Abstract
Background
The champion model is increasingly being adopted to improve uptake of guideline-based care in long-term care (LTC). Studies suggest that an on-site champion may improve the quality of care residents’ health outcomes. This review assessed the effectiveness of the champion on staff adherence to guidelines and subsequent resident outcomes in LTC homes.
Method
This was a systematic review and meta-analyses of randomised controlled trials. Eligible studies included residents aged 65 or over and nursing staff in LTC homes where there was a stand-alone or multi-component intervention that used a champion to improve staff adherence to guidelines and resident outcomes. The measured outcomes included staff adherence to guidelines, resident health outcomes, quality of life, adverse events, satisfaction with care, or resource use. Study quality was assessed with the Cochrane Risk of Bias tool; evidence certainty was assessed using the GRADE approach.
Results
After screening 4367 citations, we identified 12 articles that included the results of 1 RCT and 11 cluster-RCTs. All included papers evaluated the effects of a champion as part of a multicomponent intervention. We found low certainty evidence that champions as part of multicomponent interventions may improve staff adherence to guidelines. Effect sizes varied in magnitude across studies including unadjusted risk differences (RD) of 4.1% [95% CI: − 3%, 9%] to 44.8% [95% CI: 32%, 61%] for improving pressure ulcer prevention in a bed and a chair, respectively, RD of 44% [95% CI: 17%, 71%] for improving depression identification and RD of 21% [95% CI: 12%, 30%] for improving function-focused care to residents.
Conclusion
Champions may improve staff adherence to evidence-based guidelines in LTC homes. However, methodological issues and poor reporting creates uncertainty around these findings. It is premature to recommend the widespread use of champions to improve uptake of guideline-based care in LTC without further study of the champion role and its impact on cost.
Trial registration
PROSPERO CRD42019145579. Registered on 20 August 2019.
Similar content being viewed by others
Background
Despite its benefits, care provided to residents in long-term care (LTC) homes (e.g. oral hygiene care, pressure ulcer prevention and infection control) is not always evidence-based [1, 2]. This is in part due to the changing needs of older residents in LTC home settings (e.g. decreased ability to perform self-care and/or physical activities of daily living) resulting in the need for increasing staff education and policies about the best methods to provide this care for residents. To this end, guidelines and interventions have been developed for many problem topic areas facing LTC homes. These include, for example, guidelines for the prevention and treatment of pressure ulcers [3, 4], oral health care guidelines [5] and interventions designed to improve well-being for patients with dementia [4] or to reduce functional decline [6]. However, even with the increase of toolkits and training to assist with uptake of best practices, implementation of these practices has been sub-optimal, possibly due to multiple factors ranging from staff turnover and competing interests to forgetfulness [7]. Adherence to the guidelines or intervention protocol must be also be considered in terms of implementation outcomes since the extent to which guidelines or interventions work is directly impacted by whether or not they were implemented as intended [8].
The champion model is being increasingly adopted in areas of care that have proven resistant to improvement, e.g. oral health [9], incontinence [10] and infection control [11]. Studies suggest that having at least one on-site champion may help improve the quality of care in that area and thereby the residents’ health outcomes [11–16]. Although there is no standard definition of a champion in the implementation literature, common elements of a champion for supporting change in healthcare settings include being a staff member (who either volunteers or is assigned an additional level of responsibility), who may perform a number of different roles in order to improve staff adherence to a particular guideline, policy or intervention [17]. A champion is different than an opinion leader [18]; unlike opinion leaders, champions are typically equal to their peers or colleagues and do not have a higher social or work status [18]. Champions may fill a diverse number or combination of roles such as advocating and/or leading practice change [19, 20], building relationships and educating peers and other staff to encourage and engage them in QI initiatives [19, 21] and acting as a resource or mentoring (including modelling and reinforcing desired behaviour) to facilitate the implementation of protocol interventions [19, 22].
To date, there has been no review of the effectiveness of the champion model for improving adherence to guideline-based care in LTC homes. This systematic review assessed the effectiveness of the champion on staff adherence to guidelines and subsequent resident outcomes in LTC homes.
Methods
Here, we provide a succinct overview of our methods, a thorough description of which is included in our prospectively registered protocol (PROSPERO 2019 CRD42019145579). We developed the protocol in accordance with guidance from the Cochrane Effective Practice and Organization of Care (EPOC) group [23] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [24] (Additional file 1).
Searches
We searched four databases (CENTRAL, MEDLINE, Embase, CINAHL), two trial registries (ICTRP and ClinicalTrials.gov) and three sources of grey literature (ProQuest Dissertations and Theses, Science Citation Index Expanded, and Conference Proceedings Citation Index - ISI Web of Knowledge) from inception to July 2020 (as well as reference lists of included studies and relevant reviews). We used a sensitive search strategy with terms for champions, long-term care homes and older adults (Additional file 2).
Study characteristics
We used the Population, Intervention, Comparator, Outcomes (PICO) framework [25] to define our selection criteria.
