Abstract
Background
Acute spontaneous bleeding from renal angiomyolipoma (AML) is one of the causes of Wunderlich syndrome, a rare and potentially fatal clinical condition. Clinical deterioration will occur if there is a delay in urgent management. There are several management options for renal angiomyolipoma rupture. However, until now little is known about the case of recanalization from post-coil embolization of renal angiomyolipoma. There is no guideline about embolization technique for the management of recurrent bleeding after embolization or coil recanalization of renal angiomyolipoma.
Case presentation
A 55-year-old male has Wunderlich syndrome caused by recurrent bleeding of giant AML of the left renal due to coil recanalization compounded by a pseudoaneurysm and other bleeding site in bilateral giant renal angiomyolipoma which is showed by contrast-enhanced abdominal computed tomography scan. The patient underwent urgent transarterial embolization and some blood transfusion. Clinical improvement occurred and the patient discharged several days later.
Conclusions
Embolization for spontaneous bleeding or rebleeding of renal pseudoaneurysms may become the first choice of treatment in bilateral multiple renal angiomyolipoma rather than other managements which are available to preserve renal function.
Similar content being viewed by others
Introduction
Renal AML rupture is an emergency condition with incidence rate less than 0.3% [1]. Renal AML rupture may present as Wunderlich syndrome which is characterized by nontraumatic, spontaneous renal hemorrhage into the subcapsular, perirenal, and/or pararenal spaces [2, 3]. It consists of three Lenk’s triads which are flank pain, renal mass, and shock [3, 4]. While it is most likely to occur spontaneously, rarely it could be caused by recanalization following renal AML coil embolization which is still poorly understood [5]. A rare but dangerous side effect of coil embolization is rebleeding at the same location because of coil recanalization. Also, there is no management guideline regarding to this case.
Case presentation
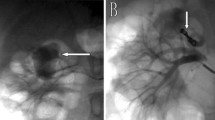
A 55-year-old male was admitted to the emergency room with sudden left abdominal pain in the last two days with a history of multiple AML of both kidneys and hypovolemic shock. Previously, his left giant renal angiomyolipoma had been ruptured and treated by embolization using a coil 8 years ago (Figs. 1,2). He did not take any oral anticoagulants or antiplatelets or history of abdominal trauma. On physical examination, his blood pressure dropped from 170/105 to 100/80 mmHg, slight tachycardia of 105 times per minute, and pale conjunctiva. His laboratory results showed a decrease of hemoglobin from previously 9.4 mg/dL to 5.3 mg/dL, a decrease in hematocrit of 15.5%, an increase in white blood cells of 18.44 × 103µL, ureum of 49 mg/dL, creatinine of 1.1 mg/dL, eGFR of 79.3 mL/minute/1.73 m2, prothrombin time 10.8 s, APTT 34.9 s, INR 1.04. After completing the abdominal CT scan with contrast (Fig. 3) and managed by several fluids to stabilize the patient’s hemodynamic, urgent transarterial embolization was carried out, while the patient was being transfused by 2 bags pack red cells (PRC) during the procedure and additional 2 bags of PRC after the procedure (Fig. 4).
A-B Axial and coronal plain abdominal CT showed coil metallic artifact at the left renal artery branch. C-D Contrast-enhanced abdominal CT showed multiple bilateral AML at both kidneys with the largest size about 11 × 12 × 15 cm at the inferior pole of left kidney . E–F Left kidney showed acute hemorrhage from the largest AML with two site of contrast pooling at the left kidney, and there was a pseudoaneurysm with maximum dome width around 0.7 cm
A-B Left kidney angiogram showed multiple hypervascular masses with two sites of contrast extravasation, C selective angiogram of left kidney interpolar region showed active bleeding from the distal site of the previous coil, D more coil inserted within the previous coil and there was no extravasation of contrast anymore, E selective arteriography of inferior pole of left kidney showed a pseudoaneurysm, F multiple coil inserted to the pseudoaneurysm and partial obliteration of the pseudoaneurysm with extravasation of contrast at the distal of the pseudoaneurysm, G more coil inserted to the pseudoaneurysm and to its proximal site, left kidney angiogram showed no coil recanalization, pseudoaneurysm nor active bleeding, H illustration for Fig. 4C-4D, I illustration for Fig. 4E-4G
The patient underwent transarterial embolization at interlobar and inferior of the renal artery branches using 2 coils of VortX Diamond-18 with variable sizes in the interlobar renal artery and 6 coils at the pseudoaneurysm to its proximal part and the bleeding stop. He transferred to the ICU for close monitoring after procedure. Few days later, the patient was hemodynamically in stable condition and laboratory results showed hemoglobin of 12.0 g/dL. The patient was discharged safely and followed up examination showed no significant change of ureum and creatinine after the procedure. Renal scintigraphy was not performed due to limited source at our hospital.
