Abstract
Background
Celiacomesenteric trunk (CMT) is one of the rare congenital anomalies of the splanchnic vasculature, characterized by the common trunk origin of the superior mesenteric artery (SMA) and celiac trunk from the abdominal aorta. Dissection of CMT with isolated SMA involvement without bowel ischemia has been rarely reported in the literature.
Case presentation
A 48-year-old male presented with generalized abdominal pain for 20 days, which increased after food intake. He also gave a history of passing loose stools on and off in the last 10–15 days, associated with melena for 4–5 days. Computed tomography (CT) and CT angiography (CTA) study of the abdomen demonstrated the common trunk origin of SMA and celiac trunk from the abdominal aorta at the D12–L1 disk level, consistent with the celiacomesenteric trunk (CMT). CTA also revealed dissection of CMT with isolated extension in the SMA origin and thrombosis in the mid and distal SMA. No bowel or mesenteric ischemia was seen due to extensive collateral supply to distal-most SMA and its branches, mainly through the inferior mesenteric artery (IMA) via an arc of Riolan and artery of Drummond. The patient was managed conservatively with bowel rest, anti-thrombolytics, and anticoagulant therapy based on imaging findings of short-segment dissection with no signs of bowel or mesenteric ischemia.
Conclusion
Diagnosis of rare vascular anomalies like CMT with associated complications of dissection and thrombosis is critical in patients with abdominal pain for prompt and precise management. CTA of the abdomen is essential for accurate diagnosis and characterization of the abnormality, which helps decide between conservative and surgical treatments.
Similar content being viewed by others
Background
The three ventral divisions of the abdominal aorta, namely the celiac trunk, SMA and IMA, and their subdivisions with numerous collateral pathways form the main vasculature supplying the gastrointestinal tract, protecting the bowel from the potential risk of ischemia or infarction in a compromised state [1]. The major collateral vessels between IMA and SMA are through the marginal artery of Drummond and the arc of Riolan. The anatomical variations of mesenteric (splanchnic) vasculature are relatively common [1]. However, the celiacomesenteric trunk (CMT), obtained from the common origin of the celiac trunk and SMA, is rare [2,3,4]. CMT has its own classification subtypes based on the segment's length and the origin of the branching arteries [4, 5]. CMT variation occurs due to regression of the origin of the 10th vitelline artery and persistent ventral anastomosis between the 12th and 13th vitelline arteries during embryological development. Arterial dissection is a long or short segment of abrupt arterial wall cleavage forming a true and false lumen with subsequent hematoma in the false lumen [6]. Spontaneous isolated dissection of visceral arteries is rare, and SMA dissection accounts for most cases [7]. In the present case study of a middle-aged male patient, we report the dissection of the rare variation of CMT with isolated extension in the SMA on CT angiography (CTA).
Case presentation
A 48-year-old male presented with generalized abdominal pain for 20 days, which increased after food intake, and generalized weakness. He also gave a history of passing loose stools on and off in the last 10–15 days, which was associated with melena and vomiting for 4–5 days. The patient had no history of fever, hematemesis, constipation, jaundice, or weight loss. There was no previous history of abdominal trauma or surgery, any comorbidities like diabetes mellitus, hypertension, tuberculosis, or addiction to alcohol or any other substance. The patient's vital signs were stable during the clinical examination. The temperature was 98.5 °F, the pulse oximetry was 98% on room air, the pulse rates were 78/min, the blood pressure was 130/80 mm Hg, and the respiration rate was 18/min. The patient had mild tenderness in the epigastric region on palpation with no guarding or rigidity. Other systemic examination findings were within the normal range. Based on clinical presentation, the possibility of pancreatitis or bowel inflammation was considered with low-grade suspicion of vascular cause leading to bowel or mesenteric ischemia.
