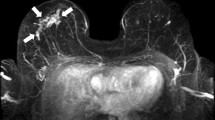
Abstract
Background
Non-mass enhancement (NME) seen on dynamic contrast enhanced breast MRI (DCE-MRI) may be caused by benign, high risk or malignant lesions. Making a clear distinction between these lesions is challenging due to the significant overlap in their imaging appearance. Our study aims to assess the various patterns of distribution, internal enhancement patterns (IEPs) and kinetics of NME using the BI-RADS lexicon fifth edition with histopathologic correlation to aid in making a more confident recommendation regarding clinical management.
Results
Sixty-six female patients with NME on DCE-MRI were included. Thirty-four lesions (51.5%) were histopathologically proven to be benign and 32 (48.5%) were malignant. Segmental distribution was the most common pattern and was found in 22 cases (33.3%), 14 of them were malignant with p-value < 0.05. Linear distribution was reported in 14 cases, (21.2%), five of which were malignant, with p-value > 0.05. Thirteen cases (19.7%) had focal distribution, only two of them were malignant with p-value < 0.05. Twelve cases (18.2%) were of regional distribution, seven of which were malignant. Multiregional and diffuse distribution were the least common and were found in 3% and 4.5% of cases respectively. As for the enhancement pattern, 30 cases (45.5%) had heterogeneous enhancement. Nineteen of which were malignant with a p-value < 0.05. Clumped enhancement was found in 24 cases (36.4%); 12 cases were found to be malignant. Nine cases (13.6%) were of homogeneous enhancement, all of them were benign and three cases (4.5%) were of clustered ring enhancement with p-value > 0.05. Restricted diffusion value was detected in 75% of malignant cases with p-value < 0.05. In terms of kinetic curve, the most frequent curve was found to be type II plateau curve (26 cases, 39.4%), 15 cases were of benign pathology and the other 11 cases were proven to be malignant. Followed by type III washout curve which was detected in 25 cases (37.9%), 20 cases were malignant and five cases were benign. And type I persistent curve was found in 15 cases (22.7%); 14 cases were histopathologically proven to be benign, and only one case was of malignant pathology, with a total p-value < 0.05.
Conclusions
Our study found that the most common distribution pattern was segmental distribution, being statistically significant with p-value < 0.05, being more common among malignant lesions. As for the enhancement pattern, heterogeneous enhancement was the most common pattern, mainly detected in malignant lesions, with p-value < 0.05. The most common type of kinetic curve was type II curve.
Similar content being viewed by others
Background
The BI-RADS lexicon fifth edition classified breast lesions into focus, mass, and non-mass enhancement (NME), [1]. NME is defined as an area of enhancement that is not associated with the three-dimensional volume of a mass, shape, and outline [1]. Pathologically, NME may be caused by either benign, high risk or malignant lesions. Benign lesions that were found to be associated with NME are pseudoangiomatous stromal hyperplasia (PASH), apocrine metaplasia and postradiation change. High-risk lesions such as radial scar, intraductal papilloma, atypical ductal hyperplasia (ADH), flat epithelial atypia or complex sclerosing lesion and malignant lesions such as ductal carcinoma in situ (DCIS), invasive ductal carcinoma (IDC), and invasive lobular carcinoma (ILC) may also appear on MRI as NME [2].
Making the distinction between benign, high risk and malignant NME lesions on MRI can be difficult due to their overlapping imaging features. Combining morphologic features including distribution and internal enhancement pattern with kinetics may help in making more confident recommendations regarding clinical management and decreasing the number of negative biopsies.
The morphological assessment of NME must include the distribution and the internal enhancing patterns (IEP). The distribution of the lesion may be either linear, focal, segmental, regional, multiple regions, or diffuse, whereas the IEPs are classified into homogeneous, heterogeneous, clustered ring, or clumped [3].
In order to increase descriptive accuracy and provide a more unified evaluation of the distribution of NME, modifications to the terminologies were made in the fifth edition of the American College of Radiology (ACR) Breast Imaging-Reporting and Data System (BI-RADS) lexicon [4]. This aimed to allow better communication with physicians and thus enhance patient care [3, 5, 6].