Discussion
Renal neoplasms are the most frequent lesions that induce Wunderlich's syndrome, with angiomyolipoma and clear cell renal cell carcinoma being the most frequent examples of each [3]. AMLs are benign tumors made up of blood arteries, fat cells, and smooth muscle cells [6, 7]. AMLs, which had greater than 4 cm, have a higher risk of hemorrhage and aneurysm larger than 5 mm associated with higher risk of rupture [3, 6, 7].
Within the general population, the incidence ranges of Wunderlich syndrome are from 0.07 to 0.3%. About 80% of cases of renal angiomyolipoma are spontaneous, while 20% are linked to tuberous sclerosis [1]. In bilateral multiple renal AML patient, it is usually related to tuberous sclerosis and pulmonary lymphangioleiomyomatosis (LAM) [6]. However, from previous examinations, there is no sign of tuberous sclerosis or LAM (Fig. 5).
There are several techniques for embolization of pseudoaneurysm [11, 12]: A percutaneous or intravascular injection of cast-forming agents, B sac-packing technique in an essential artery with a narrow neck, C embolization of the afferent artery in a dispensable artery without collateral circulation, D sandwich technique in a dispensable artery that presents collateral circulation, E covered stent excludes the pseudoaneurysm, F an uncovered stent is placed prior to the release of coils to the pseudoaneurysm
Renal pseudoaneurysm resulted from lacerated renal artery to parenchyma. In combination of hypotension, coagulation and pressure from the surrounding tissues result in temporary cessation of the bleeding [2]. Degradation of the clot and surrounding necrotic tissue results in recanalization of a pseudoaneurysm [2]. With restoration of normal blood flow, this pseudoaneurysm can grow and eventually become unstable [2].
To date, little is known about recanalization after coil embolization of renal AML. The risk of recanalization of embolized pseudoaneurysms is still unknown. Rebleeding at the same site due to coil recanalization is a rare but serious complication of coil embolization [2]. Rebleeding at the same site due to coil recanalization occurs when blood begins to flow back into the aneurysm after it has been occluded with coils [2]. This can happen for several reasons, including incomplete aneurysm occlusion, coil compaction, and aneurysm neck enlargement [2]. Rebleeding at the same site due to coil recanalization can occur at any time after coil embolization, but it is most common in the first few months after the procedure [2]. In this case, the previous coil has been for 8 years.
Most AMLs contain fat that is clearly visible on CT and MR images, so these tumors can be easily diagnosed without biopsy or surgery [13]. Approximately 5% of renal AMLs, however, have too little fat to be identified in a CT or MRI examination [13]. Therefore, renal AMLs are classified into fat-rich, fat-poor, and fat-invisible. Lesion having CT attenuation of − 10 HU or less is referred to as fat-rich AML [13]. Renal AML infrequently contains calcification, and when there is a well-circumscribed fat or macroscopic fat containing renal lesion, it is highly suggestive a renal angiomyolipoma [14].
Giant AML is a term for AML with size greater than 10 cm [15]. AMLs with size more than 4 cm tend to enlarge faster than AMLs with size less than 2 cm; it could grow up to 4 cm each year [16]. However, approximately 91% of AMLs either not grow or grow very slowly at rate of 0.02 cm/year [17]. Therefore, AML with size more than 4 cm usually needs more attention [16].
When AMLs contain too little fat which cannot be seen in CT and MRI, they are difficult to distinguish preoperatively from atypical renal cell carcinoma (RCC) which may contain small amount of fat by using radiologic examinations [13, 14]. In this case, AMLs are typically diagnosed after surgical resection and histopathological evaluation [13]. However, calcifications are less likely occurred in renal AMLs and could be a clue finding, whereas the presence of calcifications in fat containing renal lesions may be suggesting of RCC [14].
Treatment for asymptomatic AMLs smaller than 4 cm without complication is followed up. In AML with pseudoaneurysm, there are several treatments of choice including angioembolization, partial nephrectomy for AML larger than 4 cm, and whole nephrectomy as the last choice [8]. For bleeding renal AML, conservative treatment may be an alternative by treating hemodynamic and waiting for the tamponade effect to stop the bleeding. Alternative therapy involves transarterial embolization, immediate or delayed surgical procedure following embolization, and a partial or complete nephrectomy [18]. Currently, selective renal artery embolization is recommended as a first-line therapy for bleeding AML and is increasingly used as a preventive treatment for AML at risk of bleeding [6, 7]. Embolization possesses several advantages including a low complication rate, less trauma, renal function preserve, and satisfactory short-term (< 5 years) outcome [6, 7, 9]. In other circumstances, only in cases where there is a suspicion of cancer or when embolization is not possible or is not successful, can a partial or complete nephrectomy be justified to stop the bleeding in the patient [18]. The prognosis for rebleeding at the same site due to coil recanalization depends on several factors, including the severity of the initial bleeding, the patient's condition, and the time to treatment [11, 12].
In this case, we added some coil within the previous one to stop the bleeding at the interpolar region, and we treated the pseudoaneurysm at the inferior pole region by combining the sac-packing technique with embolization of the afferent artery.