Baseline blood investigations, including complete blood count (CBC), showed decreased hemoglobin levels (11.4 g/dL), decreased red blood cell count (2.76 × 106/µL), packed cell volume (32.4%), increased mean corpuscular volume (117.2 fL) and mean corpuscular Hb (41.2 gms). The peripheral blood smear showed moderate macrocytosis, mild polychromasia, and Rouleaux formation. Erythrocyte sedimentation rate (ESR) was mildly elevated (21 mm/hr). D-dimer and serum lactate levels were within normal limits, measuring 356 ng/ml and < 1 mmol/l, respectively. C-reactive protein, serum blood glucose proteins, urea, creatinine, and electrolyte levels were normal. The serum amylase, lipase, and liver function tests were within normal limits. The initial ultrasound examination of the abdomen was suboptimal due to the gaseous abdomen with inadequate visualization of the retroperitoneal structures, including the pancreas and aorta.
Multiphase CT study with angiography (CTA) of the abdomen was performed on a 128-slice multidetector CT scanner (Philips Ingenuity Core, Philips, Amsterdam, the Netherlands) to evaluate the abdominal vasculature and the gastrointestinal tract. The patient was given water as a nonopaque oral contrast for an angiography study and a better assessment of the bowel. CTA was conducted after the intravenous (IV) administration of 100 mL of non-ionic iodinated contrast (Iomeprol 400 mg, Bracco, Milan, Italy) with a power injector through a 20-gauge catheter at a rate of 4 mL/s followed by 30 ml of normal saline. The contrast bolus tracking technique in the proximal abdominal aorta after 60 ml of contrast was used to optimize the angiography study. CT images were obtained using 64 × 0.625 mm collimation and 0.8 mm thickness using 200 mAs tube current and 120kVp tube voltage with rotation time 0.75 s, Pitch 1.3, matrix 512 × 512, field of view 38.5 × 38.5 cm, in the axial plane with 0.8 mm section thickness, and 0.8 mm increments. Arterial, portal, venous, and delayed phase axial images were acquired at 17 s, 45 s, 72 s, and 90 s, respectively. These image data were used to obtain coronal and sagittal reformatted images using a 3D workstation.
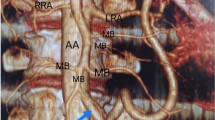
On CTA, SMA and celiac trunk both originated from the ventral aspect of the abdominal aorta at D12-L1 disc level by a common celiacomesenteric trunk (CMT), which measured approximately 29 mm in length and 12.5 mm in width (Fig. 1A). CMT was prominent and showed a linear hypodense filling defect representing a dissecting intimal flap extending in the origin of the SMA with a larger false lumen seen on the left side (Fig. 1A, B). The celiac trunk originated from the true lumen (Fig. 2A), and its branches showed normal caliber with good contrast opacification. The hypodense filling defect (thrombus) was seen in the proximal SMA extending over a length of 2.6 cm distal to origin with near complete occlusion and non-contrast opacification of the mid and distal SMA over an approximate extent of 4.2 cm (Fig. 2B). Besides, the thrombosis extended into the proximal branches of the SMA. However, the distal-most segment and branches of SMA were properly opacified with IV contrast by collateral blood supply from pancreaticoduodenal arcade and IMA via an arch of Riolan and the artery of Drummond, which appeared prominent and showed normal contrast opacification (Fig. 3A, B). The left gastric artery (LGA) was arising from the celiac trunk. In addition, an accessory renal artery was seen on the left side supplying the lower pole of the left kidney. The fluid-filled jejunal and ileal loops showed normal contrast enhancement of the wall with no abnormal wall thickening, intramural air, or adjacent mesenteric fat stranding. No signs of mesenteric and bowel ischemia were seen (Fig. 4A, B). These findings were consistent with the anatomical variant of CMT with dissection of CMT, having isolated extension in the SMA with SMA thrombosis without bowel or mesenteric ischemia due to extensive collateral formation.
