hepatitis C virus (HCV) infection, where DAA regimens are associated with a sustained virological response (SVR) rate of > 90–95% and are considered safe; nevertheless, a few complications have been reported including reactivation of hepatitis B virus (HBV) [8,9,10]. Recommendations have been made by the American Association for the Study of Liver Diseases (AASLD)/Infectious Diseases Society of America (IDSA) and European Association for the Study of the Liver (EASL) to screen all CHC patients before DAA therapy for hepatitis B surface antigen (HBsAg), hepatitis B surface antibody, and hepatitis B core antibody. However, whether serum or plasma HBV DNA measurement is necessary [4]. So, we assessed the potential risk of hepatitis B virus (HBV) reactivation in patients receiving direct-acting antiviral agent (DAA)-based therapy for patients with chronic HCV.
Methods
Study design
This prospective observational study was carried on 200 patients positive for chronic HCV (positive antibody to HCV and positive HCV RNA), from February 2019 to December 2019, who were candidates for treatment by DAA therapy according to the Egyptian guidelines and systematically assessed for their eligibility for DAA therapy through a standardized clinical and virological assessment, including complete blood count, liver profile included, alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, albumin, creatinine, prothrombin time, (MINI VIDAS®, Biomerieux, France), HBsAg, and HBc antibodies (Stat Fax 4200, Dia Sorin, USA), done for all patients, and abdominal ultrasonography (US) in addition to hepatic transient elastography by fibroscan for all participants. Some patients underwent liver biopsy. Patients with the following characteristics were ineligible for DAAs according to the national guidelines: Child-Pugh class C cirrhosis, hepatocellular carcinoma (HCC) or liver metastases, platelet counts < 50 109 cells/L, current pregnancy, or breast-feeding. Also, patients under immunosuppressive drugs or with hepatorenal syndrome were excluded. Written consent was obtained from all participants; the patients identified to carry HBsAg at baseline or with positive HBc Abs were further assessed for other HBV markers: hepatitis B e antigen (HBeAg, Stat Fax 4200, Dia Sorin, USA) at baseline and serum HBV DNA quantitative measurement (DT Lite Real Time PCR, DNA-Technology, Russia, limit of detection: 25 IU/ml) at baseline, week 4 of treatment, end of treatment, and 12 weeks post-treatment. All patients received HCV antiviral therapy according to the Egyptian guidelines. SVR was assessed at 12 weeks after the end of treatment using HCV RNA. The following definitions were considered:
-
➢ Chronic hepatitis B (CHB) according to [11]
-
HBsAg present for ≥ 6 months.
-
Serum HBV DNA varies from undetectable to several billion IU/ml.
-
Subdivided into HBeAg positive and negative. HBV DNA levels are typically > 20,000 IU/mL in HBeAg-positive CHB, and lower values (2000–20,000 IU/mL) are often seen in HBeAg-negative CHB.
-
Normal or elevated ALT and/or AST levels.
-
Liver biopsy results showing chronic hepatitis with variable necroinflammation and/or fibrosis.
-
-
➢ Virological response to HCV antiviral therapy defined by undetectable HCV-RNA during treatment and sustained virological response (SVR) at week 12 of post-treatment follow-up.
-
➢ HBV reactivation according to AASLD-IDSA recommendations [11]; loss of HBV immune control in HBsAg-positive, anti-HBc-positive, HBsAg-negative, or anti-HBc-positive patients receiving immunosuppressive therapy for a concomitant medical condition; a rise in HBV DNA compared to baseline (or an absolute level of HBV DNA when a baseline is unavailable); and reverse seroconversion (seroreversion) from HBsAg-negative to HBsAg-positive for HBsAg-negative, anti-HBc-positive patients.
Statistical analysis
Data were verified, coded by the researcher, and analyzed using IBM-SPSS 21.0 (IBM-SPSS Inc., Chicago, IL, USA). Descriptive statistics such as means, standard deviations, medians, and ranges were calculated. Test of significances: chi-square test was calculated to compare the frequencies among the groups. For continuous variables, independent t-test analysis was carried out to compare the means of normally distributed data.
-
➢ Probability (P-value)
-
P-value < 0.05 was considered significant.