Population
We included studies with participants aged 65 years and older located in LTC homes where the intervention involved designating a nursing home staff member as a champion. The staff member could include registered nurses, licensed practical nurses, personal care attendants, personal support workers or nursing aides.
Intervention
We defined a champion as an internal nursing staff member who had an implementation-related role, had received supplementary training, assumed responsibility for a specific topic area (e.g. pressure ulcer prevention) and may have acted as a key contact person with external healthcare providers (e.g. dieticians, physiotherapists, oral health specialists). Importantly, we excluded studies where the designated champion was filled by an external, high-level, educationally-influential opinion leader such as those described in Flodgren et al. [18]. Guided by the Institute of Medicine’s definition of guidelines, we included any intervention that aimed to implement a clinical practice guideline or an evidence-based recommendation that optimised patient care. Moving forward, we will use the term “guidelines” to refer to both clinical practice guidelines and evidence-based recommendations as described above.
Comparator(s)
We included the following comparison groups:
-
1.
No intervention group (no implementation strategies tested)
-
2.
Another intervention (which may or may not have included a champion)
Outcomes
We selected outcomes for this review from the list recommended by the Effective Practice and Organization (EPOC) group [26]. The primary outcome of this study was adherence to guidelines (a quality-of-care outcome outlined by the EPOC group [26]. Secondary outcomes included other EPOC-recommended outcomes such as patient outcomes (resident health outcomes, quality of life, satisfaction with care, adverse events) and resource use.
Study designs
We included only randomised controlled trials (RCTs) and cluster RCTs, as these are considered the gold-standard study design to assess the effectiveness of an intervention.
Study selection and data extraction
Titles, abstracts and full texts were independently screened by two authors in Covidence to identify RCTs that met the inclusion criteria [27]. We extracted information about the study characteristics, interventions, and outcomes [28]. Disagreements were resolved through discussion and, where necessary, adjudicated by a third author. When required, we contacted authors of studies to obtain data not available in the publication.
Quality assessment of included studies
Two authors independently assessed risk of bias (RoB) using the 9-item Cochrane risk of bias tool [29]. We considered three of the Cochrane RoB items to be essential (random sequence generation, allocation concealment, and incomplete outcome data). If we found a study to have high or unclear RoB for any of these three items, we considered it at a high risk of bias [29].
Contrasts
We assessed the following five comparisons: the effect of the champion as a stand-alone intervention compared to (i) no intervention or (ii) another intervention; (iii) the effect of the champion as part of an intervention compared to the same intervention without the champion (i.e., the additive effect of a champion); and the effect of the champion as part of a multicomponent intervention compared to (iv) no intervention or (v) another intervention.
Data coding and synthesis
We categorised the level of involvement of the champion in the interventions using the following descriptions defined by the review team:
-
(i)
Minor: Acted as role model and source of information for staff and possibly as a reminder of the intervention but was not responsible for educating staff or enacting any of the intervention components.
-
(ii)
Moderate: In addition to the responsibilities of the minor role, helped the research team to educate or mentor staff or assisted other members of the research team with activities.
-
(iii)
Major: In addition to the responsibilities of the moderate role, independently (i.e., without the research team) educated or mentored staff and enacted other components of the intervention such as action planning or using new clinical tools at the site.
For the effectiveness analysis, we pooled the results of studies with sufficient homogeneity of participants, interventions and outcomes and acceptable statistical heterogeneity (i2 < 50%) [30]. Given that the majority of our studies were cluster-RCTs, we used the adjusted between-group difference where possible, adjusted risk difference (RD) for dichotomous outcomes and adjusted mean difference (MD) for continuous outcomes. For cluster RCTs that adjusted for clustering in their analysis and reported the adjusted between-group difference, this score was used in the meta-analysis [31]. We used a conservative random-effects model for all meta-analyses using the generic inverse variance outcome method to allow for pooling of adjusted between-group differences [32]. If it was not possible to pool the results across studies due to heterogeneity, we reported a qualitative assessment of the effect [33].
Two review authors independently determined the certainty of the evidence for each outcome (high, moderate, low and very low) using the five GRADE considerations [34]. We produced a GRADE summary of findings table for each comparison [29, 35, 36].
Results
Results of the search
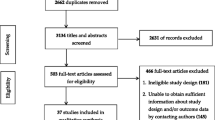
Electronic database searches identified 4367 unique citations (Fig. 1), 3860 of which were excluded following title and abstract screening. We reviewed 507 full texts; 328 were irrelevant and an additional 167 studies were excluded with reasons. Eleven cluster RCTs and one RCT were therefore included in the review [5, 6, 37–46].
