Abstract
Background
More than 90% of children with cancer live in low-resourced settings, where survival is only 20%. Sustainable evidence-based (EB) interventions yielding ongoing beneficial patient outcomes are critical to improve childhood cancer survival. A better understanding of factors promoting intervention sustainability in these settings is urgently needed. The aim of this study is to provide an empirical understanding of how clinical capacity for sustainability, or the resources needed to sustain an intervention, impacts the sustainment of Pediatric Early Warning System (PEWS), an EB intervention that improves pediatric oncology outcomes in low-resource hospitals by detecting clinical deterioration and preventing the need for more intense treatment.
Methods
We will conduct a prospective, longitudinal study of approximately 100 resource-variable hospitals implementing and sustaining PEWS participating in Proyecto EVAT, a quality improvement collaborative of Latin American pediatric oncology centers. Aim 1: We will evaluate how clinical capacity for sustainability changes over time through 5 to 9 prospective measurements of capacity via survey of clinical staff using PEWS (approximately n = 13 per center) during the phases of PEWS adoption, implementation, and sustainability using the Clinical Sustainability Assessment Tool (CSAT). Aim 2: We will determine the relationship between capacity and a) PEWS sustainment and b) clinical deterioration mortality among pediatric oncology patients at centers sustaining PEWS for 2 to 10 years using chart review and an existing patient outcomes registry. Aim 3: We will develop novel strategies to promote sustainability by gaining a deeper understanding of perceived challenges to building capacity and PEWS sustainment. In combination with quantitative outcomes, we will conduct 24 focus groups with staff (doctors, nurses, and administrators) from hospitals with both high (n = 4) and low capacity (n = 4). We will then use implementation mapping to generate theoretically driven, empirically-supported sustainability strategies.
Discussion
This study will advance implementation science by providing a theoretically driven, foundational understanding of factors that predict sustainability among a large, diverse cohort of hospitals. We will then use this knowledge to develop sustainability evidence-informed strategies that optimize capacity and promote long-term sustainment of PEWS and improvements in patient outcomes, thus promoting equity in childhood cancer care globally.
Similar content being viewed by others
Background
While much of implementation science focuses on adopting and implementing evidence-based interventions, sustainability is the least studied phase of the implementation continuum [1, 2]. Ideally, interventions should be sustained unless they are no longer effective or more effective interventions become available [3,4,5]. Many interventions are abandoned when they should be continued, often when external support, such as grant funding or collaborative assistance, is removed [6,7,8,9]. Implementing interventions is costly, and if interventions are prematurely abandoned, then initial investments are lost [10, 11]. This is especially problematic in low-resource settings where there are few opportunities to implement new interventions. Most importantly, evidence-based interventions that are not sustained cannot provide continued health benefits to patients.
The current body of scientific literature focuses primarily on conceptualizing and theorizing sustainability in health [11, 12]. A general consensus within this literature establishes the relationship between the immediate context where interventions are implemented and the likelihood of intervention sustainability [12]. Clinical capacity for sustainability, which characterizes the immediate context, is defined as the resources necessary to sustain an intervention and includes engaged staff, leadership and stakeholders, organizational readiness, workflow integration, implementation and training, and monitoring and evaluation as the most proximal contextual determinants influencing intervention sustainment [10, 13, 14]. While there are several conceptual frameworks identifying sustainability determinants, few have been evaluated empirically. A recent review of determinants of hospital intervention sustainability included no studies from low-income countries, and two-thirds of the studies were qualitative [15]. Another notable gap is a lack of theoretically informed, empirically driven sustainability strategies to modify determinants and promote intervention sustainability. A recent review of 62 sustainability strategies for health interventions noted the majority were strictly conceptual frameworks and only two were active strategies to either plan for sustainability or promote sustainability after implementation in acute care settings [11]. This existing work highlights a lack of comprehensive evaluation of factors and strategies that promote sustainability in low-resource settings, a meaningful knowledge gap that will be addressed in the current study.
This work will be conducted in the context of global pediatric oncology. The global burden of pediatric cancer is disproportionately shifted to low- and middle-income countries, which bear over 90% of childhood cancer cases [16], with a dismal survival rate of approximately 20% [17]. To reduce these disparities, the World Health Organization Global Initiative for Childhood Cancer [18] and other initiatives [19] emphasize the need to improve access to and outcomes of childhood cancer treatment globally. However, hospitals in low-resource settings frequently lack adequate infrastructure and staffing to deliver needed supportive care during cancer treatment [20,21,22], resulting in late identification of clinical deterioration events (CDEs) and high rates of preventable deaths [23, 24]. Our prior work in Latin America demonstrated high rates of CDEs among hospitalized children with cancer and a 30% mortality rate in patients with deterioration [25]. This illustrates an urgent need for effective, low-cost, and sustainable supportive care interventions, including strategies for timely identification of CDEs, to improve global childhood cancer survival. Two key challenges persist in addressing this imperative: (1) successful implementation of evidence-based interventions and (2) long-term sustainability of implemented interventions. The latter is the focus of this proposed study.
Pediatric Early Warning Systems (PEWS)
To more rapidly identify CDEs, many hospitals use PEWS: nursing-administered bedside clinical acuity scoring tools associated with escalation algorithms. PEWS accurately predict the need for pediatric intensive care unit (PICU) transfer in pediatric oncology patients in high-resource hospitals [26, 27]. Escala de Valoración de Alerta Temprana (EVAT) is a Spanish-language PEWS adapted for low-resource settings. EVAT includes a 5-component scoring tool (neurologic, cardiovascular, respiratory, staff and family concern) based on vital signs, physical examination findings, and treatment requirements [28]. Hospitalized patients are scored 0 to 11 using the PEWS tool by a bedside nurse during routine vital sign assessments. Higher scores indicate potential clinical deterioration and are addressed following an action algorithm that guides the clinical team in appropriate escalation of care. In 2014, Dr. Agulnik worked with local stakeholders to implement and validate this PEWS at a low-resource pediatric oncology hospital in Guatemala [28,29,30], resulting in a 27% reduction in CDEs, optimized PICU utilization [30], improved interdisciplinary communication, provider empowerment and perceived quality of care [31,32,33], and an annual cost-savings of over US$350,000 [34].