-
P-value < 0.001 was considered highly significant.
-
P-value > 0.05 was considered insignificant.
-
P1 refers to the statistical difference between the start of DAA and 1 month after DAA.
-
P2 refers to the statistical difference between the start of DAA and the end of DAA.
-
P3 refers to the statistical difference between 1 month after DAA and the end of DAA.
-
Results
In the current study, 200 patients were enrolled, 15% of them were aged < 40 years, 29.5% aged from 40 to 50 years, and 55.5% aged > 50 years. Of all participants, 49% were males and 51% were females. All patients were positive for HCV Abs and HCV RNA PCR quantitative test, and all were candidates for DAA therapy. There is a highly statistically significant difference (p-value < 0.05) between HCV RNA PCR (at the beginning, at the end of 4 weeks, and at the end of 12 weeks) in the studied patients (Table 1). There is no statistically significant difference (p-value > 0.05) between HBV DNA PCR quantitative test results (at the beginning, at the end of 4 weeks, and at the end of 12 weeks) (Table 2). The means of liver function prior to DAA therapy were ALT (60), AST (55.6), ALP (285.3), GGT (60), bilirubin (2.3), Alb (2.2), and, lasty, INR (1.2) (Table 4).
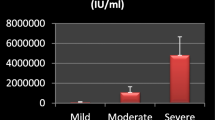
No statistically significant differences were found between liver ultrasound findings and fibroscan at the beginning, at the end of 4 weeks, and at the end of 12 weeks of treatment by DAAs (p-value > 0.05) (Table 3). A highly statistically significant difference was found between liver function tests at the beginning, at the end of 4 weeks, and at the end of 12 weeks of treatment (p-value < 0.001) (Table 4), where it shows improvements except for albumin; there was no statistically significant difference observed in the studied patients (Table 4). At beginning of the study, there were 34 patients who are co-infected with HCV and HBV with quantitative PCR test for HBV DNA ≥ 20 IU/ml, 30 patients with positive HBsAg, and 4 patients with positive HBc Ab (IgM). In addition to 5 cases with occult HBV infection with negative HBs Ag and positivity of anti-HBc IgG, 6 patients were immunized against HBV with positive anti-HBs Ab (Table 5). Fourteen patients were with HBV PCR < 2000 IU/ml with normal enzymes and not received antiviral therapy, and 20 patients were on antiviral drugs for HBV; their fibroscan range from f0 to f1 in 15 patients and from f1 to f2 in 5 patients. Abdominal ultrasound shows fatty liver in 17 patients and liver cirrhosis in 3 patients.
After 1 month of starting (DAAs), laboratory and serological investigations for all patients show that PCR for HCV RNA was still positive in 10 (5%) patients and became negative in all other patients. At the end of DAA therapy, only 4 (2%) patients were still positive for HCV PCR (Table 6).
Associated improvement in liver functions was observed (Table 4). Abdominal ultrasound throughout the study was normal in 115 patients, fatty infiltration in 57 patients, and cirrhotic changes in 28 patients (Table 4). Also, the fibroscan ranged between f3 and f4 in 32 patients, f1 and f2 in 55 patients, and f0 and f1 in 113 patients throughout the study without changes (Table 6).
As regards other hepatitis B virology markers, no statistically significant difference was found at the beginning, at the end of 4 weeks, and at the end of 12 weeks of treatment by DAAs (p-value > 0.05), except for HBc Ab (IgM), where it increased with statistically significant difference between the studied patients (during and after treatment) (Table 5). As regards laboratory finding at the start of the study, there was a positivity of HBsAg in 34 cases co-infected with HBV (with positive HBs Ag and detectable HBV DNA > 20 IU/ml) in addition to 5 occult HBV (with negative HBs Ag and positivity of HBc IgG and detectable levels of HBV DNA) (Table 7).
After 1 month of DAA therapy, reactivation was detected in 6 cases (4 occult cases show reverse seroconversion (became HBs Ag positive), and 2 co-infected cases show increased HBV DNA > 1000 IU/L above the baseline level). In addition, 3 new cases acquired recent infection with the positivity of HBc IgM and detectable levels of HBV DNA (Table 7).