Description of included studies (Table 1)
The included studies were conducted in Australia [37, 38], Belgium [5, 39], Canada [40], France [41], the Netherlands [42, 43], the UK [44, 45] and the USA [6]. There was also one multi-country study [46]. Ten studies targeted nursing staff, five of which also targeted additional care staff including physicians or allied health professionals (e.g. physiotherapists, pharmacists) [5, 38, 39, 41, 45] and two studies targeted nursing aides exclusively [6, 40]. The behaviours targeted by the interventions included adherence to guidelines for provision of oral hygiene [5, 40, 42], dementia care [43, 44], function-focused care [6] and palliative care [46], as well as assessment and management of malnutrition [37], detection of delirium [45], detection of depression [38], prevention of pressure ulcers [39] or infections [41].
Description of the champion intervention (Table 2; Additional file 3)
All included studies evaluated the effects of a champion as part of a multicomponent intervention. Where reported, the duration of the intervention ranged from 4 to 16 months. The frequency of how often the different intervention components were administered was poorly reported.
Training of and duties performed by the champion
In eight studies, a single staff member was appointed as a champion, while in four studies, a team of two or more champions was appointed [5, 43, 44, 46]. Details on how the champions were appointed were not provided. Training intensity (e.g. frequency, number and length of sessions) was only reported in two studies, which ranged from 2.5–15 h [6, 45].
In addition to receiving training and providing general oversight regarding the implementation of the recommendations, some champions were tasked with extra responsibilities. Most commonly, this included delivery of some or all of the education sessions to LTC home staff [5, 37, 39–43, 45, 46] and liaising with the research team from one-off sessions to develop an initial action plan to weekly sessions for implementation support [5, 6, 37, 39–46]. All additional duties are outlined in Table 2. Overall, we found the champion to play a major role in ten studies as they were responsible for enacting the majority of the intervention components [5, 6, 37, 38, 40–43, 45, 46]. In the remaining two studies, the champions had either a moderate [39] or minor role [44].
Other intervention components (affecting all LTC staff, including the champion)
All studies included education or training sessions for LTC home staff as one of the main intervention components. One study did not use any additional components beyond education [40, 46]. Amongst the 11 remaining studies, six also provided some form of mentoring or motivation training [5, 6, 38, 42, 44, 45], seven included monitoring via direct observation [5, 6, 39, 41–44], five provided written or oral feedback on performance [5, 39, 42–44], three used goal setting or action planning [6, 37, 43], three included staff reminders via posters [37, 39, 41] and one used pocket cards [39]. Six studies provided tools to help enact the desired behaviour [5, 38, 39, 41, 42, 45]. These included new screening tools to identify depression [38], delirium [45] and people at risk of pressure ulcers [39], as well as tools to improve the use of hand sanitiser [41] and oral hygiene products (for use with residents) [5, 42].
Risk of bias in included studies (Table 1; Additional file 4)
We found that all studies were at high risk of bias. Amongst the three pre-specified criteria (appropriate sequence generation, concealed allocation and complete outcome data), most were judged to have an unclear risk of bias on randomisation (n = 5) and/or allocation (n = 10) due to lack of information to make an accurate judgement. In addition, more than half of the studies (n = 7) were found to have incomplete outcome data on the primary outcome of staff adherence and/or the resident outcomes. Also, 4 of the 11 cluster RCTs did not adjust for clustering in their analysis placing them at risk of presenting misleading results.
Effectiveness of the champion interventions
We found no studies assessing the effect of a champion as a stand-alone intervention compared to no intervention or another intervention
Effect of an intervention with a champion compared to the same intervention without the champion (Table 3)
Staff adherence
One RCT (69 staff) with low certainty evidence suggested that adding a champion to an implementation intervention may improve adherence (RD = 23% [95% CI: 5%, 52%]) to correctly detecting depression amongst residents [38]. No other outcomes were assessed in this comparison.
Effects of champions as part of multicomponent interventions compared to no intervention (Table 3)
Staff adherence
Staff adherence was assessed objectively by members of the research team in three studies (2 clusters RCTs and 1 staff-randomised RCT including 15 clusters and a total of 260 staff). Heterogeneity in the type of guidelines assessed, target behaviour, and adherence measures used across studies meant that meta-analysis was inappropriate. Overall, we found low certainty evidence that champions as part of multicomponent interventions may improve staff adherence to guidelines. The effect sizes varied in magnitude across studies including unadjusted risk differences (RD) of 4.1% [95% CI: -3%, 9%] to 44.8% [95% CI: 32%, 61%] for improving pressure ulcer prevention in a bed and a chair respectively [39], an RD of 44% [95% CI: 17%, 71%] for improving depression identification [38] and an RD of 21% [95% CI: 12%, 30%] for improving function-focused care to residents [6]. All results were unadjusted for baseline differences.