Proyecto EVAT implementation strategy
These results led St. Jude Global [19] at St. Jude Children’s Research Hospital to establish Proyecto EVAT, a quality improvement collaborative to improve survival in hospitalized children with cancer in Latin America [19, 35]. Hospitals that care for children with cancer are recruited to Proyecto EVAT through collaboration with the St. Jude Global Alliance [19] or via learning about the program from others. Hospitals apply to an annual cohort, obtain institutional approval to participate, and are assigned to one of twelve mentor training centers. Each hospital assembles a local PEWS implementation leadership team, including at minimum a pediatric oncology nurse, pediatric oncology ward physician, and intensivist, adjusting the size to local needs.
Proyecto EVAT hospitals are guided through a 3-phase implementation process via bimonthly virtual mentorship meetings. During the planning phase, hospitals implement a de-identified prospective registry of CDEs in pediatric oncology patients, collecting 6–12 months of baseline data. To maintain effectiveness, fidelity with no changes is recommended to the validated components of the PEWS tool [28, 36]. Validated components of PEWS considered to be essential to its effectiveness include the scoring system, an algorithm, and using the tool with every vital sign assessment. Hospitals, however, are encouraged to adapt other elements of PEWS to their setting, including adjusting the wording of the PEWS tool and details of the PEWS algorithm to better fit with local medical language, available resources, and processes for care escalation in hospitalized children [37].
After completing these activities, hospitals move to the implementation phase. Experts from St. Jude and the mentor centers teach local implementation teams PEWS implementation strategies using a standardized curriculum. Implementation teams then conduct local training with clinicians, pilot PEWS, and assess its effectiveness. From the start of the pilot, local leaders track PEWS use and fidelity (measured by the three types of PEWS errors) and patient outcomes (CDE registry), which are sent to St. Jude monthly. Implementation is considered successful (i.e., implementation completion) when a hospital achieves sufficient PEWS use and fidelity, defined as < 15% PEWS errors for two consecutive months. Hospitals then move to the sustainability phase, with the expectation of indefinite PEWS sustainment through continued PEWS use and fidelity, resulting an ongoing positive impact on patient outcomes. During this phase, hospitals continue collaborating with Proyecto EVAT through monthly virtual meetings and/or as mentor centers.
Since 2017, regional enthusiasm for Proyecto EVAT has grown, with 10–15 new hospitals enrolling in the program annually and more expressing interest. In an ongoing evaluation of PEWS at participating hospitals, we found that local implementation leadership teams successfully overcame implementation barriers and initially achieved excellent PEWS fidelity as well as improvements in patient outcomes, including a reduction in deterioration morality in participating centers [38,39,40,41,42,43,44].
Challenges sustaining PEWS
Preliminary data from Proyecto EVAT demonstrates hospitals improve clinical capacity during implementation. Hospitals may struggle to sustain PEWS, but adequate clinical capacity supports PEWS sustainment. In a preliminary analysis of hospitals using PEWS for up to 24 months, approximately 30% reported PEWS error rates above the 15% threshold for one or more months, indicating a lack of PEWS sustainment. A qualitative study of barriers and enablers to PEWS implementation at five Proyecto EVAT hospitals sustaining PEWS demonstrated several capacity-related barriers to sustainability, including the COVID-19 pandemic, fluctuations in human and material resources needed for PEWS, staff turnover resulting in insufficient training, difficulty obtaining leadership buy-in, and lack of internal systems for ongoing PEWS monitoring [45,46,47]. After controlling for individual, hospital, and intervention factors, clinical capacity as measured by the Clinical Sustainability Assessment Tool (CSAT) was significantly associated with PEWS sustainment (OR 3.27, p < 0.0001). Marginal effects from the final model indicate that an increasing capacity score was positively associated with PEWS sustainment (11% greater likelihood of sustainment for every additional CSAT point on a scale from one to five). These results suggest that not all hospitals have sufficient capacity for sustainability, a notable portion do not sustain PEWS, and higher clinical capacity makes PEWS sustainment more likely. However, the relationship between capacity and sustainment may be dynamic over time and longitudinal examinations are needed to understand these relationships. The INvestigating Sustainability of PEWS In REsource-limited hospitals (INSPIRE) study will build on this prior work by examining clinical capacity beyond implementation to understand its impact on PEWS sustainability over time.
Methods and design
The INSPIRE study attempts to answer the overall question of what are relevant components of clinical capacity that determine PEWS sustainability in resource-variable hospitals providing childhood cancer care (Table 1). To answer this question, we will conduct a longitudinal observational study of a cohort of pediatric oncology centers implementing and sustaining PEWS over five years. Upon completing this study, we will establish how clinical capacity changes over time (Aim 1), determine the influence of capacity on sustainment and patient outcomes (Aim 2), and use a mixed-method approach to understand staff perspectives on challenges to capacity building and sustainability and develop novel strategies to promote sustainability (Aim 3).
EVAT steering committee and external advisory board
This study will leverage the Proyecto EVAT Steering Committee (EVAT SC), a 33-member multidisciplinary team of nurses and physicians from 12 hospitals in 9 Latin American countries. EVAT SC members are experts in PEWS implementation and are selected from regional PEWS training centers. The EVAT SC reviewed the CSAT, the primary measure for the studey, for conceptual appropriateness, was involved in the translation and piloting of the CSAT, and approved this proposed study as feasible, important, and regionally acceptable. For the duration of the proposed work, the EVAT SC will be updated on project progress twice annually to provide oversight and feedback and to ensure regional appropriateness and applicability. This work will also be supported by an External Advisory Board composed of three senior investigators with expertise in implementation research, quality improvement, and global pediatric oncology.
Capacity and sustainability framework
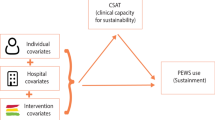
Our conceptual model (Fig. 1) is guided by the dynamic sustainability framework [14] and the public health capacity for sustainability framework [13]. The dynamic sustainability framework posits that interventions are implemented within a clinical context nested in a broad ecological system with a complex interplay between sustainability determinants, intervention sustainment, and intervention outcomes. We define sustainment as the continued use of evidence-based intervention elements after implementation, without external support; and sustainability to more broadly include intervention sustainment, ongoing beneficial patient outcomes, and intervention adaptation to improve sustainment (both gray boxes) [5]. We theorize that hospitals’ clinical capacity for sustainability, which refers to the resources needed to sustain an intervention, is the primary set of determinants of intervention sustainability. To promote sustainability, interventions may be adapted or capacity may be changed to support ongoing intervention use, resulting in a feedback loop between the intervention and capacity over time.