After 3 months of study, reactivation was detected in one patient with co-infection (where increased HBV DNA > 1000 IU/L above the baseline level), and 5 new cases acquired recent infection late in the study (Table 7).
Discussion
HCV is a worldwide infection affecting about 180 million persons with the highest prevalence in Egypt. ASALD (2015) demonstrated that the American Association Study of Liver Disease suggests that all HCV patients who are about to initiate DAA therapy should be assessed for HBV co-infection by checking for the presence of HBsAg, anti-HBs, and anti-HBc. In addition, patients with positive HBsAg should be tested for HBV DNA viral load before the initiation of DAA therapy. The patients who meet the criteria for HBV treatment due to active HBV infection should initiate the HBV treatment before or during the HCV treatment. The patients with low or undetectable HBV DNA levels should be monitored at regular intervals (usually no more than once every 4 weeks) for HBV reactivation, and the patients with HBV DNA levels that meet treatment criteria should initiate HBV therapy. For those patients with positive anti-HBc or anti-HBs and anti-HBc, there are no sufficient recommendations; however, there is a risk of HBV reactivation in the case of elevated liver enzymes during or after DAA therapy. This prospective study was carried on 200 patients with chronic HCV infection who visited our outpatient clinic in Al-Azhar University Hospital and Aswan antiviral unit who started DAA-based therapy, and the patients were evaluated for HBV reactivation during and after DAA therapy; 34 of them had chronic HBV.
In the current study, 15% of cases aged < 40 years, 29.5% aged between 40 and 50 years, and 55.5% aged > 50 years, and 49% were males and 51% were females; this agreed with the Egyptian Demographic Health Survey (EDHS) that estimated the prevalence of HCV antibodies and HCV RNA in HCV patients, commonest among the 15–59 years age group [12, 13], which may be as a result of continuing exposure and increased risk of infection in this age group [14], and this was nearly in agreement with Kawagishi and Suda’s study that included 191 patients with HCV infection who received IFN-free DAA therapies where the mean age was 69 years and 86% of cases were males; also, our documented age was agreed with Lin et al.’s study [15] where the mean age of studied cases was 59.9 years and males represented 58% of cases. In the current study, no statistically significant differences were found between ultrasonographic and fibroscan findings in the enrolled cases at the start of the study, 1 month after, and after treatment (p-value > 0.05), while in Lin et al.’s study [15], they used APRI score (this is an AST to Platelet Ratio Index) that was the main tool used to determine liver cirrhosis status. In a meta-analysis of 40 studies, investigators concluded that an APRI score > 1.0 had a sensitivity of 76% and a specificity of 72% for predicting cirrhosis. In addition, APRI score > 0.7 had a sensitivity of 77% and a specificity of 72% for predicting significant hepatic fibrosis [16]. Our findings were agreeing with Yeh et al.’s study [17], where they did not observe any HBV-related ALT flare (abrupt rise of ALT level to > 5 times the upper limit of normal during chronic (HBV) infection or hepatic decompensation, but were antagonistic as regards to ALT, where there was no ALT elevation before or at the peak of HBV DNA levels in HBsAg-positive patients with HBV reactivation, indicating that on-treatment ALT monitoring may not be sensitive enough to detect HBV reactivation) [17]. Most patients in Belperio et al.’s study [18] appeared to have “silent” or “mild” HBV reactivation characterized by normal ALT or less than a 2-fold change in ALT. The occurrence of HBV reactivation without hepatitis in the setting of DAA treatment has also been observed as the most common presentation by others. The observed incidence of HBV reactivation among HBsAg-positive patients of 8.3% (7/84) and the incidence of HBV reactivation with evidence of biochemical hepatitis of 2.4% (2/84) warrant use of HBV prophylaxis