Assessment of the kinetic enhancement curve has also been described as a useful tool which has shown high sensitivity in assessing the vascularity of lesions detected in repeated DCE-MRI scans. The time-signal intensity curves were classified as: persistent enhancing (Type 1), plateau (Type 2), or washout (Type 3) [7].
Additionally, DWI is another functional technique of DCE-MRI, used to enhance diagnostic accuracy owing to its high specificity. Several studies explained the ability of DWI to distinguish malignant from benign breast lesions, by characterization of diffusion restriction in cases with breast cancer [8].
Material and methods
Study design
This cross-sectional study included 66 consecutive patients who were referred for breast MRI examination at the Radiology department in Assiut University hospital either for screening, evaluation of suspicious lesions on sonomammography or postoperative follow-up and had NME on DCE-MRI during the period from February 2020 to February 2021.
The exclusion criteria were (a) Patients with contraindications to MRI as patients with pacemaker, aneurysmal clipping, retained metallic foreign body. (b) Patients who suffer from claustrophobia. (c) Pregnant women especially in the first trimester. Each participant signed an informed written consent before being included in the study. Approval of the Ethical Committee of Faculty of Medicine (approval number 17100879) and clinical trial approval (NCT04083027) were obtained before commencing this study.
Clinical assessment
Patient preparation
Participants within the childbearing period were examined between day 5 and 15 of the menstrual cycle, to reduce any residual background parenchymal enhancement that may interfere with image interpretation. A brief explanation of the technique was given, including clear instructions of avoiding unnecessary motion during the examination.
MRI technique
MR characteristics were evaluated by two radiologists with 10–15 years of experience in breast imaging according to the fifth edition of the Breast Imaging and Reporting and Data system (BI-RADS). Biopsy was then taken by different methods either by Tru-cut needle biopsy or surgical biopsy and sent to experienced pathologists for histopathological assessment.
MRI protocol
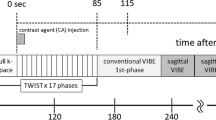
A 1.5-T MR imaging machine (Siemens Magnetom Sempra, Siemens Healthineers, Germany) was used for the MR imaging procedure. An IV route via the cubital vein was typically secured prior to inspection. After that, the patient was then asked to stay in the prone position, and both breasts were securely positioned in a double breast coil (four-channel phased array coil). Fast spin echo sequence with axial T1 weighting (TR/TE, 311/4.6 ms; FOV read 400; 3 mm thickness; matrix 340 × 512). Axial T2-weighted fast spin echo sequence (340 × 512 matrix, slice thickness of 3 mm, field of view 380 mm, TR/TE, 4550/93 ms) Axial turbo short time inversion recovery (STIR) T2-weighted images (matrix 340 × 512, slice thickness 3mm, field of view 380mm, TR/TE, 5180/74 ms). The following parameters were used to acquire the diffusion-weighted images (DWI): TR/TE milliseconds, 6730/50; 120 × 120 matrix; three mm for section thickness, 1.5 mm for intersection gap, and 50 and 1000 s/mm2 for b values. Axial fat suppressed 3D spoiled gradient recalled echo (SPGR) sequences [TR:4.9 ms, TE:2.3 ms, flip angle: 10, and FOV: 160 mm] were used in the dynamic contrast enhanced study of the breast. One sequence was conducted prior to, and seven sequences were performed seventy-second intervals following the injection of contrast medium. After the first sequence, a bolus injection of Gd-DTPA (gadopentetate dimeglumine, Magnevist) (0.1 mmol/kg) was manually given, and 20 ml of saline were added afterward.
Statistical analysis
Data analyzing and calculation of means, medians, standard deviations, percentages, ranges, and frequencies as descriptive statistics were done using IBM-SPSS 24.0 (IBM-SPSS Inc., Chicago, IL, USA).Footnote 1 The normality of continuous variables was tested using Kolmogorov–Smirnov test/Shapiro–Wilk test. The Chi-square/Fisher’s exact test was used to compare between the study groups regarding the difference in distribution of frequencies. To compare the means of dichotomous parametric data, student t test analysis was used. MRI findings with statistical significance from the univariate analyses were further included in the multivariable logistic regression models. A p-value of < 0.05 was considered as statistically significant.