The clinical context for this study includes both the hospital and pediatric oncology unit. To conceptualize the clinical context in sufficient detail, we use the Clinical Capacity for Sustainability Model [48]. This model suggests that clinical capacity for sustainability falls within 7 domains: (1) engaged staff and leadership—frontline and administrative staff who are supportive of the intervention; (2) engaged stakeholders—other individuals, such as patients or parents, who are supportive of the intervention; (3) organizational readiness—organizational internal support and the resources needed to effectively manage the intervention; (4) workflow integration—how well the intervention fits into work that is done or will be done; (5) implementation and training—the process of implementing and training to deliver and maintain an intervention; (6) monitoring and evaluation—a process to evaluate the intervention to determine its effectiveness; and (7) outcomes and effectiveness—using monitoring and evaluation to determine outcomes for clinicians or patients.
In our conceptual model, clinical capacity for sustainability is the primary predictor of PEWS sustainability, including both PEWS sustainment and continued benefit to patient outcomes. Capacity growth, PEWS adaptation, and PEWS implementation will co-occur during the initial implementation process with support from Proyecto EVAT. Hospitals are encouraged to adapt some elements of PEWS to suit local capacity but are expected to maintain fidelity to the PEWS tool and how it is used in patient care. Once implementation is complete, hospitals sustain PEWS, including both PEWS use and fidelity, independently of Proyecto EVAT. Hospitals may continue to build capacity or experience capacity declines. We hypothesize that a hospital’s baseline capacity and ability to increase or maintain capacity (Aim 1) leads to a greater likelihood of PEWS sustainability (Aim 2). We expect PEWS to be sustained indefinitely, specifically by continuing to use and maintain fidelity to the PEWS tool, despite minor fluctuations in overall capacity, its individual components, or appropriate PEWS adaptation. Through PEWS sustainability, we expect maintenance of lower CDE mortality rates long-term (Aim 2). If capacity drops or organizations cannot use PEWS with fidelity, PEWS may not be sustained (i.e., abandoned), resulting in increased CDE mortality rates. By identifying challenges to capacity, we will develop sustainability strategies targeting these challenges, which will subsequently improve PEWS sustainability (Aim 3).
Setting
The proposed work will be conducted with resource-variable pediatric oncology hospitals participating in Proyecto EVAT. Currently, this includes 82 hospitals in 20 Spanish- and Portuguese-speaking countries in Latin America, representing over 10,500 new annual pediatric cancer diagnoses and more than 42,000 hospital admissions per year (Fig. 2). We expect that additional hospitals will be incorporated as we enroll new Proyecto EVAT cohorts during years 1 and 2 of the study period.
Data collection
For Aims 1 and 2, we will use a longitudinal observational research design. This will allow us to follow the development of capacity and the impact of capacity on PEWS sustainability in a variety of natural contexts. Over the study period, we will capture hospitals at various points in the PEWS implementation and sustainment process, ranging from those newly adopting PEWS to ones sustaining PEWS for over 10 years. We anticipate having between 5 and 9 observations of clinical capacity per hospital, dependent upon where hospitals are in the PEWS implementation process. We have structured data collection to occur at two relevant milestones in Proyecto EVAT to capture potential capacity increase during the adoption, initial implementation, and sustainment of PEWS. Once hospitals complete PEWS implementation and are sustaining PEWS, we will collect data every 6 months over the 4-year study data collection period (Fig. 3). We selected this interval to allow us to capture major changes in capacity and sustainability while minimizing participant burden. Among hospitals that have completed PEWS implementation, the primary outcomes, PEWS sustainment and CDE mortality rate, will be assessed in the 2 months prior to capacity assessments. For Aim 3, we will use a sequential mixed-method design by nesting qualitative data collection among hospitals who exhibit high and low capacity and have been using PEWS for at least 2 years. We will use focus groups of implementation leaders, clinicians, and hospital administrators to understand staff perspectives of the influence of capacity on PEWS sustainability and identify strategies that may develop capacity and support sustainability. We will then use an intervention mapping approach to identify critical capacity components and develop novel sustainability strategies for low-resource hospitals.
Recruitment
All current Proyecto EVAT hospitals will be recruited for participation at the start of the project, and we expect to recruit two additional annual cohorts during the study period, resulting in approximately 102 study hospitals (82 current hospitals + 10 new hospitals per year × 2 years in subsequent cohorts). Each hospital’s local PEWS implementation leadership team will be contacted for participation in the study and asked to identify a site lead. Site leads will be responsible for obtaining hospital approval for participation and guide data collection at their hospitals. If hospitals do not wish to participate, they will have the option to opt out of the study but remain in Proyecto EVAT.
All PEWS implementation leadership team members (mean 7, range 4–15) and frontline clinical staff who routinely use PEWS (mean 20, range 9–61) will be eligible and invited to participate at each survey data collection time point (anticipated 27 participants per time point). Based on preliminary data, we conservatively expect at least a 50% response rate (currently 65%), resulting in at least 13 responses per time point (current mean is 19 per hospital).
Study measures
Study measures and collection methods are summarized in Table 2 which aligns with our conceptual model. In alignment with our concept of sustainability, our primary outcomes are PEWS sustainment (i.e., sustainment outcome) and CDE mortality rate (i.e., patient outcome). Our primary quantitative covariates and predictors include hospital characteristics, participant characteristics, clinical capacity for sustainability, and PEWS adaptation.
We operationalize PEWS sustainment as 2 consecutive months of PEWS use and fidelity, defined as < 15% PEWS errors (Table 2). Based on our prior work, we expect that some hospitals will have errors above this threshold and thereby be considered not sustaining. We will follow these hospitals to understand whether they resume PEWS sustainment or experience continued decline and PEWS abandonment. The primary patient outcome will be measured by the CDE mortality rate, calculated based on the de-identified quality improvement CDE registry (Table 2). We selected this patient outcome because it is the most reliable, easily collected, and most reflective of the effects of PEWS on patient care [44]. Implementation leaders will collect sustainment and CDE mortality data for 2 months before each data collection time point.