Resident clinical health outcomes
Eleven studies reported residents’ clinical health outcomes [5, 6, 37, 39–46]. Three assessed oral hygiene [5, 40, 42], two assessed agitation [43, 44] and the remaining five assessed either physical function [6], comfort in the last week of life [46], pressure ulcer prevalence [39], malnutrition [37], delirium [45] or infection rate [41]. Meta-analysis was not suitable for outcomes of oral hygiene and agitation (Fig. 2). We found moderate certainty evidence that residents in LTC homes with the champion intervention had slight reductions in dental plaque (adjusted MD = − 0.28 [95% CI: − 0.55, 0.00]; 37 clusters, 167 residents) and denture plaque (adjusted MD = − 0.34 [95% CI: − 0.50, − 0.18]; 37 clusters, 388 residents) and low certainty evidence of little or no effect of champion interventions on agitation levels (adjusted MD = 0.49 [95% CI: − 2.39, 3.37], 31 clusters, 503 residents). Amongst the other clinical outcomes, we found either no significant difference (malnutrition, comfort in the last week of life, delirium, infection rate, category II–IV pressure ulcer prevalence) or a slight improvement in the clinical outcome (physical function, category I–IV pressure ulcer prevalence) for those in the LTC facilities with the champion intervention. These results, however, were uncertain as they were based on very low certainty evidence from single studies (data presented in Table 3).
Quality of life
Results from three studies (45 clusters, 653 residents) provide very low certainty evidence to suggest that champions as part of multicomponent interventions improve care for dementia and prevention of delirium, but have no effect on resident quality of life (unadjusted MD = 0.03 [95% CI: − 0.01, 0.07]).
Adverse outcome
We found very low certainty evidence from one study (4 clusters, 169 residents) of no significant difference on resident adverse events related to a function-focused care programme between groups receiving the multicomponent intervention with a champion or no intervention. Unadjusted RDs for (i) injury (RD = 7% [95% CI: − 5%, 20%]), (ii) falls (RD = 1% [95% CI: − 14%, 16%]) and (iii) ED visits related to falls (RD = 4% [95% CI: − 2%, 10%]) [6].
Satisfaction with care
We found very low certainty evidence from one study (73 clusters, 913 residents) that there is no significant difference in residents’ satisfaction with care between those receiving the champion intervention or no intervention (adjusted MD = 1.72 [95% CI: − 0.15, 3.59]) [46].
Resource use
We found very low certainty evidence from two studies (18 clusters, 261 residents) of a reduction in hospital admissions for those groups receiving the champion as part of a multicomponent intervention. Meta-analysis was not performed due to differences in how hospital admissions were defined and timepoint assessed. Overall, the reductions reported as unadjusted RD ranged from 7% [95% CI: − 15%, 0%] [6] to 22% [95% CI: − 37%, − 7%] for those in the champion intervention group [6, 45].
Discussion
Summary of findings
This is the first systematic review assessing the effect of a champion intervention for improving adherence to guideline-based care in LTC homes. We found 12 RCTs testing a champion as part of a multicomponent intervention compared to no intervention. However, only three provided data on adherence; the majority instead assessed resident clinical health outcomes. Overall, our findings from the three studies in this comparison suggested that a champion as part of a multicomponent intervention may improve adherence to guidelines compared to no intervention. Importantly, since these interventions were multicomponent in nature, it is impossible to isolate the effectiveness of the champion from the other components. However, within each of these three studies, the champion played either a moderate or major role in the delivery of the intervention. For example, in all three studies, the champion delivered staff education/training and liaised with the research team to monitor progress and problem solve implementation issues as well as provide feedback to staff. Therefore, it is likely that they may have been a contributing factor to the effects on staff adherence. In addition, one of the three studies also assessed the effects of the intervention with and without a champion, which allowed us to estimate the additive effect of a champion [38]. The results of this study indicate that adherence to recommendations was greater when a champion was used, providing further support for the potential effectiveness of a champion as an implementation strategy. Taken together, we believe the evidence suggests that interventions that involve a champion and staff education and feedback on performance may improve staff adherence. However, given the moderate sample sizes of these three studies and the poor reporting of key risk of bias items, this estimate is considered to be of low to moderate certainty. Moreover, while we found one study that isolated the role of the champion and found it to be an effective strategy, this result is also very low certainty and needs further study.
With the exception of oral hygiene, there was either no significant difference or a slight improvement on resident outcomes for LTC homes with the champion intervention. For oral hygiene outcomes, we found moderate-quality evidence in favour of the champion intervention. It is perhaps not surprising that there is unclear evidence on resident clinical outcomes, since we would only anticipate change on these outcomes if the implementation intervention was successful at changing staff behaviour to provide the recommended guideline-based care. For eight of 10 studies, this information was not available and thus, it is unclear why resident outcomes remained unchanged. In the two studies that did measure guideline adherence and resident outcomes, the champion intervention had a positive effect on improving both staff adherence and residents’ clinical health outcomes [6, 39].
Findings in relation to other research
A recent integrative review [48] examining the role of the champion in supporting the implementation of evidence-based interventions into practice also found that champions, as a vehicle for implementation, exerted a positive influence on adherence to guidelines, recommendations and other relevant outcomes. Of the four randomised studies considered in this review, three were in areas we did not cover in the present review (neonatal units, schools, acute care hospital wards). The fourth was McCabe et al. [38], which is included in our review. Similar to our review, each of these studies found the presence of a champion led to a favourable outcome [48]. Thus, it would seem that our findings, although limited by the number of studies in this comparison, are in line with findings in other settings.