A, B Contrast-enhanced axial CT image (A) at the level of D12-L1 disk reveals celiacomesenteric trunk (CMT) showing a linear hypodense dissecting intimal flap (white arrow) with a larger false lumen on the left side. Multiplanar reformation in the sagittal plane (B) shows an extension of this linear dissecting intimal flap in the origin of the SMA (white arrow)
A, B Reconstructed CT images using volume rendering technique (A) and maximum intensity projection (B) showing good contrast opacification of the distal-most portion of SMA and its branches by collateral supply through the IMA via an arc of Riolan (short thick white arrows) and artery of Drummond (long white arrows) and pancreaticoduodenal arcade (white arrowhead) with prominent gastroduodenal artery (paired white arrows)
A, B Contrast-enhanced axial (A) and coronal (B) reformatted CT images taken in the venous phase reveal near complete occlusion of SMA and its proximal branches (white arrows). Fluid-filled jejunal and ileal loops show normal contrast enhancement of the bowel walls with no signs of mesenteric and bowel ischemia
An interventional radiologist's opinion was obtained regarding the patient's ongoing care. The patient was advised against emergent endovascular therapy for revascularization because the dissection was a short segment, uncomplicated, with adequate collateralization, and did not result in intestinal or mesenteric ischemia. The patient's symptoms improved after a week of conservative treatment consisting of bowel rest, antithrombolytics, and anticoagulant medicine. Following two weeks of medical supervision with antithrombolytics and anticoagulant maintenance, he was released from the hospital. The patient had been on regular follow-up and is doing clinically better, as recorded at his last follow-up at the end of 4 months. He had also been counseled by a vascular surgeon for the future need for an aorto-SMA/Ileo-mesenteric bypass grafting based on the evolving clinical scenario.
Discussion
Celiacomesenteric trunk (CMT), which represents the common origin of the SMA and the celiac trunk, is an uncommon anatomical variant of abdominal vasculature having an incidence varying from a range of 0.54–3.4% with male preponderance [4]. SMA and celiac trunk are the ventral divisions of the abdominal aorta supplying the foregut and midgut, respectively, and frequently show anatomical variations. The primitive fetal blood supply to the abdomen is via multiple vitelline arteries originating from the fused, paired dorsal aortas, joined by a ventral anastomotic channel. The 10th vitelline artery and its ventral anastomosis give origin to the celiac trunk with its three branches during normal fetal development. The origin of the 11th and 12th vitelline arteries and ventral anastomosis between the 12th and 13th vitelline arteries disappears from the dorsal aorta, with SMA developing from the 13th vitelline artery (Fig. 5). CMT is formed due to regression of the origin of the 10th vitelline artery and persistence of ventral anastomosis between the 12th and 13th vitelline arteries (Fig. 5) [1].
Schematic diagram showing normal embryology and anatomy of the mesenteric vasculature and variant celiac-SMA common trunk. Normally, the 10th and 13th vitelline arteries persist to individually form the celiac trunk and SMA, whereas the rest of the segments regress before birth. If the 10th to 12th vitelline arteries regress with abnormal persistence of ventral anastomosis, a celiacomesenteric trunk is formed. [CA—celiac axis, SMA—superior mesenteric artery, HA—hepatic artery, SA—splenic artery, LGA—left gastric artery] (Note: Figure adapted from Alam et al. [18])
Various studies have classified the anatomical variations of the celiac trunk based on the branching pattern and SMA origin [1, 2, 4, 5, 8,9,10,11]. The most common classical type (Fig. 5), which defined the celiac trunk as the artery originating from the aorta till its trifurcation in common hepatic (CHA), splenic (SA), and left gastric (LGA) arteries, is reported to be present in about 90% of the patients [2, 8, 9]. Wang et al. [2], in their study of 1500 subjects, redefined the celiac axis as the artery giving origin to at least two of its main branches and proposed ten anatomical variations of the celiac axis and the SMA origins on CTA. They classified CMT as the arterial trunk containing at least two main arterial branches of the celiac trunk and SMA, with a reported incidence of 3.4% in their study population [2]. CMT was further classified into type 1: Hepatogastrosplenomesenteric (HGSM) trunk (Fig. 5), type II: Hepatosplenomesenteric (HSM) trunk and LGA origin from aorta, type III: Gastrosplenomesenteric (GSM) trunk with CHA origin from aorta, type IV: Hepatogastromesenteric (HGM) trunk with SA arising from the aorta [2].