This study leverages the Clinical Sustainability Assessment Tool, a reliable measure based on the Clinical Capacity for Sustainability Model, to evaluate clinical capacity for sustainability among the Proyecto EVAT hospitals. The team developed the CSAT to assess the sustainability of clinical practices across 7 domains specific to health care and clinical settings [51]. Initial testing of the CSAT showed excellent internal consistency and preliminary evidence for discriminant validity (i.e., differences in CSAT scores by academic vs. nonacademic organizations and by inpatient vs. outpatient settings) and took about 15 min to complete [48, 51]. A Spanish version of the CSAT measure and associated report has been translated, regionally adapted, and validated for use in low-resource settings. An electronic version of the tool was piloted among 19 EVAT SC members to establish acceptability within the context of Proyecto EVAT, and feedback was used to create the final tool [49]. This work confirms the CSAT is culturally and contextually appropriate and discriminates between high- and low-capacity hospitals. Based on positive participant feedback, the CSAT was integrated into the Proyecto EVAT timeline in 2021, with two standardized CSAT measurements during the PEWS implementation phase (1: after the PEWS pilot to inform full-scale PEWS implementation, and 2: at implementation completion).
Aim 1 analyses: changes in clinical capacity to sustain PEWS over time
We will investigate how overall capacity and its components change through the phases of PEWS adoption, implementation, and sustainment. We hypothesize that capacity will develop during early implementation and increase over time using PEWS. Primary data for Aim 1 will be hospital characteristics, participant demographics, and clinical capacity. Data will be examined for missingness and outliers and tested for normality, linearity, and homoscedasticity. Corrective strategies will be used as appropriate but may not be necessary given the robustness of mixed-effects modeling to various assumption violations [50, 52]. Data will be analyzed to generate descriptive statistics (e.g., frequencies, central tendencies, and variabilities) and diagnostic plots (e.g., bar charts and contingency tables) of capacity. Descriptive analyses will include variability of capacity across hospitals over time. All data management and analyses will be conducted using R (v4.3.1 or later).
We will use a multi-level modeling approach to build a series of growth curve models of capacity (CSAT scores) over time. The growth curve models will allow us to assess individual- and hospital-level associations with changes in capacity. Moreover, because capacity will be measured at many time points [5,6,7,8,9], the growth curve models will allow us to identify the linear and nonlinear change patterns of sustainability [52]. This rich description of changes in capacity is an important contribution of this study. The multi-level model template that will be used for these growth curve analyses is: \({SustCap}_{tij}={{IC}_{tij}+{HC}_{tj}+ IC}_{tij}*{HC}_{tj}\), where SustCap is capacity measured by the total or domain-specific CSAT scores; IC is the set of individual-level covariates (e.g., staff role); and HC is the set of hospital-level covariates (e.g., size). Time0 defines the start of the data collection; observations will be collected at time t for each outcome variable and time-varying covariate. The interaction term between the individual and hospital-level covariates allows us to explore cross-level interactions between setting and clinical staff characteristics. Multi-level modeling has many advantages for this type of organizational-level observational study [53]. It will allow us to build models that can appropriately handle staff- and patient-level data clustering within hospitals, examine the effects of both individual and hospital characteristics on the dependent variables, and analyze patterns of change over time.
Aim 2 analyses: determine clinical capacity components that predict PEWS sustainability
We will identify capacity components that influence long-term sustainability. We hypothesize that greater overall capacity makes PEWS sustainment and continued benefits to patient outcomes more likely. Key variables include hospital characteristics, clinical capacity, PEWS adaptation, PEWS sustainment, and patient outcomes (Table 2).
We will follow a similar modeling strategy as described in Aim 1. We will build a series of multi-level growth curve models to assess changes in PEWS sustainment and patient outcomes over time, as a function of individual- and hospital-level, as well as capacity characteristics. As above, multi-level modeling has many advantages for this type of longitudinal study [53]. The first set of models will focus on PEWS sustainment as the primary outcome:\({SustOutcome}_{tj}={{IC}_{tij}+{HC}_{tj}+ IC}_{tij}*{HC}_{tj}+{CSAT}_{tij}\), where SustOutcome measured at time t for hospital j is one of the PEWS sustainment variables (Table 2); IC is the set of individual-level covariates (e.g., staff role); HC is the set of hospital-level covariates (e.g., size), and CSAT is the set of total and domain-specific CSAT scores. Sustainment outcomes are either binary or percentages, so generalized mixed-effects modeling will be used [53]. The second set of models will then look at patient outcomes: \({PatientOutcome}_{tj}={{IC}_{tij}+{HC}_{tj}+ IC}_{tij}*{HC}_{tj}+{CSAT}_{tij}+{SustOutcome}_{tj}\). The interpretation of this model is similar to the previous one, but adding the PEWS sustainment outcomes as additional covariates. This will allow us to assess the extent to which success in PEWS sustainment is associated with downstream clinical outcomes. We will use either general or generalized mixed-effects modeling depending on the dependent variable (e.g., a Poisson model will be used for CDE mortality rates).
Power estimates (Aims 1 and 2)
We used a simulation approach for power analysis in mixed-effects models according to the proposed analytic models [54]. We selected 2 prototypic models for estimating power: a multilevel model in which individual clinical staff are nested within hospitals (corresponding to secondary research questions that include cross-sectional multilevel analyses) and a longitudinal model in which hospital-level covariates and outcomes are measured over time (corresponding to the primary research questions in Aims 1 and 2). We obtained parameter estimates from study design decisions (e.g., number of hospitals) and analysis of the pilot data (e.g., means and variability of CSAT scores and hospital CDE mortality rates). We used conservative estimates of the number of participants, hospitals, time points, and intraclass correlation values. For the longitudinal models, we will have between 5 and 9 observations for each hospital. For power analyses, we assume 7 time points, which is a conservative estimate of the minimum number of observations we will have from most hospitals.
We conducted the power analyses with the SIMR package in R (Table 3) [55]. For each prototypic model, we calculated the power for detecting small or medium effect sizes for level 1, level 2, and cross-level interaction effects (L1, L2, CLI, respectively). Small and medium effect sizes are based on standardized estimates [55, 56]. The estimated power for the study ranged from good to excellent. Although the L2 main effects had lower power due to the number of hospitals, the more important effects for both models were CLIs (e.g., how clinical outcomes vary over time for different types of hospitals), which were excellent (> 95%) for both analyses.
Aim 3: develop strategies to target clinical capacity and sustainability challenges
We will evaluate the perspectives of clinical staff and hospital administrators on capacity development, PEWS sustainment, and impact on patient outcomes in a subset of Proyecto EVAT hospitals exhibiting high- and low-capacity for sustainability. Using a sequential mixed-methods design, we will qualitatively determine staff perspectives on changes to capacity over time and how this relates to PEWS sustainment and patient outcomes. We will triangulate this with our quantitative assessment of capacity to provide a deeper understanding of how capacity relates to sustainability. We will then use an established implementation mapping process [57] to develop novel strategies to support PEWS sustainability in low-resource hospitals and address identified capacity challenges.