Conclusion
Early diagnosis and management of Wunderlich’s syndrome in giant AML hemorrhage is important due to prompt treatment-affecting prognosis. Endovascular treatment currently is recommended as a first-line therapy for bleeding AML. Until now, little is known about recurrent bleeding after embolization or coil recanalization of renal AML, which can be a fatal complication when it is occurred. Embolization of spontaneous bleeding from renal pseudoaneurysms may become the first choice of treatment in bilateral multiple renal AMLs rather than total nephrectomy to preserve renal function. In the case of recurrent AML bleeding at the same site due to recanalization of pseudoaneurysm from previous management, adding some coils within the previous coils could be an option, and a combination of sac-packing technique with embolization of the afferent artery could be an additional technique in the management of pseudoaneurysm.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Medda M, Picozzi SC, Bozzini G, Carmignani L (2009) Wunderlich’s syndrome and hemorrhagic shock. J Emerg Trauma Shock 2(3):203–205
Shah JN et al (2023) Wunderlich syndrome: comprehensive review of diagnosis and management. Radiographics 43(6):1–16
Larbi H, Hassan I, Cherraqi A, Saouab R, Mohammed A, Ahmed A (2022) Wunderlich syndrome: rare and unrecognized emergency. Urology Case Report 43:102093
Parmar N, Langdon J, Kaliannan K, Mathur M, Guo Y, Mahalingam S (2022) Wunderlich syndrome: wonder what it is. Curr Probl Diagn Radiol 51(2):270–281
Yang HK, Koh ES, Shin SJ, Chung S (2013) Incidental renal artery pseudoaneurysm after percutaneous native renal biopsy. BMJ Case Rep 2013:bcr2012006537
Álvarez-Restrepo JC et al (2022) New trends and evidence for the management of renal angiomyolipoma: a comprehensive narrative review of the literature. J Kidney Cancer VHL 9(1):33–41
Gong M, Liu Z, Su H, Zhao B, Kong J, He X (2021) Urgent transcatheter arterial embolization for Wunderlich syndrome with hypovolemic shock secondary to ruptured renal angiomyolipoma. Front Surg 8:704478
Ramirez-Limon D, Gonzaga-Carlos N, Angulo-Lozano J et al (2022) Wunderlich Syndrome Associated With Angiomyolipomas. Cureus 14(4):e23861
Esmat HA, Naseri MW (2021) Giant renal pseudoaneurysm complicating angiomyolipoma in a patient with tuberous sclerosis complex: an unusual case report and review of the literature. Ann Med Surg (Lond) 62:131–134
Ahmed M et al (2022) Sporadic renal angiomyolipoma: can we adopt a uniform management protocol? Colombian Urol J. https://doi.org/10.1055/s-0042-1759625
Wiśniewski K, Tyfa Z, Tomasik B, Reorowicz P, Bobeff EJ, Posmyk BJ et al (2021) Risk factors for recanalization after coil embolization. J Pers Med 11(8):793
Madhusudhan KS, Venkatesh HA, Gamanagatti S, Garg P, Srivastava DN (2016) Interventional radiology in the management of visceral artery pseudoaneurysms: a review of techniques and embolic materials. Korean J Radiol 17(3):351–363
Park BK (2017) Renal angiomyolipoma: Radiologic Classification and imaging features according to the amount of fat. Am J Roentgenol 209(4):826–835
Yousaf A, Nabi U, Hussein ML, Twair A, Gashir MB (2020) Fat-containing renal cell carcinoma mimicking angiomyolipoma: a radiological and histopathological diagnostic challenge. Cureus. https://doi.org/10.7759/cureus.6721
Chen P, Jin L, Yang Y, Chen Z, Ni L, Yang S, Lai Y (2017) Giant renal angiomyolipoma: a case report. Mol Clin Oncol 7(2):298–300
Seyam RM, Bissada NK, Kattan SA et al (2008) Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology 72:1077–1082
Amy Lim E, Abel J (2016) Contemporary management of renal angiomyolipomas. Transl Cancer Res 5(S6):S1241–S1247. https://doi.org/10.21037/tcr.2016.11.57
Kervancioglu S, Yilmaz F (2015) Urgent arterial embolization of ruptured renal angiomyolipoma. Open Med (Wars) 10(1):233–237
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
The individual contributions of authors to the manuscript should be specified in this section. Koesbandono, Prijo Sidipratomo, Raditya Utomo, Christiano Tansol, Yohanes Chandra Kurniawan analyzed and interpreted the patient data regarding the CT scan and angiogram of left renal artery, all of whom made equal contributions to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript (No. 144/K-LKJ/ETIK/XII/2023), Prof Dr. dr. Cucu, Sp.MK(K).
Consent for publication
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Competing Interests
The authors declare that they have no competing interests
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koesbandono, Sidipratomo, P., Utomo, R. et al. Transarterial embolization in Wunderlich syndrome due to recanalization of giant renal angiomyolipoma pseudoaneurysm: a case report and literature review. Egypt J Radiol Nucl Med 55, 132 (2024). https://doi.org/10.1186/s43055-024-01301-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01301-3