Abstract
Background
Aortic valve repair in rheumatic patients is not well-studied. We aimed to present our initial Egyptian experience in the aortic valve repair and compare it with the aortic valve replacement. The study included 85 patients who had an aortic valve surgery for aortic regurgitation (AR) in a single center from 2018 to 2020. We assigned the patients to either aortic valve repair (n= 39) or aortic valve replacement (n= 46). Fifty-nine patients (69.4%) had rheumatic heart disease. Study outcomes were hospital complications and the degree of aortic regurgitation after 6 months in patients who had aortic valve repair.
Results
Patients who had replacement were significantly older (49.6± 7.2 vs. 43.8± 8.6 years: P= 0.002) and had more advanced New York Heart Association (P<0.001) and Canadian Cardiovascular Scoring (P= 0.03) classes. Hypertension (31 (67.4%) vs. 17 (43.6%); P= 0.03) and hypercholesteremia (18 (40%) vs. 17 (18.9%); P= 0.04) were more common in the replacement group. Patients who had replacement had a significantly higher percentage of valve retraction (P<0.001). Cardiopulmonary bypass (54.5 (49.5–60) vs. 45 (41–49) min; P<0.001) and ischemic times (36.5 (31–40) vs. 30 (28–33) min; P<0.001) were longer in patients who had an aortic valve replacement. Blood transfusion (28 (60.9%) vs. 11 (282%); P= 0.003) and ICU stay (24.5 (24–48) vs 23 (20–31) h; P= 0.01) were higher in the replacement group. Hospital mortality was non-significantly different between groups. Four patients had trivial AR (10.3%), and six had mild AR (15.4%) in the repair group. There was no difference in valve pathology or outcomes in aortic valve repair patients for degenerative versus rheumatic pathologies. After a 6-month follow-up, four patients had trivial AR (10.3%), and six patients had mild AR (15.4%) in the repair group.
Conclusions
Aortic valve repair could be an alternative to replacement in selected patients with rheumatic heart disease. Shorter cardiopulmonary bypass and ischemic times may improve repair outcomes compared to replacement.
Similar content being viewed by others
Background
Aortic valve repair (AVr) could be an alternative to aortic valve replacement (AVR) in selected patients. The technique has several potential benefits over the conventional AVR, including a lower risk of infective endocarditis, reduced anticoagulation-related complications compared to the mechanical valves, and better durability compared to bioprosthetic valves [1].
The European Association for Cardio-Thoracic Surgeons/European Society of Cardiology guidelines for heart valve disease published in 2017 stated that “Heart Team discussion” for selecting patients suitable for AVr. AVr can be considered in patients with pliable, non-calcified valves (Class IC indication) [2]. Aortic valve repair is technically challenging, and the new guidelines recommended aortic valve repair in experienced centers only [3].
The aortic valve repair techniques are still evolving, and several modifications have been described [4]. The procedure’s outcome may be affected by the center experience and the valve pathology. There is a paucity of studies comparing repair and replacement, and patients with rheumatic heart disease are still under-presented in the published series. Therefore, we aimed to present our initial Egyptian experience in aortic valve repair and compare it with aortic valve replacement performed during the same timeline.
Methods
Study design and patients
We performed a retrospective study comparing aortic valve repair and replacement. The study included 85 patients who had aortic valve surgery in a single center from July 2018 to July 2020. Patients were assigned to either aortic valve repair or replacement by the heart team. Patients were selected to either group after comprehensive echocardiographic evaluation of the aortic valve. Patients with aortic valve regurgitation and pliable cusps with no calcification or retraction with or without annular dilatation or cusp prolapse were assigned to the repair group. Patients with extensive valve pathology, cusp calcification, retraction, or valve stenosis were assigned to the replacement group. All patients had severe aortic regurgitation as an indication of isolated aortic valve surgery. We excluded patients with end-organ failure (renal or hepatic failure), severe neurological disease, infective endocarditis, associated coronary artery or other valve diseases, previous cardiac surgery, emergency operation, and patients with severe aortic stenosis or bicuspid aortic valve.
Ethical considerations
The local Ethical Committee approved the data collection for this study, and they waived the need for the patient’s consent due to the retrospective design.