Recruitment and enrollment
Three focus groups (physicians, nurses, and administrators, separately) will be conducted at each of 8 Proyecto EVAT hospitals that have been using PEWS for at least 2 years (24 focus groups). The hospitals will be sampled purposively with a modified positive and negative deviant approach [58] to include four high-capacity and four low-capacity hospitals (using upper and lower quartiles of CSAT scores to recruit two high- and low-capacity hospitals in years 2 and 3). Based on our prior work indicating variation in capacity, this approach will allow us to explore how differences in capacity relate to sustainability and staff perspectives on identified capacity challenges. We will recruit participants using a purposive sampling approach [59] to include implementation leaders and clinical staff recruited for Aim 1 and 2, and hospital administrators identified by site leads, aiming to enroll 5–7 participants per focus group (total 120–168 participants). We will use homogenous grouping by participant roles to help ensure honest discussions [60, 61].
Data collection
Focus groups will be conducted using the video conferencing platform Zoom in Spanish or Portuguese by two native-speaking facilitators from St. Jude unknown to participants, and audio-recorded [49, 62]. The facilitation guide will be based on our conceptual framework (Fig. 2) and assess perceived challenges to capacity in the 7 CSAT domains, PEWS adaptation, PEWS sustainment, impact on patient outcomes and potential strategies to promote sustainability. We expect focus groups to last 60–90 min.
Analysis plan
Audio recordings will be translated into English and transcribed through a certified service [31,32,33, 62, 63]. English transcripts will be de-identified, segmented, and uploaded to MAXQDA software for analysis. A qualitative analysis team will develop an initial codebook with a priori codes informed by the CSAT domains and conceptual framework as well as inductive codes developed using a constant comparative approach with iterative memoing of transcripts to allow for emergent themes [64]. Transcripts will be coded independently by two coders. Interrater reliability will be monitored, and discrepancies resolved through consensus and a separate adjudicator. We expect to use two broad analytic approaches: categorical coding, which will group data conceptually according to the domains of our framework, and thematic coding, which will describe the relations among the concepts (e.g., the dynamic between capacity and PEWS sustainability) [65]. Because our approach is guided by a structured framework and a previously employed method for inductive codes, we are confident that this strategy will achieve analytic saturation [66].
Data synthesis
The results from quantitative assessment of capacity using the CSAT will be triangulated with qualitative participant perspectives on capacity development and PEWS sustainability to provide convergence (i.e., to assess how different data answer the same question) [67, 68]. We will further explicate primary quantitative findings through joint displays, facilitating comparisons of quantitative and qualitative results [69]. Specifically, qualitative results will be used to gain a deeper understanding of capacity strengths and challenges, as well as how capacity relates to PEWS sustainability [70, 71].
Strategy development
In year 5, we will use results from the above analyses to develop sustainability strategies by leveraging implementation mapping, which applies intervention mapping to implementation strategy development [57]. To date, sustainability strategies for clinical settings have been primarily developed based on literature review and without a systematic process [11]. Intervention mapping is widely used to design and adapt behavioral interventions and provides a systematic process to development interventions using five steps: (1) conduct a needs assessment; (2) identify sustainability outcomes, performance objectives, determinants, and create matrices of change; (3) choose theories of change and select strategies; 4) produce strategy protocols and materials; (5) evaluate outcomes [72, 73]. Our activities and analyses from the three study aims will serve as the needs assessment and address step 1: identify capacity barriers and needs. To accomplish the second step, our research team will use study results to identify performance objectives and create matrices of change. Performance objectives are actions that will accomplish the intended sustainability outcome. For instance, a simple performance objective to improve PEWS sustainment would be to have nurses use PEWS more often. Matrices of change are an analytic technique that help integrate determinants, theories of change, performance objectives, and sustainability outcomes to select strategies that directly address critical determinants in a theoretically-sound manner (third step). For instance, we may identify nurse turnover and lack of PEWS knowledge (determinants) as primary barriers to PEWS use among nurses (performance objective) impacting overall PEWS sustainment (outcome). To address this barrier, we may select an educational strategy, based on the theoretical assumption that knowledge leads to behavior change, such as booster PEWS training sessions for new nurses. We will present our strategy development progress to the EVAT SC for feedback. By the end of the study period, we will have developed materials and protocols needed for these strategies (fourth step) using recommendations for specifying strategies [74] and be positioned to evaluate these strategies (step 5) in future work using a design, such as a hybrid type III trial, appropriate for strategy evaluation.
Dissemination plan
Our dissemination plan considers various audience, including researchers, hospital administrators, clinical staff, and funders. We will share our findings through conference presentations and open-access publications. All versions of the CSAT are publicly available at the SustainTool.org website [75], which will be updated with study results and resources for study partners. Individual center results of the capacity assessment will be shared in real-time with centers using the CSAT reports [49]; we will also develop an interactive dashboard to share findings and developed strategy materials with study participants and the public. Making these tools freely available will expedite their use as common measures in future studies and by clinicians and other stakeholders looking to assess the clinical capacity of their organizations.
Discussion
The INSPIRE study prospectively follows capacity and its impact on sustainability over time. By choosing this design, we accept loss of control over recruitment and measurement conditions. However, our design is strengthened by the diversity in location, size, and capacity of the participating hospitals, allowing us to longitudinally follow the natural course of PEWS sustainability over many years. A longitudinal observational study is the most appropriate design to provide empirical evidence for the interrelations between capacity, PEWS sustainment, and patient outcomes in real-world low-resource hospitals.