While our dataset is not sufficient to carry out post hoc analyses to explore which types of guidelines or interventions might benefit most from using a champion to boost implementation, we can draw on behaviour change theory and related evidence to infer how champions may be most effective. Perhaps the most comprehensive resources for designing theory-informed behaviour change interventions were produced by Michie and colleagues [49–51]. These include the Theoretical Domains Framework (TDF) the Behaviour Change Technique (BCT) Taxonomy, and the theory and techniques tool) [49–51]. The TDF framework is a synthesis of 33 different theories and includes 14 domains that represent the main drivers of behaviour change (e.g. knowledge, skills, social influences) [49]. The BCT Taxonomy provides a list of 93 techniques that can be used to change behaviour; these form the active components of an intervention (e.g. instruction on how to perform the behaviour, modelling, goal-setting, social support) [50]. The theory and techniques tool indicates which BCTs have been shown to be effective for each of the 14 TDF domains [51]. From a theoretical perspective, if we have an understanding of the TDF domains that are relevant to the implementation of a particular guideline or intervention, as well as an understanding of which domains are likely to be impacted by a champion, we can, at least conceptually, understand whether a champion is likely to be a useful implementation strategy to support adherence to that guideline or intervention.
We used the resources developed by Michie et al. [50] to first identify the behaviour change techniques at work in a typical champion-based intervention (see Additional file 5 for a list of common champion roles and responsibilities, the implicated BCTs and the TDF domains to which they relate). Using the theory and techniques tool, we then determined which TDF domains were linked with those techniques. The BCTs identified amongst champion roles and responsibilities were most commonly related to 4 key TDF domains that would determine behaviour change:
-
1.
Beliefs about capabilities (e.g. verbal persuasion about capability which could be involved in a mentoring role)
-
2.
Knowledge (e.g. provision of information commonly delivered through education sessions to staff members)
-
3.
Beliefs about consequences (e.g. salience of consequences which would occur when delivering staff education)
-
4.
Social influences (e.g. social support which would be a part of communication and building relationships with staff).
Therefore, from a theoretical perspective, the champions in these studies (with roles as described in Additional file 5) would be best placed to support teh implementation of and adherence to guidelines in which there were would likely be issues with, for example, lack of confidence to follow the guidelines, knowledge, social support or problems related to incorrect or unhelpful beliefs about the outcomes of following the guideline. For guidelines that may have other obstacles for implementation such as the ability of the healthcare professionals to retain required information (memory, attention and decision processes) the champion strategy, as commonly used in the literature and as enacted in the included studies in this review, may not yield the desired impacts on guideline adherence.
Strengths and limitations of the review
Only RCTs and cluster RCTs were included in this review. Other study designs, more susceptible to bias, were excluded. To avoid selection bias, all references were screened, data-extracted and RoB assessed by two reviewers. There is also the possibility of publication bias, where studies reporting a null effect of the intervention are not submitted for publication, or if submitted are not accepted for publication. While we did try to mitigate this by searching for grey literature, we were unable to assess the possible extent of publication bias due to the heterogeneous nature of the interventions.
Limitations of included studies
It is important to note that while the general duties of the champion were reported in most studies, many aspects of the interventions were not reported in sufficient detail to allow for replication or a more comprehensive understanding of intervention procedures for choosing or training the champions. For example, none of the included studies indicated how the champion was chosen or described the training provided to the champion (beyond the number of hours of training provided). While most studies provided the general role of the champion, the day to day procedures of how they enacted their role was missing, limiting our understanding of what the champions actually did. Most studies included small sample sizes and few actually measured adherence to guidelines which is the main aim of any implementation intervention, limiting the ability to determine its effectiveness. Moreover, important outcomes such as costing or resource use were rarely assessed so there was little information available for those who would wish to replicate or adopt the intervention.
Implications for clinical practice
While this review found some evidence to support the use of champions in multicomponent interventions to implement guidelines, at this time, the evidence is not strong enough to recommend their widespread use without further understanding their role and the impact on cost. For example, in each of these interventions, the champion held major responsibilities and extra duties which appear consistent with the definitions of champions in the wider LTC literature [17]. However, we have to consider the impact of any additional duties a champion role may have and what resource implications that may have. Without knowing the exact benefit of the champion portion of the intervention it is premature to suggest that this is a reliable implementation strategy. Moreover, the varied nature of the champion role across studies in terms of the scope of their duties also makes it hard to recommend a champion since we do not know which duties are most effective for change.
Future research
Future research should focus on designing studies with larger sample sizes and more robust methods to isolate the effects of the champion. For example, given that the use of champions may have resource implications, future studies should consider evaluating the additive clinical and cost-effectiveness of a champion to ascertain any added value for LTC homes. Additionally, investigators using cluster RCTs are popular in LTC settings should ensure they adjust for clustering in their analysis as per guidelines by Campbell et al. [52] to reduce risk of misleading results [52]. Of particular importance is the assessment of staff adherence in combination with resident clinical outcomes which was missing from the majority of studies. Finally, investigators should report (a) interventions in line with TiDier guidelines [28] to allow us to better understand the exact role of the champion and replicate or scale-up the intervention and (b) methodological components in line with the CONSORT statement to enable accurate risk of bias assessment.