Data and endpoints
We collected data for this study from paper and electronic patients records. Preoperative data included demographics, comorbidities, symptoms, laboratory results, and valve pathology. Preoperative echocardiographic evaluation of the aortic valve was performed and included the number and location of cusp prolapses, root dilatation, and cusp retraction.
Operative data included the incision, cardiopulmonary bypass, and cross-clamp times. Postoperative complications and study outcomes were low cardiac output, blood transfusion, re-exploration, pulmonary, neurological, and infective complications, ICU stay, and hospital mortality. The degree of residual aortic regurgitation was evaluated in patients who had aortic valve repair.
Operative technique
Both techniques were performed under general anesthesia and trans-esophageal echocardiographic guidance. All patients had a median sternotomy. We performed ascending aorta common atrial cannulation for all patients and gave retrograde cardioplegia followed by direct coronary ostea antegrade cardioplegia. Aortic valve repair or replacement was performed through a transverse aortotomy.
The aortic valve repair technique was tailored according to the aortic valve pathology. Cusps prolapse was repaired with plication stitches in the cusp free edge (one or multiple stitches) to raise the cusp’s free edge to the proper position (Fig. 1). In case of annular dilatation, reduction annuloplasty, or commissural plication, was done. The sub-commissural triangles were closed with horizontal mattress sutures (4/0 polypropylene or 2/0 polyster with Teflon pledgets) (Fig. 2).
Statistical analysis
Categorical data were expressed as frequencies and percentages and compared with the chi-square test or Fisher’s exact test when appropriate. Friedman test was used to compare predischarge and follow-up aortic regurgitation grades. Continuous data were tested for normality distribution visually using histograms and statistically using the Shapiro-Wilk test. Normally distributed data were compared parametrically using the Student t test and presented as mean and standard deviation. Non-normally distributed data were compared non-parametrically using the Man-Whitney test and described as 25th, 50th (median), and 75th percentiles. Statistical analysis was performed using SPSS v.23 (IBM Corp- Armonk- NY- USA), and a P value of less than 0.05 was considered statistically significant.
Results
Preoperative data
Patients who had AVR were significantly older (49.6± 7.2 vs. 43.8± 8.6 years: P= 0.002) and had more advanced New York Heart Association (NYHA) and Canadian Cardiovascular Scoring (CCS) classes. Hypertension and hypercholesteremia were more common in the AVR group. There were no differences in gender, body mass index, laboratory results, and ejection fraction between groups. The distribution of valve pathology was comparable between both groups with no significant difference. Patients who had replacement had a significantly higher percentage of valve retraction (Table 1).
Operative and postoperative data
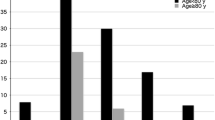
There was no difference in the surgical approach between groups. Cardiopulmonary bypass and ischemic times were shorter in patients who had aortic valve repair. There were no differences in the postoperative complication between groups. Patients who had replacement required more blood transfusion (28 (60.9%) vs. 11 (28.2%); P= 0.003) and had longer ICU stay (24.5 (24–48) vs. 23 (20–31) h; P= 0.01). Five (12.8%) patients had trivial aortic regurgitation (AR) after aortic valve repair, and10 (25.6%) patients had a mild repair. Before hospital discharge, 12 (30.8%) patients had mild AR, and 1 (2.6%) had trivial AR (Table 2).
After a 6-month follow-up, there was no difference in left ventricular end-diastolic and end-systolic diameter between both groups. Four patients had trivial AR (10.3%), and six patients had mild AR (15.4%) in the repair group with significant improvement of the degree of AR at 6 months compared to the predischarge grade (P= 0.001). There were no events in the aortic replacement group (Table 2).
Repair in degenerative versus rheumatic pathology
In patients with degenerative AR, six patients had right coronary cusp prolapse, five had non-coronary cusp prolapse, one had two-cusp prolapse, and three had root dilatation. In patients with rheumatic AR, three patients had right cusp prolapse, three had left cusp prolapse, 13 had non-coronary cusp prolapse, and five had root dilation (P= 0.11).