Results
In this study, MRI evaluation was performed to characterize 66 NME lesions in 66 different female patients their age ranging between 25 and 64 years with mean age (± SD) of 41.61 ± 8.7 years. Patients were also evaluated by clinical examination and sonomammography.
Thirty-four lesions (51.5%) were histopathologically proven to be benign and 32 (48.5%) were malignant (Table 1). The benign lesions included granulomatous mastitis (14.7%), PASH (14.7%), ADH (14.7%), fibroadenosis (11.8%), apocrine metaplasia (8.8%), ductal hyperplasia (5.9%), fat necrosis (5.9%), periductal mastitis (5.9%), duct ectasia (2.9%), papillomatosis (2.9%), flat epithelial atypia (2.9%) and radial scar (2.9%) (Fig. 1).
The malignant lesions were histopathologically diagnosed as DCIS (40.6%), IDC (21.9%), invasive carcinoma (18.8%), inflammatory carcinoma (12.5%) and ILC (6.3%) (Fig. 2).
When the distribution of the NME in the studied cases was evaluated, segmental distribution was the most common as in Fig. 3 and it was found in 22 cases (33.3%). The incidence of segmental distribution was significantly higher in malignant lesions with p-value <0.05 and PPV about 63%. Focal distribution was significantly more common in benign lesions as in Fig. 4 with p-value <0.05 and PPV about 15.4%. However, diffuse, linear, regional and multiregional distributions did not show statistically significant difference between benign and malignant lesions (Table 2).
A 54-year-old female patient complained of right breast lump. Sonomammography showed a malignant-featuring mass in the para-areolar region at 12–1 o’clock, with clustered microcalcification in the upper inner quadrant. She was sent for DCE-MRI for preoperative assessment. Axial T2WI showed area of architectural distortion extending from a para-areolar malignant-featuring mass toward the nipple, being hypointense. Associated with skin thickening and nipple retraction a axial T2WI showed enlarged rounded ipsilateral axillary LNs with lost hilum b isointense in axial STIR c restricted in DWI (motion artifact) d axial post-contrast fat suppressed T1WI showed NME in the retro-areolar region extending from the mass, of segmental distribution, and of heterogeneous enhancement pattern. Noted skin thickening and enhancement e type II plateau TIC f The final histopathological diagnosis was DCIS
A 47-year-old patient complained of right breast lump. She had a strong family history, and she was sent for MRI. Mammography was insignificant and US showed area of focal fibroadenosis in the upper outer quadrant with few small cysts. Axial T1WI showed isointense ill-defined lesion in the upper outer quadrant of the right breast a hypointense in axial T2WI b isointense in axial STIR c no diffusion restriction detected d axial post-contrast fat suppressed T1WI showed NME of focal distribution in the upper outer quadrant of the right breast, of clumped enhancement e type I TIC f The final histopathological diagnosis was (PASH)
As for the enhancement pattern, only homogenous and heterogeneous enhancement patterns showed statistically significant difference between benign and malignant lesions. Homogenous enhancement (13.6%) was found exclusively in benign lesions while heterogenous pattern (45.5%) was more common in malignant lesions as in Fig. 5 with PPV 63.3%. Clumped and clustered ring enhancement showed no statistically significant difference between benign and malignant lesions with p-values >0.05. (Table 3).