Sustainability of evidence-based interventions is perhaps the most important aspect of the implementation continuum, yet has not been rigorously examined, particularly in low-resource settings [10]. This study will address this significant scientific gap by moving beyond conceptual frameworks to empirical testing to understand the relationship between clinical capacity, intervention sustainment, and patient outcomes in variably-resourced hospitals over time. A better understanding of how to sustain evidence-based interventions like PEWS is urgently needed to increase global survival of childhood cancer, particularly in low-resource settings. Upon completing the proposed work, we will establish how capacity changes over time, determine its impact on intervention sustainment and patient outcomes, and use staff perspectives on capacity building to develop novel sustainability strategies. This work is significant by providing a theoretically driven, longitudinal understanding of factors that predict sustainability in a large cohort of low-resource hospitals delivering the same intervention in a variety of settings. It is innovative by moving beyond a cross-sectional exploration towards empirical, longitudinal evidence supporting the dynamic relationships between capacity and intervention sustainability. Furthermore, we will leverage this knowledge to address a widely identified need to develop sustainability strategies that optimize capacity, promote intervention sustainability, and encourage health equity in childhood cancer outcomes in low-resource settings [1, 76]. Ultimately, these results will launch a trajectory of research that improves childhood cancer survival by effectively sustaining evidence-based interventions like PEWS and promoting equity by focusing on low-resource hospitals where preventable mortality remains high.
Availability of data and materials
Not applicable.
Abbreviations
- CDEs:
-
Clinical deterioration events
- CLI:
-
Cross level interaction
- CSAT:
-
Clinical Sustainability Assessment Tool
- EB:
-
Evidence-based
- EVAT:
-
Escala de Valoración de Alerta Temprana
- EVAT SC:
-
EVAT Steering Committee
- INSPIRE:
-
INVestigating Sustainability of PEWS In REsource-limited hospitals
- L1:
-
Level one
- L2:
-
Level two
- PEWS:
-
Pediatric Early Warning System
- PICU:
-
Pediatric intensive care unit
- WHO:
-
World Health Organization
References
Proctor E, Luke D, Calhoun A, McMillen C, Brownson R, McCrary S, et al. Sustainability of evidence-based healthcare: research agenda, methodological advances, and infrastructure support. Implement Sci. 2015;10(1):88.
Braithwaite J, Ludlow K, Testa L, Herkes J, Augustsson H, Lamprell G, et al. Built to last? The sustainability of healthcare system improvements, programmes and interventions: a systematic integrative review. BMJ Open. 2020;10(6):e036453.
McKay VR, Morshed AB, Brownson RC, Proctor EK, Prusaczyk B. Letting go: conceptualizing intervention de-implementation in public health and social service settings. Am J Community Psychol. 2018;62(1–2):189–202.
Brownson RC, Allen P, Jacob RR, Harris JK, Duggan K, Hipp PR, et al. Understanding mis-implementation in public health practice. Am J Prev Med. 2015;48(5):543–51.
Shelton RC, Cooper BR, Stirman SW. The sustainability of evidence-based interventions and practices in public health and health care. Annu Rev Public Health. 2018;39(1):55–76.
Freedman AM, Kuester SA, Jernigan J. Evaluating public health resources: what happens when funding disappears? Prev Chronic Dis. 2013;14(10):E190.
Wiltsey Stirman S, Kimberly J, Cook N, Calloway A, Castro F, Charns M. The sustainability of new programs and innovations: a review of the empirical literature and recommendations for future research. Implement Sci. 2012;7(1):17.
Hailemariam M, Bustos T, Montgomery B, Barajas R, Evans LB, Drahota A. Evidence-based intervention sustainability strategies: a systematic review. Implement Sci. 2019;14(1):57.
Schechter S, Jaladanki S, Rodean J, Jennings B, Genies M, Cabana MD, et al. Sustainability of paediatric asthma care quality in community hospitals after ending a national quality improvement collaborative. BMJ Qual Saf. 2021;30(11):876–83.
Gruen RL, Elliott JH, Nolan ML, Lawton PD, Parkhill A, McLaren CJ, et al. Sustainability science: an integrated approach for health-programme planning. Lancet. 2008;372(9649):1579–89.
Lennox L, Maher L, Reed J. Navigating the sustainability landscape: a systematic review of sustainability approaches in healthcare. Implement Sci. 2018;13(1):27.
Birken SA, Haines ER, Hwang S, Chambers DA, Bunger AC, Nilsen P. Advancing understanding and identifying strategies for sustaining evidence-based practices: a review of reviews. Implement Sci. 2020;15(1):88.
Schell SF, Luke DA, Schooley MW, Elliott MB, Herbers SH, Mueller NB, et al. Public health program capacity for sustainability: a new framework. Implement Sci. 2013;8(1):15.
Chambers DA, Glasgow RE, Stange KC. The dynamic sustainability framework: addressing the paradox of sustainment amid ongoing change. Implement Sci. 2013;8(1):117.
Cowie J, Nicoll A, Dimova ED, Campbell P, Duncan EA. The barriers and facilitators influencing the sustainability of hospital-based interventions: a systematic review. BMC Health Serv Res. 2020;20(1):588.
Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Atun R. Estimating the total incidence of global childhood cancer: a simulation-based analysis. Lancet Oncol. 2019;20(4):483–93.
Ward ZJ, Yeh JM, Bhakta N, Frazier AL, Girardi F, Atun R. Global childhood cancer survival estimates and priority-setting: a simulation-based analysis. Lancet Oncol. 2019;20(7):972–83.
The Global Initiative for Childhood Cancer. Available from: https://www.who.int/initiatives/the-global-initiative-for-childhood-cancer. [cited 2023 Aug 14].
St. Jude Global. Available from: https://www.stjude.org/global.html. [cited 2023 Aug 7].
Ceppi F, Antillon F, Pacheco C, Sullivan CE, Lam CG, Howard SC, et al. Supportive medical care for children with acute lymphoblastic leukemia in low- and middle-income countries. Expert Rev Hematol. 2015;8(5):613–26.
Duke T, Cheema B. Paediatric emergency and acute care in resource poor settings paediatric acute care in developing countries. J Paediatr Child Health. 2016;52(2):221–6.
Dray E, Mack R, Soberanis D, Rodriguez-Galindo C, Agulnik A. Beyond supportive care: a collaboration to improve the intensive care management of critically ill pediatric oncology patients in resource-limited settings. Pediatr Blood Cancer. 2017;64(1545–500964):S354.
Friedrich P, Ortiz R, Fuentes S, Gamboa Y, Ah Chu-Sanchez MS, Arambú IC, et al. Barriers to effective treatment of pediatric solid tumors in middle-income countries: can we make sense of the spectrum of nonbiologic factors that influence outcomes? Cancer. 2014;120(1):112–25.
Rodriguez-Galindo C, Friedrich P, Morrissey L, Frazier L. Global challenges in pediatric oncology. Curr Opin Pediatr. 2013;25(1):3.