Pre-discharge, six patients (40%) had mild AR in patients with degenerative AR, and six patients (25%) had mild AR, and one patient (4.2%) had trivial AR in patients with rheumatic AR (P= 0.68). After 6 months, one patient (6.7%) had trivial AR, and two patients (13.3%) had mild AR in the degenerative pathology compared to three patients (12.5%) with trivial AR and four patients with mild AR (16.7%) in the rheumatic pathology (P˃0.99).
Discussion
Aortic valve replacement is the optimal treatment for managing severe isolated aortic regurgitation. The technique is safe with proven efficacy over the last decades. However, mechanical valves are associated with anticoagulation-related complications, and tissue valves have limited durability. All these factors reduced survival in patients after aortic valve replacement compared to the normal population [2]. Aortic valve repair was introduced as an alternative approach to replacement with the proposed benefits of better durability compared to tissue valves with fewer valve-related complications [5]. Currently, there is no randomized trial comparing aortic valve repair versus replacement, and patients with rheumatic heart disease are under-presented in the literature.
We retrospectively compared patients who underwent aortic valve repair and replacement. The majority of our patients had rheumatic heart disease with no difference in valve pathology between groups. Rheumatic pathology still presents the leading cause of valve surgery in our country [6], and few studies recommended valve repair in selected rheumatic patients. Afifi and colleagues recommended valve repair in mild and moderate lesions concomitant with other valve surgery and in younger patients to avoid the drawbacks of prosthetic materials [7]. However, another study recommended against using valve extension technique in young patients with rheumatic heart disease because of the high re-operation rate [8]. Patients’ assignment to either group in our study was based on the heart team discussion. Patients who had aortic valve repair were younger and had lower comorbidities and symptoms. Those patients had cusp prolapse as the main valve lesion; however, patients in the replacement group had a high percentage of cusp retraction. This finding could indicate that repair was done at an early disease stage than replacement. It is crucial to appropriately select patients for repair as the long-term outcomes depend on the quality of the cusp and the repair technique [9]. We did not find a difference in the aortic valve pathology between degenerative and rheumatic pathology in the repair group. There were no differences in the outcomes between both pathologies. This finding could indicate that the outcomes of aortic valve repair are related to the valve lesion rather than the valve pathology.
Aortic valve repair is technically demanding, and several recent modifications have been introduced with unknown long-term outcomes [10]. We repaired cusp prolapse with suspension suture, and we did not use cusp extension in our series. This is because we repaired patients with good pliable cusp with no cusp retraction. The immediate repair outcome was satisfactory; however, a long-term follow-up is recommended. In patients with annular dilatation, we performed reduction annuloplasty with sub-commissural plication. The effect of sub-commissural annuloplasty could disappear with recurrence of aortic regurgitation [11, 12]. Therefore, an external annuloplasty ring was proposed to improve the long-term outcomes of aortic repair [1, 4]. The indication of external annuloplasty was an annular diameter of 25 mm or greater, which is not common in patients with rheumatic heart disease. Therefore, no patient in our study had external annuloplasty.
Few studies compared aortic valve repair versus replacement, and all were retrospective. Regeer and colleagues found that more patients with bicuspid aortic valves had valve replacement than repair. They reported comparable left ventricular reverse remodeling in repair and replacement groups [13]. Mayer and associates found better survival after aortic valve repair than replacement in aortic valve infective endocarditis patients. The use of patches and bicuspid aortic valves predicted late failure [14]. The outcomes in our study were comparable between both groups apart from the need for blood transfusion and length of ICU stay, which were more in patients who had a replacement. These findings can be explained by the shorter cardiopulmonary and ischemic times in the repair group. Our study showed the feasibility of aortic valve repair in rheumatic patients and the need for a larger randomized study to confirm our findings and report the long-term outcomes. Additionally, patients who had repair were younger; this could warrant earlier intervention in young patients with moderate to severe aortic regurgitation if the aortic valve repair is feasible.
Study limitations
The results of the study should be interpreted in the context of the limitations. The study is a single-center and retrospective in nature. Patients’ assignment was confounded by indication since not all patients were suitable for repair. This assignment method led to differences in the baseline characteristics, affecting the study outcomes. The small sample size also limits the study, but this was attributed to the strict inclusion criteria of patients with single valve disease. Moreover, the study is limited by the short-term follow-up. A longer follow-up study is recommended.