A 25-year-old female patient was presented with left pleural effusion, and by cytology, it was proven to be of malignant nature. By examination, the left breast was found to be swollen, tender with pitting edema of the skin. In USA, there was subdermal lymphatic thickening with underlying interstitial edema of the breast parenchyma. Axial T1WI showed diffuse architectural distortion of the left breast parenchyma, being hypointense, with diffuse skin thickening with rounded axillary LNs and lost hilum a isointense in axial STIR b facilitated DWI c axial post-contrast fat suppressed T1WI showed NME of diffuse distribution of the left breast with heterogeneous enhancement, associated with diffuse skin thickening and enhancement d type II plateau TIC e The final histopathological diagnosis was inflammatory carcinoma
Kinetic curve evaluation revealed that type II plateau curve was the most common (26 cases, 39.4%). All three kinetic curve types showed statistically significant difference between benign and malignant lesions with p-value <0.001. Type I curve was significantly more common in benign lesions, being detected in 14 (4`.2%) benign lesions versus only 1 (3.1%) malignant lesions. the incidence of type II curve was higher in benign lesions (15, 44.1%) as in Fig. 6 than in malignant lesions (11, 34.4%) as in Fig. 7. Type II curve was detected in 20 (62.5%) malignant lesions and only 5 (14.7%) benign lesions (Table 4).
A 30-year-old female patient was referred to our unit with a tender left breast lump. Sonomammography revealed architectural distortion in the left breast, and magnetic resonance imaging (MRI) was recommended due to positive family history of cancer breast. Axial T2WI showed area of architectural distortion in the upper outer quadrant of the left breast, being hypointense with multiple central areas of hyperintense signal (suggesting inflammatory process), with left axillary LNs; rounded in shape with lost hilum, and diffuse skin thickening a hyperintense in axial STIR b restricted in DWI c axial post-contrast fat suppressed T1WI showed NME of segmental distribution in the upper outer quadrant of the left breast, with heterogeneous enhancement d type II plateau TIC e The final histopathological diagnosis was granulomatous mastitis
A 52-year-old patient was presented with a left breast lump. In sonomammography, there was an architectural distortion in the lower outer quadrant of the left breast, and the patient was sent for DCE-MRI. Axial T1WI showed area of architectural distortion in the lower outer quadrant of the left breast, being isointense a hypointense in axial T2WI b isointense in axial STIR, with mild skin edema c restricted in DWI d axial post-contrast fat suppressed T1WI showed regional NME enhancement in the lower outer quadrant of the left breast with extension to the upper outer quadrant, of clumped enhancement pattern e and f type III washout TIC g The final histopathological diagnosis was ILC
Diffusion restriction was seen in 24 (75%) malignant lesions and in 10 (29.4%) benign lesions which was statistically significant with a p-value <0.001 (Table 5).
Discussion
Despite the high sensitivity of MRI in detecting breast cancers, when diagnosing NME the differentiation between benign and malignant lesions can be challenging due to overlapping imaging findings, which may require unnecessary biopsy [9]. NME on breast MRI refers to areas that show enhancement that are not corresponding to a mass in the pre-contrast sequence. It is important to distinguish NME from background parenchyma enhancement (BPE) which is usually bilateral and symmetrical with diffuse distribution. BPE is usually mild, having a slow and persistent kinetic curve [6, 10].
Of the 66 cases of NME included in this study, 34 cases (51.1%) were histopathologically proven to be benign and 32 cases (48.5%) were malignant. The study of Liu et al. [9] had similar results to our study where 52.5% of the cases were benign and 47.5% were malignant. In other studies, the NME was caused by a significantly higher number of malignant than benign cases like the study done by Yang et al. [6], in which only 38.1% of the cases were benign and 61.9% were malignant and the study of Asada et al. [11] also found that 14% of the cases were benign and 84% of the cases were malignant. On the contrary, the study of Aydin [12] showed that 76.7% of the enrolled cases were benign, and the other 23.2% cases were malignant.
The current study showed that three benign pathologies were the most common to present as NME. These were granulomatous mastitis, PASH and atypical ductal hyperplasia each was found in five (7.8%) of the cases included in our study [13]. The most common malignant pathology was DCIS which was found in 13 (19.6%) of the cases. However, these results were in discordance with other studies such as the study of Yang et al. [6], which reported that fibrocystic hyperplasia was the most common benign pathology, seen in 19% of the cases, and IDC was the most common malignant pathology, which was found in 28.6% of the cases. Moreover, Liu et al. [9] found that the most common benign pathology was adenosis in 11.8% of the cases, and the most common malignant pathology was DCIS in 27.1% of the cases.