in this setting. Notably, there was an apparent lack of association with baseline HBV DNA levels as 3 of the 8 with reactivation had undetectable pre-DAA HBV DNA levels. HBV reactivation was detected in 6 patients (12.8%) during treatment and reach to 7 patients (17.9%) after treatment, and this was higher than Belperio et al.’s study [18]; 62,290 patients infected with HCV were retrospectively assessed having completed oral DAA treatment. Among the 377 patients (0.6%) who were known to be HBsAg-positive prior to DAA initiation, 96 (25.5%) were on HBV treatment at the start of DAA therapy. HBV reactivation was defined by a > 1000 IU/mL increase in HBV DNA occurring while on DAA treatment. Eight of the cases occurred in patients known to be HBsAg positive, and 1 case occurred in a patient known only to be isolated anti-HBc-positive whereas HBV reactivation occurred in HCV infected patients with and without detectable HBV DNA prior to DAA initiation. HBV reactivation did not appear to be impacted by baseline HCV RNA level, presence of cirrhosis, or HCV DAA regimen. The rate of reactivation of HBV infection in the current study is higher than the reported HBV reactivation rate of 1–2% per year in persons with inactive disease. Thus, providers should recognize that patients with isolated anti-HBc are at some risk, albeit less, and that identifying these patients prior to DAA treatment and assessing HBV DNA status can heighten recognition of reactivation [19]. Our study was in line with Cheng et al.’s study [20], which reported 327 patients receiving pan oral DAA agents for HCV infections in areas endemic for HBV in China. Ten patients were positive for hepatitis B surface antigen (HBsAg), and 124 patients had occult HBV infection. HBV reactivation was determined by measuring HBV DNA and HBsAg status in serial serum samples collected every 2 weeks during DAA treatment and then every 4 weeks after treatment until week 12. In the total study population, 10 patients (3.1%) had hepatitis; 3 cases were associated with HBV reactivation (1 case not in the icteric phase, 1 case in the icteric phase, and 1 case with liver failure) and 7 from other causes. Testing positive for HBsAg before DAA treatment was a strong risk factor for developing hepatitis during treatment (hazard ratio, 15.0; P < .001). The study had some limitations; were we are unable to identify some detailed information that might have had an effect on HBV reactivation such as HBsAg level, anti-HBs titer, and HBV genotype. In addition, the type of DAAs is not included in the study which may play a role in reactivation of previous HBV infection.
Conclusion
Screening for HBV infection prior to DAA therapy is required to detect recent infection and reactivation of previous infection during or after DAA therapy which may affect the outcome of DAA therapy.
Availability of data and materials
Available
Abbreviations
- DAAs:
-
Direct antiviral agents
References
Kao JH et al (2016) Hepatitis C virus infection in Taiwan: past, present, and future. J Formos Med Assoc 115(2):65–66. https://doi.org/10.1016/j.jfma.2015.06.012
Abo-Amer YE et al (2018) Declining prevalence of hepatitis C virus among university students in one of the main governorates in Egypt. Infect Drug Resist 11:2435–2441. https://doi.org/10.2147/IDR.S183462 eCollection 2018. PMID: 30538509
Abd-elsalam S et al (2019) Sofosbuvir, pegylated interferon and ribavirin in the treatment of an Egyptian cohort with hepatitis C virus infection in real-life clinical practice. Infect Disord Drug Targets 19(2):179–184. https://doi.org/10.2174/1871526518666180912121835 PMID: 30207250
Liu C-H, Liu C-J, Su T-H, Fang Y-J, Chen et al (2017) Hepatitis B virus reactivation in patients receiving interferon-free direct-acting antiviral agents for chronic hepatitis C virus infection. Infect Dis Soc Am. 2017;4(1):ofx028.
World Health Organization (2019) Progress report on HIV, viral hepatitis, and sexually transmitted infections 2019. Accountability for the global health sector strategies, 2016–2021. Geneva: World Health Organization; 2019 (WHO/CDS/HIV/19.7). License: CC BY-NC-SA 3.0 IGO.