Conclusions
Aortic valve repair could be an alternative to replacement in selected patients with rheumatic heart disease. Shorter cardiopulmonary bypass and ischemic times may improve the outcomes of repair compared to replacement.
Availability of data and materials
The authors declare that the data supporting the findings of this study are available upon request.
Abbreviations
- AR:
-
Aortic regurgitation
- AVr:
-
Aortic valve repair
- AVR:
-
Aortic valve replacement
- CCS:
-
Canadian Cardiovascular Scoring classes
- LCC:
-
Left coronary cusp
- NCC:
-
Non-coronary cusp
- NYHA:
-
New York Heart Association
- RCC:
-
Right coronary cusp
References
Lansac E, Di Centa I, Sleilaty G, Lejeune S, Khelil N, Berrebi A et al (2016) Long-term results of external aortic ring annuloplasty for aortic valve repair. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg 50(2):350–360
Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ et al (2017) 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 38(36):2739–2791 Available from: https://doi.org/10.1093/eurheartj/ehx391
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J et al (2021) 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg 60(4):727–800
de Kerchove L, Mastrobuoni S, Boodhwani M, Astarci P, Rubay J, Poncelet A et al (2016) The role of annular dimension and annuloplasty in tricuspid aortic valve repair. Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-thoracic Surg 49(2):428
Lansac E, de Kerchove L (2018) Aortic valve repair techniques: state of the art. Eur J Cardiothorac Surg 53:1101–1107
Yacoub M, Mayosi B, ElGuindy A, Carpentier A, Yusuf S (2017) Eliminating acute rheumatic fever and rheumatic heart disease. Lancet (London, England) 390(10091):212–213
Afifi A, Hosny H, Yacoub M (2019) Rheumatic aortic valve disease-when and who to repair? Ann Cardiothorac Surg 8(3):383–389
d'Udekem Y, Sharma V (2013) Repair options in rheumatic aortic valve disease in young patients: potential problems with pericardial cusp extension. World J Pediatr Congenit Heart Surg 4(4):392–396
Schneider U, Hofmann C, Aicher D, Takahashi H, Miura Y, Schäfers H-J (2017) Suture Annuloplasty significantly improves the durability of bicuspid aortic valve repair. Ann Thorac Surg 103(2):504–510
Przybylski R, Pawlak S, Śliwka J, Urlik M, Maruszewski M, Kukulski T et al (2015) Aortic cusp extension valvuloplasty: repair with an extracellular patch. Kardiochirurgia i Torakochirurgia Pol = Polish J Cardio-Thorac Surg 12(4):314–317
de Kerchove L, Boodhwani M, Glineur D, Vandyck M, Vanoverschelde J-L, Noirhomme P et al (2011) Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 142(6):1430–1438
le Polain de Waroux J-B, Pouleur A-C, Goffinet C, Vancraeynest D, Van Dyck M, Robert A et al (2007) Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation. 116(11 Suppl):I264–I269
Regeer MV, Versteegh MIM, Klautz RJM, Stijnen T, Schalij MJ, Bax JJ et al (2015) Aortic valve repair versus replacement for aortic regurgitation: effects on left ventricular remodeling. J Card Surg 30(1):13–19
Mayer K, Aicher D, Feldner S, Kunihara T, Schäfers H-J (2012) Repair versus replacement of the aortic valve in active infective endocarditis. Eur J cardio-thoracic Surg Off J Eur Assoc Cardio-thoracic Surg 42(1):122–127
Acknowledgements
Not applicable.
Funding
No funding was received for this project. This research did not receive any grants from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Contributions
Mohamed amr: conceived and designed the analysis, collected th e data and data analysis, and took part in writing the paper. Elsayed Fayad: data collection and took part in writing the paper. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
IRB approval number consent to participate was waived by the Ethical Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Amr, M.A., Fayad, E. Early outcomes of aortic valve repair versus replacement for aortic regurgitation: a single-center experience. Cardiothorac Surg 30, 2 (2022). https://doi.org/10.1186/s43057-021-00063-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43057-021-00063-2