In this study, segmental distribution of NME was found to be the most frequent type and was diagnosed in 22 of the 66 cases (33.3%). It was also the most frequent distribution type among the malignant lesions, and it was statistically significant (p < 0.05) with PPV 63% in favor of malignancy. In our study, the most common malignant pathology displaying segmental distribution was DCIS [6, 12, 16, 17]. This is rational since DCIS strictly involves the milk ducts causing the characteristic segmental NME depending on the number of ducts involved and the typical proliferation pattern of DCIS [14]. This is concordant with the results reported by Yang et al., Chou et al., Aydin and Lunkiewicz et al. [6, 12, 15, 16], however, they reported higher PPV which ranged between 67-100%.
The second most common type of distribution was the linear type, found in 14 patients (21.2%). Linear distribution was more common among benign lesions; however, this was statistically insignificant (p > 0.05). Lunkiewicz et al. [16], Asada et al. [11] and Yang et al. [6] also reported statistically insignificant results linking linear distribution with benign lesions. On the other hand, Liu et al. and Aydin showed in their studies its significance with benign lesions [9, 12].
Furthermore, focal type of distribution was found in 13 (19.6%) patients, with statistically significant results in favor of benign lesions (p < 0.05) and PPV of 66.7%. This was discordant with the studies of Liu et al., Yang et al. Aydin and Asada et al. [6, 9, 11, 12] which showed the insignificance of the focal type of distribution (p-value > 0.05).
Regional, multiregional, and diffuse types of distribution were statistically insignificant. This was in agreement with the results of Liu et al., Yang et al., Lunkiewicz et al. and Asada et al. [6, 9, 11, 16].
When the internal enhancement pattern was evaluated, our study demonstrated that heterogeneous pattern of enhancement was found in 30 of the 66 cases (45.5%), and this result was statistically significant (p < 0.05) with PPV 63.3%. However, in the studies done by Yang et al. [6], Uematsu et al. [17], Thomassin et al. [18], Aydin [12] and Asada et al. [11], the prevalence of the heterogeneous enhancement pattern was lower, while the cluster-ring pattern had the highest prevalence with a p-value of > 0.05. This can be explained by the intra- and interobserver variability in interpreting the findings and the difference in study group size between different studies.
The homogenous enhancement pattern was seen in nine cases (13.6%), most commonly found in cases with fibroadenosis. This result was statistically significant (p-value < 0.05). In the study conducted by Aydin et al. [12], homogenous enhancement was also related to benign lesions. Clumped pattern of enhancement was detected in 12 (36.6%) benign cases; however, it was statistically insignificant (> 0.05).
The clustered ring pattern of enhancement, in this study, was found only in 3 cases (4.5%), 2 of them were histopathologically proven to be granulomatous mastitis and the other was DCIS. These results were discordant with the studies of Aydin [12], Liu et al. [9], Lunkiewicz et al. [16] and Yang et al. [6] which reported that the clustered ring pattern ranged from 18 to 34.5% with p-value < 0.05 and being in favor for predicting malignant lesions.
We also evaluated the dynamic contrast-enhanced (DCE) characteristics of NME as well as time intensity curves (TIC). This agreed with results of Yang et al. [6], Liu et al [9] and Aydin [12] who reported that benign lesions had persistent dynamic curve (Type I) and malignant lesions exhibited wash-out curve (Type III), which was statistically significant (p < 0.05). This was concordant with the study done by Zhou et al. [19] who reported that type II kinetic curve was more common in non mass enhancing benign papillary neoplasms (7/12) than malignant (5/12) papillary neoplasms with p-value = 0.04. This suggests discrepancy among the various studies regarding the benign versus malignant nature of NME lesions showing type II TIC. This highlights the importance of including the distribution and pattern of enhancement during the assessment of such lesions.