Tang W et al. added type V to the CMT classification provided by Wang Y et al. to define other variation patterns not covered in type I to IV, which included LGA origin from other arteries excluding aorta and celiac, CHA origin from SMA and single trunk origin for all arteries [5]. Based on the common trunk length, Tang W et al. classified CMT into long-segment type with common trunk length between 17 and 39 mm and short-segment type with common trunk length of 6 to 14 mm [5]. They also divided LGA into ‘a’ to ‘d’ types based on its origin from the celiac axis, aorta, single common trunk, and other arteries, respectively. According to the nomenclature system by Tang W et al. [5], Type Ia (long type) CMT variant was seen in our case as CMT originated from the ventral aspect of the abdominal aorta, measuring 29 mm in length and 12.5 mm in width and further divided into two branches, superiorly celiac trunk (with LGA origin from celiac trunk) and inferiorly SMA.
The celiac trunk and SMA form a vast mesenteric network of collateral pathways that protect viscera from potential ischemia or infarction during a compromised vascular state by providing a rich collateral blood supply in a typical patient [1, 10,11,12]. The pancreaticoduodenal arcade, formed by the inferior and superior pancreaticoduodenal arteries, is a crucial anastomotic channel during celiac trunk or SMA occlusion. Other important anastomotic channels during SMA or IMA occlusion are the marginal artery of Drummond (between branches from the left, middle, and right colic arteries) that runs the entire length of the colon, and Arc of Riolan, which runs through the midportion of the mesenteric arcade radially, close to the inferior mesenteric vein. The presence of a common trunk in a patient is of paramount clinical implications due to the lack of collateral protection of a dual vascular supply to the abdominal organs, leaving a sizeable segment of the bowel with a single dominant vascular supply [13]. In our case, the post-thrombotic distal portion of SMA and its branches revealed good contrast opacification by collateral supply through the pancreaticoduodenal arcade and IMA via an arc of Riolan and artery of Drummond with no signs of bowel or mesenteric ischemia.
Patients with CMT variant splanchnic vasculature may be asymptomatic without any complications and do not require any treatment. However, CMT can be accompanied by pathological abnormalities, including aneurysms, dissections, occlusions, stenosis, and extrinsic compression, which completely stop the splanchnic artery supply and cause severe mesenteric ischemia as well as ischemia of the supra-mesocolic organs [13, 14].
Isolated visceral artery dissection, which has not been accompanied by an aortic dissection or pathology in the past, is most frequently reported in renal and carotid arteries; however, it is seen rarely in the splanchnic arteries [7]. The SMA, followed by the celiac trunk, is the most common site for visceral arterial dissections [7]. In 1947, Bauersfeld reported the first isolated dissection involving SMA, defined as SMA wall dissection not accompanied by aortic dissection [15]. The patient in the present case had dissection of the origin of the CMT, which extended into the origin of SMA with near complete thrombotic occlusion of the mid and distal SMA, approximately 2.6 cm distal to origin (Fig. 6). The pathogenesis of a spontaneous dissection had yet to be fully discovered; however, proposed associations with hypertension, atherosclerosis, smoking, pregnancy, vasculitis, trauma, iatrogenic, connective tissue diseases, fibromuscular dysplasia, and malignancy have been noted [16, 17]. No such predisposing factor was present in our case.