Conclusions
The findings suggest that champions may improve staff adherence to evidence-based guidelines in LTC homes. These results align with evidence from champion interventions in other settings. However, the certainty around these findings remains low due to methodological issues and poor reporting of the included studies. It is premature to recommend the widespread use of champions to improve uptake of guideline-based care in LTC homes without further study of the champion(s)’ role and its impact on resources and cost.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Feldman PH, Kane RL. Strengthening research to improve the practice and management of long-term care. Milbank Q. Wiley Online Library. 2003;81:179–220.
Specht JK. Evidence based practice in long term care settings. J Korean Acad Nurs. 2013;43(2):145–53. https://doi.org/10.4040/jkan.2013.43.2.145.
Haesler E. National pressure ulcer advisory panel, european pressure ulcer advisory panel and pan pacific pressure injury alliance. Prevention and treatment of pressure ulcers: quick reference guide. Australia: Cambridge Media Perth; 2014. p. 14–32.
Kitwood T, Bredin K. Towards a theory of dementia care: personhood and well-being. Ageing Soc. 1992;12(03):269–87. https://doi.org/10.1017/S0144686X0000502X Cambridge University Press.
De Visschere L, Schols J, van der Putten G-J, de Baat C, Vanobbergen J. Effect evaluation of a supervised versus non-supervised implementation of an oral health care guideline in nursing homes: a cluster randomised controlled clinical trial. Gerodontology. 2012;29(2):e96–106. https://doi.org/10.1111/j.1741-2358.2010.00418.x.
Resnick B, Galik E, Gruber-Baldini A, Zimmerman S. Testing the effect of function-focused care in assisted living. J Am Geriatr Soc. 2011;59(12):2233–40. https://doi.org/10.1111/j.1532-5415.2011.03699.x.
Jablonski A, Ersek M. Nursing home staff adherence to evidence-based pain management practices. J Gerontol Nurs. 2009;35:28–34 SLACK Incorporated.
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Mental Health Mental Health Serv Res. 2011;38:65–76 Springer.
Bassim CW, Gibson G, Ward T, Paphides BM, DeNucci DJ. Modification of the risk of mortality from pneumonia with oral hygiene care. J Am Geriatr Soc. 2008;56(9):1601–7. https://doi.org/10.1111/j.1532-5415.2008.01825.x.
Ouslander JG. Quality improvement initiatives for urinary incontinence in nursing homes. J Am Med Dir Assoc. 2007;8(3):S6–11. https://doi.org/10.1016/j.jamda.2006.12.020.
Damschroder LJ, Banaszak-Holl J, Kowalski CP, Forman J, Saint S, Krein SL. The role of the “champion” in infection prevention: results from a multisite qualitative study. BMJ Qual Saf. 2009;18(6):434–40. https://doi.org/10.1136/qshc.2009.034199.
Lee R, Scott F. Competent to care. A train-the-trainer method of teaching as a way of implementing the correct use of the’Malnutrition Universal Screening Tool’in Norfolk: is it effective? Proc Nutr Soc. 2009;68(3):300–5. https://doi.org/10.1017/S002966510900127X.
Nicol R, Petrina Sweeney M, McHugh S, Bagg J. Effectiveness of health care worker training on the oral health of elderly residents of nursing homes. Community Dent Oral Epidemiol. 2005;33(2):115–24. https://doi.org/10.1111/j.1600-0528.2004.00212.x.
Shaw EK, Howard J, West DR, Crabtree BF, Nease DE, Tutt B, et al. The role of the champion in primary care change efforts: from the State Networks of Colorado Ambulatory Practices and Partners (SNOCAP). J Am Board Fam Med. 2012;25(5):676–85. https://doi.org/10.3122/jabfm.2012.05.110281.
Wårdh I, Berggren U, Hallberg LR-M, Andersson L, Sörensen S. Dental auscultation for nursing personnel as a model of oral health care education: development, baseline, and 6-month follow-up assessments. Acta Odontol Scand. 2002;60(1):13–9. https://doi.org/10.1080/000163502753471943.
Wårdh I, Hallberg LR-M, Berggren U, Andersson L, Sörensen S. Oral health education for nursing personnel; experiences among specially trained oral care aides: one-year follow-up interviews with oral care aides at a nursing facility. Scand J Caring Sci. 2003;17(3):250–6. https://doi.org/10.1046/j.1471-6712.2003.00214.x.
Woo K, Milworm G, Dowding D. Characteristics of Quality Improvement Champions in Nursing Homes: A Systematic Review With Implications for Evidence-Based Practice. Worldviews Evid Based Nurs. 2017;14(6):440–6. https://doi.org/10.1111/wvn.12262.