Agulnik A, Cárdenas A, Carrillo AK, Bulsara P, Garza M, Alfonso Carreras Y, et al. Clinical and organizational risk factors for mortality during deterioration events among pediatric oncology patients in Latin America: a multicenter prospective cohort. Cancer. 2021;127(10):1668–78.
Agulnik A, Forbes PW, Stenquist N, Rodriguez-Galindo C, Kleinman M. Validation of a pediatric early warning score in hospitalized pediatric oncology and hematopoietic stem cell transplant patients. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2016;17(4):e146-153.
Dean NP, Fenix JB, Spaeder M, Levin A. Evaluation of a pediatric early warning score across different subspecialty patients. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(7):655–60.
Agulnik A, Méndez Aceituno A, Mora Robles LN, Forbes PW, Soberanis Vasquez DJ, Mack R, et al. Validation of a pediatric early warning system for hospitalized pediatric oncology patients in a resource-limited setting. Cancer. 2017;123(24):4903–13.
Agulnik A, Soberanis Vasquez DJ, García Ortiz JE, Mora Robles LN, Mack R, Antillón F, et al. Successful implementation of a pediatric early warning score in a resource-limited pediatric oncology hospital in Guatemala. J Glob Oncol. 2016;2(3_suppl):60s.
Agulnik A, Mora Robles LN, Forbes PW, Soberanis Vasquez DJ, Mack R, Antillon-Klussmann F, et al. Improved outcomes after successful implementation of a pediatric early warning system (PEWS) in a resource-limited pediatric oncology hospital. Cancer. 2017;123(15):2965–74.
Graetz D, Kaye EC, Garza M, Ferrara G, Rodriguez M, SoberanisVásquez DJ, et al. Qualitative study of pediatric early warning systems’ impact on interdisciplinary communication in two pediatric oncology hospitals with varying resources. JCO Glob Oncol. 2020;6:1079–86.
Graetz DE, Giannars E, Kaye EC, Garza M, Ferrara G, Rodriguez M, et al. Clinician emotions surrounding pediatric oncology patient deterioration. Front Oncol. 2021;25(11):626457.
Garza M, Graetz DE, Kaye EC, Ferrara G, Rodriguez M, SoberanisVásquez DJ, et al. Impact of PEWS on perceived quality of care during deterioration in children with cancer hospitalized in different resource-settings. Front Oncol. 2021;11:660051. https://doi.org/10.3389/fonc.2021.660051.
Agulnik A, Antillon-Klussmann F, Soberanis Vasquez DJ, Arango R, Moran E, Lopez V, et al. Cost-benefit analysis of implementing a pediatric early warning system at a pediatric oncology hospital in a low-middle income country. Cancer. 2019;125(22):4052–8.
Agulnik A, Garza M, Ruiz AG, Soberanis D, Rivera J, Martinez A, et al. Successful implementation of a pediatric early warning system (PEWS) in 10 resource-limited pediatric oncology centers in Latin America and the Caribbean. In: Pediatric Blood & Cancer. Hoboken: Wiley; 2019. p. S512–3.
Brown SR, Martinez Garcia D, Agulnik A. Scoping Review of Pediatric Early Warning Systems (PEWS) in resource-limited and humanitarian settings. Front Pediatr. 2019;8(6):410.
Agulnik A, Ferrara G, Puerto-Torres M, Gillipelli SR, Elish P, Muniz-Talavera H, et al. Assessment of barriers and enablers to implementation of a pediatric early warning system in resource-limited settings. JAMA Netw Open. 2022;5(3):e221547.
Martinez A, Baltazar M, Loera A, Rivera R, Aguilera M, Garza M, et al. Addressing barriers to successful implementation of a pediatric early warning system (PEWS) at a pediatric oncology unit in a general hospital in Mexico. In: Pediatric Blood & Cancer. Hoboken: Wiley; 2019. p. S533–4.
Rivera J, Hernandez C, Mata V, Espinoza S, Nuñez M, Perez Y, et al. Improvement of clinical indicators in hospitalized pediatric oncology patients following implementation of a pediatric early warning score system. In: Pediatric Blood & Cancer. Hoboken: Wiley; 2019. p. S536–7.
Vergara P, Saez S, Palma J, Soberanis D, Agulnik A. Implementation of a pediatric early warning system in pediatric patients undergoing hematopoietic stem cell transplantation in Latin America. In: Pediatric Blood & Cancer. Hoboken: Wiley; 2017. p. S24–S24.
Diaz-Coronado R, Pascual Morales C, Rios Lopez L, Morales Rivas R, Muniz-Talavera H, Carrillo A, et al. Reduce mortality in children with cancer after implementation of a pediatric early warning system (PEWS): a multicenter study in Peru. In: Pediatric Blood & Cancer. Hoboken: Wiley; 2021. p. S52–3.
Fing E, Tinoco R, Paniagua F, Marquez G, Talavera HM, Agulnik A. Decrease in mortality is observed after implementing a pediatric early warning system in a pediatric oncology unit of the General Hospital of Celaya, Mexico. In: Pediatric Blood & Cancer. Hoboken: Wiley; 2021. p. S327–8.
Mirochnick E, Graetz DE, Ferrara G, Puerto-Torres M, Gillipelli SR, Elish P, et al. Multilevel impacts of a pediatric early warning system in resource-limited pediatric oncology hospitals. Front Oncol. 2022;12:1018224.
Agulnik A, Muniz-Talavera H, Pham LTD, Chen Y, Carrillo AK, Cárdenas-Aguirre A, et al. Effect of paediatric early warning systems (PEWS) implementation on clinical deterioration event mortality among children with cancer in resource-limited hospitals in Latin America: a prospective, multicentre cohort study. Lancet Oncol. 2023;24(9):978–88.
Agulnik A, Ferrara G, Puerto M, Gillipelli S, Elish P, Muniz-Talavera H, et al. Barriers and enablers of implementation of Pediatric Early Warning Systems (PEWS) in resource-limited pediatric oncology centers. Hoboken: Wiley; 2021. p. S74.
Wiphatphumiprates PP, Graetz DE, Ferrara G, Puerto-Torres M, Gillipelli SR, Elish P, et al. The COVID-19 Pandemic’s impact on sustainability and expansion of a pediatric early warning system in resource-limited hospitals. Cancer Med. 2023;12(10):11878–88.
Agulnik A, Schmidt-Grimminger G, Ferrara G, Puerto-Torres M, Gillipelli SR, Elish P, et al. Challenges to sustainability of pediatric early warning systems (PEWS) in low-resource hospitals in Latin America. Front Health Serv. 2022;2:1004805.