Our study found that the DWI was statistically significant (p-value > 0.05) in differentiating between the benign and malignant cases. Diffusion restriction was observed in 51.5% of the cases, in 24 cases (36.3%) with malignant pathology and ten cases (15.1%) with benign pathology. The absence of restriction was detected in 48.5% of the cases, more in the cases with benign pathology (24 cases) than the cases with malignant pathology (8 cases). The results were in agreement with the study of Aydin [12], Kul et al. [20] and An et al. [21] but discordant with the results of the study of Liu et at. [9] that stated there was no significant difference on DWI between DCIS and benign lesions.
Conclusions
The routine DCE-MRI protocol can provide a qualitative assessment of the NME lesions based on their distribution and enhancement pattern, for better characterization of benign and malignant lesions with significantly higher specificity and overall diagnostic performance. Based on our MRI findings, segmental distribution and heterogeneous enhancement patterns are more consistent with malignant NME lesions. Furthermore, TIC Type I and type III support the diagnosis of benign or malignant NME, respectively, while the type II curve is inconclusive. Also, DWI added more value in differentiating between benign and malignant lesions. In summary, combining morphologic features including distribution and internal enhancement pattern with kinetics and diffusion may help in making more confident decisions regarding clinical management. Benign morphologic features can be used in future studies to recommend follow-up instead of histopathological assessment, to avoid unnecessary biopsies.
Our study had some limitations, such as lack of MRI-guided biopsy, and the fact that patients who underwent breast MRI at our unit were those who were strongly suspected to have malignancies. Small sample size is another limitation, thus further studies with larger sample sizes should be conducted to confirm our conclusions.
Availability of data and materials
All data analyzed during this research are included in this published article.
Notes
IBM_SPSS. Statistical Package for Social Science. Ver.24. Standard version. Copyright © SPSS Inc., 2012-2016. NY, USA. 2016.
References
Torous VF, Resteghini NA, Phillips J, Dialani V, Slanetz PJ, Schnitt SJ et al (2021) Histopathologic correlates of nonmass enhancement detected by breast magnetic resonance imaging. Arch Pathol Lab Med 145:1264–1269. https://doi.org/10.5858/arpa.2020-0266-OA
Chadashvili T, Ghosh E, Fein-Zachary V, Mehta TS, Venkataraman S, Dialani V et al (2015) Nonmass enhancement on breast MRI: Review of patterns with radiologie-pathologie correlation and discussion of management. Am J Roentgenol 204:219–227. https://doi.org/10.2214/AJR.14.12656
Shin K, Phalak K, Hamame A, Whitman GJ (2017) Interpretation of Breast MRI Utilizing the BI-RADS Fifth Edition Lexicon: how are we doing and where are we headed. Curr Probl Diagn Radiol 46:26–34. https://doi.org/10.1067/j.cpradiol.2015.12.001
Morris EA, Comstock CE, Lee CH et al. Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® Magnetic Resonance Imaging. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology; 2013., n.d.
Ha GW, Yi MS, Lee BK, Youn HJ, Jung SH (2011) Clinical outcome of magnetic resonance imaging-detected additional lesions in breast cancer patients. J Breast Cancer 14:213–218. https://doi.org/10.4048/jbc.2011.14.3.213
Yang QX, Ji X, Feng LL, Zheng L, Zhou XQ, Wu Q et al (2017) Significant MRI indicators of malignancy for breast non-mass enhancement. J Xray Sci Technol 25:1033–1044. https://doi.org/10.3233/XST-17311
Yang SN, Li FJ, Chen JM, Zhang G, Liao YH, Huang TC (2016) Kinetic curve type assessment for classification of breast lesions using dynamic contrast-enhanced mr imaging. PLoS One. https://doi.org/10.1371/journal.pone.0152827
Mendez AM, Fang LK, Meriwether CH, Batasin SJ, Loubrie S, Rodríguez-Soto AE et al (2022) Diffusion breast MRI: current standard and emerging techniques. Front Oncol. https://doi.org/10.3389/fonc.2022.844790