Soliman H, Ziada D, Salama M, Hamisa M, Badawi R, Hawash N, Selim A, Abd-Elsalam S (2020) Predictors for fibrosis regression in chronic HCV patients after the treatment with DAAS: results of a real-world cohort study. Endocr Metab Immune Disord Drug Targets 20(1):104–111. https://doi.org/10.2174/1871530319666190826150344 PMID: 31448717
Hanafy AS, Soliman S, Abd-Elsalam S (2019) Rescue therapy for chronic hepatitis C virus infection after repeated treatment failures: impact on disease progression and risk of hepatocellular carcinoma. Hepatol Res 49(4):377–384. https://doi.org/10.1111/hepr.13303 Epub 2019 Jan 28. PMID: 30570817
European Association for the Study of the Liver (2017) EASL Recommendations on treatment of hepatitis C 2016. J Hepatol 66(1):153–194. https://doi.org/10.1016/j.jhep.2016.09.001
Elfert A et al (2020) Treatment of hepatitis C cirrhotic patients with directly acting antivirals: a multicenter study. Infect Disord Drug Targets. https://doi.org/10.2174/1871526520666201019122205 Online ahead of print. PMID: 33076813
Abdel-Noor R, Watany M, Abd-Elsalam S, ElKhalawany W, Soliman S, Badawi R (2020) Is hepatitis B surface antigen (HB s Ag) enough alone as a screening test for HBV infection in rheumatic disease patients before starting immunosuppressive therapies? A cross-sectional study. Infect Disord Drug Targets 20(6):878–883. https://doi.org/10.2174/1871526519666191212094141 PMID: 31830889
AASLD-IDSA (2016) recommendations for testing, managing and treating adults infected with hepatitis C virus. Hepatology 62:932–954
Kandeel A, Genedy M, El-Refai S, Funk A, Fontanet A, Talaat M (2016) The prevalence of hepatitis C virus infection in Egypt 2015: implications for future policy on prevention and treatment. Liver Int 37(1):45–53
Mohamed AA, el-Toukhy NETR, Said EM, Gabal HMR, AbdelAziz H, Doss W, el-Hanafi H, el Deeb HH, Mahmoud S, Elkadeem M, Shalby HS, Abd-Elsalam S (2020) Hepatitis C virus: efficacy of new DAAs regimens. Infect Disord Drug Targets 20(2):143–149. https://doi.org/10.2174/1871526519666190121114003 PMID: 30663575
MatheÏ C, Buntinx F, Van Damme P (2001) Is the prevalence of hepatitis c virus (HCV) RNA in anti-HCV–positive injection drug users positively correlated with age? J Infect Dis 184(5):659–660. https://doi.org/10.1086/322795
Lin ZH, Xin YN, Dong QJ et al (2015) Evaluation of aspartate aminotransferase-to-platelet ratio index as a non-invasive marker for liver cirrhosis. Clin Diagn Res 9(11):OC22–OC24
Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY (2011) Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology 53(3):726–736. https://doi.org/10.1002/hep.24105
Yeh ML, Huang CF, Hsieh MH, Ko YM, Chen KY, Liu TW, Lin YH, Liang PC, Hsieh MY, Lin ZY, Chen SC, Huang CI, Huang JF, Kuo PL, Dai CY, Yu ML, Chuang WL (2017) Reactivation of hepatitis B in patients of chronic hepatitis C with hepatitis B virus infection treated with direct acting antivirals. J Gastroenterol Hepatol 32(10):1754–1762. https://doi.org/10.1111/jgh.13771
Belperio PS, Shahoumian TA, Mole LA, Backus LI (2017) Evaluation of hepatitis B reactivation among 62,920 veterans treated with oral hepatitis C antivirals. Hepatology 66:27–36
Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH (2016) American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 63(1):261–283. https://doi.org/10.1002/hep.28156
Wang C, Ji D, Chen J, Shao Q, Li B, Clin JL (2016) Hepatitis due to reactivation of hepatitis B virus in endemic areas among patients with hepatitis C treated with direct-acting antiviral agents. Gastroenterol Hepatol 15(1):132–136
Acknowledgements
None
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
HA is the corresponding author for the submission and revised the manuscript. AM was responsible for the analysis of the data. Laboratory investigations were performed by AS. AH was responsible for the data collection. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The local ethical committee (Al-Azhar University Hospitals, Assiut) approved the study, but the reference number is not applicable, and a written consent for participation was taken from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azeem, H.A., Alkabeer, A.M., Mohammed, A.S. et al. Study of hepatitis B virus infection, reactivation among patients with chronic hepatitis C infection treated by direct antiviral agents (DAAs). Egypt Liver Journal 11, 53 (2021). https://doi.org/10.1186/s43066-021-00121-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-021-00121-8