Malone S, Prewitt K, Hackett R, Lin JC, McKay V, Walsh-Bailey C, et al. The clinical sustainability assessment tool: measuring organizational capacity to promote sustainability in healthcare. Implement Sci Commun. 2021;2(1):77.
Agulnik A, Malone S, Puerto-Torres M, Gonzalez-Ruiz A, Vedaraju Y, Wang H, et al. Reliability and validity of a Spanish-language measure assessing clinical capacity to sustain Paediatric Early Warning Systems (PEWS) in resource-limited hospitals. BMJ Open. 2021;11(10):e053116.
Bosker R. Multilevel analysis: an introduction to basic and advanced multilevel modeling. 2011. p. 1–368.
Luke D. The clinical sustainability assessment tool (CSAT): assessing sustainability in clinical medicine settings. Academy Health; 2018. Available from: https://academyhealth.confex.com/academyhealth/2018di/meetingapp.cgi/Paper/28543 [cited 2023 Aug 7].
Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. USA: Oxford University Press; 2003. p. 672.
Luke DA. Multilevel modeling. California: SAGE Publications; 2019. p. 114.
Arnold B, Hogan D, Colford J, Hubbard A. Simulation methods to estimate design power: an overview for applied research. BMC Med Res Methodol. 2011;20(11):94.
Arend M, Schäfer T. Statistical power in two-level models: a tutorial based on Monte Carlo simulation. Psychol Methods. 2018;24:1–19.
Cohen J. Statistical power analysis for the behavioral sciences. United States: Academic Press; 2013. p. 459.
Fernandez ME, Ten Hoor GA, van Lieshout S, Rodriguez SA, Beidas RS, Parcel G, et al. Implementation mapping: using intervention mapping to develop implementation strategies. Front Public Health. 2019;7:158.
Rose AJ, McCullough MB. A practical guide to using the positive deviance method in health services research. Health Serv Res. 2017;52(3):1207–22.
Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Policy Ment Health. 2015;42(5):533–44.
Jayasekara RS. Focus groups in nursing research: methodological perspectives. Nurs Outlook. 2012;60(6):411–6.
Okuyama A, Wagner C, Bijnen B. Speaking up for patient safety by hospital-based health care professionals: a literature review. BMC Health Serv Res. 2014;14(1):61.
Graetz DE, Sniderman E, Villegas CA, Kaye EC, Ragab I, Laptsevich A, et al. Resilient health care in global pediatric oncology during the COVID-19 pandemic. Cancer. 2022;128(4):797–807.
Graetz D, Rivas S, Fuentes L, Cáceres-Serrano A, Ferrara G, Antillon-Klussmann F, et al. The evolution of parents’ beliefs about childhood cancer during diagnostic communication: a qualitative study in Guatemala. BMJ Glob Health. 2021;6(5):e004653.
Glaser BG. The constant comparative method of qualitative analysis. Soc Probl. 1965;12(4):436–45.
Saldaña J. The coding manual for qualitative researchers. United States: Sage; 2009. p. 223.
Hennink M, Kaiser BN. Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med. 2022;292:114523.
Aarons GA, Fettes DL, Sommerfeld DH, Palinkas L. Mixed methods for implementation research: application to evidence-based practice implementation and staff turnover in community based organizations providing child welfare services. Child Maltreat. 2012;17(1):67–79. https://doi.org/10.1177/1077559511426908.
Creswell J, Clark V, Gutmann M, Hanson W. Advanced mixed methods research designs. In: Handbook of mixed methods in social and behavioral research. 2003. p. 209–40.
Guetterman TC, Fetters MD, Creswell JW. Integrating quantitative and qualitative results in health science mixed methods research through joint displays. Ann Fam Med. 2015;13(6):554–61.
Palinkas LA, Aarons GA, Horwitz S, Chamberlain P, Hurlburt M, Landsverk J. Mixed method designs in implementation research. Adm Policy Ment Health Ment Health Serv Res. 2011;38(1):44–53.
Brown CH, Curran G, Palinkas LA, Aarons GA, Wells KB, Jones L, et al. An overview of research and evaluation designs for dissemination and implementation. Annu Rev Public Health. 2017;38(1):1–22.
Bartholomew LK, Parcel GS, Kok G. Intervention mapping: a process for developing theory and evidence-based health education programs. Health Educ Behav. 1998;25(5):545–63.
Bartholomew Eldredge LK. Planning health promotion programs: an intervention mapping approach. 4th ed. San Francisco: Jossey-Bass & Pfeiffer Imprints, Wiley; 2016. p. 678. Jossey-Bass Public Health.
Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. 2013;8(1):139.
Clinical Sustainability Assessment Tool. Available from: https://sustaintool.org/csat/. [cited 2023 Aug 8].
NOT-CA-20–025: Notice of Special Interest (NOSI): Dissemination and Implementation Science for Cancer Prevention and Control in Low Resource Environments. Available from: https://grants.nih.gov/grants/guide/notice-files/NOT-CA-20-025.html. [cited 2023 Aug 8].
Acknowledgements
We would like to acknowledge the support for this work from the Proyecto EVAT Steering Committee, represented by Dr. Carlos Acuña, and the grant External Advisory Board (Drs. Carlos Rodriguez-Galindo, Rachel Shelton, and Christopher Dandoy).
Funding
This study is supported by the National Cancer Institute grant R37CA276215-01 awarded to Drs. Agulnik and McKay.
Author information
Authors and Affiliations
Contributions
VM and AA drafted the initial sections of the manuscript. SM, DL, MD, and DG supported the conceptualization of the study, and MPT AC, and KP supported hospital and practitioner collaborations on the project. BC, YC, MD, and SM have supported analyses leading to the development of this project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All protocols and procedures were approved by the Institutional Review Board at St. Jude Children’s Research Hospital. PEWS implementation and the CDE quality improvement registry were determined to be non-human subjects research (quality improvement); survey data and qualitative data collection were determined as Exempt.
Consent for publication
Not applicable.
Competing interests
The authors declare that they do not have any competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
McKay, V., Carothers, B., Graetz, D. et al. Sustainability determinants of an intervention to identify clinical deterioration and improve childhood cancer survival in Latin American hospitals: the INSPIRE study protocol. Implement Sci Commun 4, 141 (2023). https://doi.org/10.1186/s43058-023-00519-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43058-023-00519-y