Liu G, Li Y, Chen S-L, Chen Q (2022) Non-mass enhancement breast lesions: MRI findings and associations with malignancy. Ann Transl Med 10:357. https://doi.org/10.21037/atm-22-503
Giess CS, Yeh ED, Raza S, Birdwell RL (2014) Background parenchymal enhancement at breast MR imaging: Normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 34:234–247. https://doi.org/10.1148/rg.341135034
Asada T, Yamada T, Kanemaki Y, Fujiwara K, Okamoto S, Nakajima Y (2018) Grading system to categorize breast MRI using BI-RADS 5th edition: a statistical study of non-mass enhancement descriptors in terms of probability of malignancy. Jpn J Radiol 36:200–208. https://doi.org/10.1007/s11604-017-0717-9
Aydin H (2019) The MRI characteristics of non-mass enhancement lesions of the breast: associations with malignancy. Br J Radiol. https://doi.org/10.1259/bjr.20180464
Aydiner A, Iğci A, Soran A (2019) Breast disease: diagnosis and pathology. Springer, Berlin
Grimm LJ, Rahbar H, Abdelmalak M, Hall AH, Ryser MD. Ductal carcinoma in situ: state-of-the-art review. Radiology. 2022;302:246–55. https://doi.org/10.1148/radiol.21183
Chou SHS, Romanoff J, Lehman CD, Khan SA, Carlos R, Badve SS, et al. Preoperative breast MRI for newly diagnosed ductal carcinoma in situ: imaging features and performance in a multicenter setting (ECOG-ACRIN E4112 Trial). Radiology 2021;301:66–77. https://doi.org/10.1148/RADIOL.2021204743
Lunkiewicz M, Forte S, Freiwald B, Singer G, Leo C, Kubik-Huch RA (2020) Interobserver variability and likelihood of malignancy for fifth edition BI-RADS MRI descriptors in non-mass breast lesions. Eur Radiol 30:77–86. https://doi.org/10.1007/s00330-019-06312-7
Uematsu T, Kasami M (2012) High-spatial-resolution 3-T breast MRI of nonmasslike enhancement lesions: an analysis of their features as significant predictors of malignancy. Am J Roentgenol 198:1223–1230. https://doi.org/10.2214/AJR.11.7350
Thomassin-Naggara I, Trop I, Chopier J, David J, Lalonde L, Darai E et al (2011) Nonmasslike enhancement at breast MR imaging: the added value of mammography and US for lesion categorization. Radiology 261:69–79. https://doi.org/10.1148/radiol.11110190
Zhou J, Li M, Liu D, Sheng F, Cai J. Differential diagnosis of benign and malignant breast papillary neoplasms on MRI with non-mass enhancement. Acad Radiol. 2023;30(Suppl 2):S127–32. https://doi.org/10.1016/J.ACRA.2023.02.010
Kul S, Eyuboglu I, Cansu A, Alhan E (2014) Diagnostic efficacy of the Diffusion weighted imaging in the characterization of different types of breast lesions. J Magn Reson Imag 40:1158–1164. https://doi.org/10.1002/jmri.24491
An YY, Kim SH, Kang BJ (2017) Differentiation of malignant and benign breast lesions: added value of the qualitative analysis of breast lesions on diffusionweighted imaging (DWI) using readoutsegmented echo-planar imaging at 30 T. PLoS One. https://doi.org/10.1371/journal.pone.01746813
Acknowledgements
No acknowledgement.
Funding
This research did not receive specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EA suggested the research idea, ensured the original figures and data in the work, minimized the obstacles to the team of work, correlated the study concept and design and the major role in analysis: SM collected data in all stages of manuscript and performed data analysis. NA supervised the study with significant contribution to design the methodology, manuscript revision and preparation. SA correlated the clinical data of patients and matched it with the findings, drafted and revised the work. All authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed written consents taken from the patients and healthy volunteers, and the study was approved by ethical committee of Assiut University Hospitals, faculty of medicine. Committee’s reference number: 17100879.
Consent to publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
All participants included in the research gave written consent to publish the data included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, S., Elhamd, E.A. & Attia, N.M. Non-mass enhancement on breast MRI: Clues to a more confident diagnosis. Egypt J Radiol Nucl Med 55, 87 (2024). https://doi.org/10.1186/s43055-024-01231-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01231-0