Flodgren G, Parmelli E, Doumit G, Gattellari M, O'Brien MA, Grimshaw J, Eccles MP. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2019;6:CD000125.
Soo S, Berta W, Baker GR. Role of champions in the implementation of patient safety practice change. Healthc Q. 2009;12 Spec No Patient:123-8. https://doi.org/10.12927/hcq.2009.20979.
Fesmire FM, Peterson ED, Roe MT, Wojcik JF. Early use of glycoprotein IIb/IIIa inhibitors in the ED treatment of non-ST-segment elevation acute coronary syndromes: a local quality improvement initiative. Am J Emerg Med. 2003;21:302–8 Elsevier.
Rantz MJ, Zwygart-Stauffacher M, Hicks L, Mehr D, Flesner M, Petroski GF, et al. Randomized multilevel intervention to improve outcomes of residents in nursing homes in need of improvement. J Am Med Dir Assoc. 2012;13:60–8 Elsevier.
Kaasalainen S, Brazil K, Akhtar-Danesh N, Coker E, Ploeg J, Donald F, et al. The evaluation of an interdisciplinary pain protocol in long term care. J Am Med Dir Assoc. 2012;13:664. e1–8.
Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for review authors. 2017. Available from: epoc.cochrane.org/epoc-resources-review-authors
Moher D, Liberati AA, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(jul21 1):b2535. https://doi.org/10.1136/bmj.b2535.
Thomas J, Kneale D, McKenzie JE, Brennan SE, Bhaumik S. Determining the scope of the review and the questions it will address. In Cochrane Handbookfor Systematic Reviews of Interventions (eds Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA). 2019. https://doi.org/10.1002/9781119536604.ch2.
Cochrane Effective Practice and Organisation of Care (EPOC). What outcomes should be reported in Cochrane Effective Practice and Organisation of Care (EPOC) reviews?. 2017 [cited 2021 Jun 14]. Available from: https://epoc.cochrane.org/resources/epoc-resources-review-authors
Covidence systematic review software. Melbourne: Veritas Health Innovation; Available from: www.covidence.org
Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, Altman DG, Barbour V, Macdonald H, Johnston M, Lamb SE, Dixon-Woods M, McCulloch P, Wyatt JC, Chan AW, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. https://doi.org/10.1136/bmj.g1687.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928 British Medical Journal Publishing Group.
Effective Practice and Organisation of Care (EPOC). Analysis in EPOC reviews. Oslo: Norwegian Knowledge Centre for the Health Services; 2014.
Deeks JJ, Higgins JP, Altman DG. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions (eds Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA). 2019. https://doi.org/10.1002/9781119536604.ch10.
Higgins JPT, Green S. Extracting study results and converting to the desired format. In: Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2009.
Effective Practice and Organisation of Care (EPOC). Synthesising results when it does not make sense to do a meta-analysis. Oslo: Norwegian Knowledge Centre for the Health Services; 2014.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD British Medical Journal Publishing Group.
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. https://doi.org/10.1016/j.jclinepi.2010.09.011.
Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Cochrane Handbook for Systematic Reviews of Interventions Version, vol. 5; 2011.
Gaskill D, Isenring EA, Black LJ, Hassall S, Bauer JD. Maintaining nutrition in aged care residents with a train-the-trainer intervention and Nutrition Coordinator. J Nutr Health Aging. 2009;13(10):913–7. https://doi.org/10.1007/s12603-009-0251-2.
McCabe MP, Karantzas GC, Mrkic D, Mellor D, Davison TE. A randomized control trial to evaluate the beyondblue depression training program: does it lead to better recognition of depression? Int J Geriatr Psychiatry. 2013;28(3):221–6. https://doi.org/10.1002/gps.3809.
Beeckman D, Clays E, Van Hecke A, Vanderwee K, Schoonhoven L, Verhaeghe S. A multi-faceted tailored strategy to implement an electronic clinical decision support system for pressure ulcer prevention in nursing homes: a two-armed randomized controlled trial. Int J Nurs Stud. 2013;50(4):475–86. https://doi.org/10.1016/j.ijnurstu.2012.09.007.
MacEntee MI, Wyatt CCL, Beattie BL, Paterson B, Levy-Milne R, McCandless L, et al. Provision of mouth-care in long-term care facilities: an educational trial. Community Dent Oral Epidemiol. 2007;35(1):25–34. https://doi.org/10.1111/j.1600-0528.2007.00318.x.
Chami K, Gavazzi G, Bar-Hen A, Carrat F, de Wazières B, Lejeune B, et al. A short-term, multicomponent infection control program in nursing homes: a cluster randomized controlled trial. J Am Med Dir Assoc. 2012;13:569. e9–569. e17.
van der Putten G-J, Mulder J, de Baat C, De Visschere LM, Vanobbergen JN, Schols JM. Effectiveness of supervised implementation of an oral health care guideline in care homes; a single-blinded cluster randomized controlled trial. Clin Oral Investig. 2013;17(4):1143–53. https://doi.org/10.1007/s00784-012-0793-2.
van de Ven G, Draskovic I, Adang EM, Donders R, Zuidema SU, Koopmans RT, et al. Effects of dementia-care mapping on residents and staff of care homes: a pragmatic cluster-randomised controlled trial. PLoS One. 2013;8:e67325.