Schematic diagram for index case showing dissecting flap in the CMT, which is extending into the origin of SMA and thrombosis causing near complete occlusion of the mid and distal SMA, of which distal-most portion and proximal SMA filled up with collateral supply through the IMA via an arc of Riolan, and artery of Drummond (C1, C2). Other collaterals depicted in the image are of the inferior pancreaticoduodenal artery with the CA (C3). [CA—celiac axis, SMA—superior mesenteric artery, HA—hepatic artery, SA—splenic artery, LGA—left gastric artery, C—collateral] (Note: Figure adapted from Alam et al. [18])
The symptomatic presentation of visceral arterial dissections depends on the etiology, location, and size of the pathology. The most frequent presentation is abdominal pain, often associated with nausea, vomiting, melena, hematemesis, and weight loss. Common complications in a symptomatic patient are in the form of acute bowel ischemia/infarction, acute peritonitis, or fatal aneurysmal rupture with intra-abdominal hemorrhage leading to hemorrhagic shock [17, 18]. Preoperative knowledge of the vascular variant, correct classification, and presence of collateral circulation, and complications are essential for any surgical, transplant, oncological, and interventional procedures for better management and favorable outcomes without unexpected complications or iatrogenic injuries [19, 20]. Ultrasonography (USG) of the abdomen, being quick and cost-effective, is often the first imaging investigation performed in patients presenting with abdominal pain. USG can demonstrate an intimal dissection flap, arterial dimension, and associated thrombus with the absent color flow on Doppler [21,22,23]. SMA dissecting aneurysm often shows alternate blue and red color flow patterns in the true lumen and false lumen on either side of the intimal flap on color Doppler representative of opposite directional flow [24]. The presence of echogenic foci in the bowel wall representing pneumatosis intestinalis on USG suggests bowel ischemia [23]. Duplex scanning can be helpful in the assessment of bowel viability and the need for vascular intervention for SMA dissection, follow-up of symptomatic patients, and surveillance of surgical or endovascular treatment [21, 24]. The utility of duplex ultrasound (DUS) could be affected by various factors like the deep location of the abdominal aorta and its branches, obesity, and limited sonic window due to bowel gases.
CTA is the modality of choice in evaluating arterial and venous anatomy, with high diagnostic accuracy for detecting arterial variations [3]. The volumetric acquisition of data allows the creation of isotropic volume rendering, multi-planar reconstruction, formation of the 3D anatomical model, and establishment of the relationship with adjacent organs. CTA also helps evaluate the associated pathologies like dissection with identification of the intimal flap and its extension, segmental lumen visualization, aneurysm, and thrombosis with segmental fat infiltration [6, 17, 18, 25]. Detection of true and false lumens (double lumen sign) is the most characteristic sign of the dissection on CTA [17]. CT also provides an estimate of the size and severity of the lesion and associated complications like bowel ischemia and ruptured dissecting aneurysm leading to mesenteric hematoma and hemorrhagic ascites. CT features of acute bowel ischemia include the absence of gut wall enhancement and mesenteric edema, and intramural bowel gas, mesenteric or portal venous gas associated with bowel necrosis [6, 23]. Digital subtraction angiography (DSA) is an invasive test that can provide anatomical variations in detail, luminal details, and complications of dissection such as thrombosis, embolization, and measurement of the gradient across the artery [18, 25, 26]. DSA-guided minimally invasive endovascular treatment, such as thrombolysis and stenting, can also be planned at the same time in suitable patients without additional procedures or contrast requirements [25, 26]. DSA, as a diagnostic tool, has limitations by being an invasive procedure that can further cause embolization or dissection [6]. CTA is noninvasive and better depicts the arterial luminal and other relevant abdominal anatomical details and associated complications. Sakamoto et al. classified the morphology of isolated SMA dissection into four types: type I, patent type with an entry and re-entry; type II, “cul-de-sac” type without re-entry; type III, with a thrombosed false lumen with ulcer-like projection (ULP); and type IV, with a completely thrombosed false lumen without ULP [27]. Zerbib et al. added type V accompanied by dissection and stenosis of the SMA and type VIa and VIb with complete or partial thrombotic occlusion [28]. Isolated SMA dissection with thrombosis, in our case, falls under Type VIb according to Modified Sakamoto’s classification.