Livingston G, Barber J, Marston L, Stringer A, Panca M, Hunter R, et al. Clinical and cost-effectiveness of the managing agitation and raising quality of life (MARQUE) intervention for agitation in people with dementia in care homes: a single-blind, cluster-randomised controlled trial. Lancet Psychiatry. 2019;6(4):293–304. https://doi.org/10.1016/S2215-0366(19)30045-8 Elsevier.
Siddiqi N, Cheater F, Collinson M, Farrin A, Forster A, George D, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing. 2016;45(5):652–61. https://doi.org/10.1093/ageing/afw091.
Van den Block L, Honinx E, Pivodic L, Miranda R, Onwuteaka-Philipsen BD, van Hout H, Pasman HRW, Oosterveld-Vlug M, Ten Koppel M, Piers R, Van Den Noortgate N, Engels Y, Vernooij-Dassen M, Hockley J, Froggatt K, Payne S, Szczerbinska K, Kylänen M, Gambassi G, Pautex S, Bassal C, De Buysser S, Deliens L, Smets T; PACE trial group. Evaluation of a Palliative Care Program for Nursing Homes in 7 Countries: The PACE Cluster-Randomized Clinical Trial. JAMA Intern Med. 2020;180(2):233-42. https://doi.org/10.1001/jamainternmed.2019.5349.
Beeckman D, Clays E, Van Hecke A, Vanderwee K, Schoonhoven L, Verhaeghe S. A multi-faceted tailored strategy to implement an electronic clinical decision support system for pressure ulcer prevention in nursing homes: a two-armed randomized controlled trial. Int J Nurs Stud. 2013;50(4):475-86. https://doi.org/10.1016/j.ijnurstu.2012.09.007. Epub 2012 Oct 1.
Miech EJ, Rattray NA, Flanagan ME, Damschroder L, Schmid AA, Damush TM. Inside help: an integrative review of champions in healthcare-related implementation. SAGE Open Med. 2018;6:2050312118773261.
Cane J, O’Connor D, Michie S. Validation of the theoretical domains framework for use in behaviour change and implementation research. Implement Sci. 2012;7:1–17 Springer.
Michie S, Wood CE, Johnston M, Abraham C, Francis JJ, Hardeman W. Behaviour changetechniques: the development and evaluation of a taxonomic method for reporting and describing behaviour change interventions (a suite of five studies involving consensus methods, randomised controlled trials and analysis of qualitative data). Health Technol Assess. 2015;19(99):1-188. https://doi.org/10.3310/hta19990.
Human Behaviour Change Project. The Theory and Techniques Tool. Theory and Techniques Tool. [cited 2021 Jun 14]. Available from: https://theoryandtechniquetool.humanbehaviourchange.org/tool
Campbell MK, Mollison J, Steen N, Grimshaw JM, Eccles M. Analysis of cluster randomized trials in primary care: a practical approach. Fam Pract. 2000;17(2):192–6. https://doi.org/10.1093/fampra/17.2.192 Oxford University Press.
Acknowledgements
We would like to acknowledge the support of several research personnel at the Cochrane EPOC group and Memorial University. The review authors gratefully acknowledge the support of the Cochrane EPOC Group’s UK Satellite, which is supported by an NIHR Cochrane Programme Grant in collaboration with the Cochrane Effective Practice and Organization of Care (EPOC) Group’s Information Specialist, Paul Miller, who developed the original search strategy. We acknowledge Michelle Swab, Public Services Librarian at Memorial University who reviewed and updated the original search. We acknowledge Andrea Pike, Senior Researcher at the Faculty of Medicine at Memorial University for her contribution for reviewing and copy-editing the manuscript in preparation for publication.
Funding
This review does not have an official sponsor or funding.
Author information
Authors and Affiliations
Contributions
SW, AS, GF and Ah were involved in writing the protocol. AS, SW, AH, BF, JT and HR were involved in screening and data extraction. AH, HR and BF were involved in the analysis. AH, GF, HR, BF and SW were involved in writing the results and the manuscript. All authors were involved in reviewing and providing comment on the manuscript. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflicts of interest to report.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
PRISMA checklist.
Additional file 2.
Example of search strategy (MEDLINE).
Additional file 3.
Summaries of included studies.
Additional file 4.
Risk of bias table.
Additional file 5.
Champion roles linked with relevant behavior change techniques and associated theoretical domains.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hall, A.M., Flodgren, G.M., Richmond, H.L. et al. Champions for improved adherence to guidelines in long-term care homes: a systematic review. Implement Sci Commun 2, 85 (2021). https://doi.org/10.1186/s43058-021-00185-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43058-021-00185-y