Medical or surgical treatment of isolated mesenteric arterial dissection depends on acute to chronic clinical presentation, assessment type and extent of the vascular involvement, end-organ injury, and associated complications [6, 17, 29, 30]. The intervention decision in asymptomatic patients will be based on the site and extent of dissection and the presence of aneurysm on CTA. The short segment dissection with no aneurysm or a small stable aneurysm is managed conservatively with bowel rest, antihypertensives, antiplatelets, and anticoagulation treatment with close surveillance. Long segment dissection, with or without enlarging aneurysm, is treated with endovascular treatment or surgical revascularization, including balloon dilation, thrombus suction, thrombolysis, stent graft placement, stenting, and endarterectomy [6, 29]. Symptomatic patients with no or mild bowel ischemia can be managed conservatively with close surveillance and step up to endovascular or surgical management in case of worsening symptoms [22, 29, 30]. However, endovascular treatment or laparotomy with resection of the affected bowel segment and anastomosis is needed in patients with severe ischemia and bowel infarction [29].
Although surgical techniques and outcomes have improved, there are still difficulties in approaching the lesion and determining the best way to reconstruct the vessel. Therefore, CTA plays a significant role in the excellent delineation of the pathology, collateralization, and associated complications, which helps in deciding between the conservative or surgical treatment option and in planning the endovascular or vascular anastomosis procedures. The symptomatic patient in the index case was treated conservatively with bowel rest, thrombolytics, and anticoagulant therapy given short segment dissection in CMT and SMA with SMA thrombosis without any aneurysm or signs of mesenteric/bowel ischemia due to rich collateral supply (Fig. 6).
Conclusion
The celiacomesenteric trunk (CMT) is a rare anatomical variation of mesenteric vasculature characterized by the common trunk aortic origin of the celiac trunk and superior mesenteric artery (SMA). Isolated dissection of splanchnic arteries is also an uncommon condition and may lead to serious complications like bowel ischemia. We have reported a rare case of dissection of the CMT with isolated involvement and thrombosis of SMA without bowel or mesenteric ischemia on CT angiography. Radiologists need to be aware of isolated visceral artery dissection as a possible underlying etiology for a common but nonspecific complaint of abdominal pain. CTA is crucial in diagnosing rare vascular anomalies and associated complications for prompt and precise management.
Availability of data and materials
All data generated or analyzed during this study are included in this published article. Any required information is available upon reasonable request from the corresponding author, R.K.
Abbreviations
- CMT:
-
Celiacomesenteric trunk
- SMA:
-
Superior mesenteric artery
- IMA:
-
Inferior mesenteric artery
- LGA:
-
Left gastric artery
- CHA:
-
Common hepatic artery
- SA:
-
Splenic artery
- HGSM:
-
Hepatogastrosplenomesenteric
- HSM:
-
Hepatosplenomesenteric
- GSM:
-
Gastrosplenomesenteric
- HGM:
-
Hepatogastromesenteric
- CTA:
-
Computed tomography angiography
References
Walker TG (2009) Mesenteric vasculature and collateral pathways. Semin Intervent Radiol 26(3):167–174. https://doi.org/10.1055/s-0029-1225663
Wang Y, Cheng C, Wang L, Li R, Chen JH, Gong SG (2014) Anatomical variations in the origins of the celiac axis and the superior mesenteric artery: MDCT angiographic findings and their probable embryological mechanisms. Eur Radiol 24(8):1777–1784. https://doi.org/10.1007/s00330-014-3215-9
Winston CB, Lee NA, Jarnagin WR, Teitcher J, DeMatteo RP, Fong Y, Blumgart LH (2007) CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR Am J Roentgenol 189(1):W13–W19. https://doi.org/10.2214/AJR.04.1374
Ramesh Babu CS, Joshi S, Gupta KK, Gupta OP (2015) Celiacomesenteric trunk and its variants a multidetector row computed tomographic study. J Anat Soc India 2015(64):32–41. https://doi.org/10.1016/j.jasi.2015.04.007
Tang W, Shi J, Kuang LQ, Tang SY, Wang Y (2019) Celiomesenteric trunk: New classification based on multidetector computed tomography angiographic findings and probable embryological mechanisms. World J Clin Cases 7(23):3980–3989. https://doi.org/10.12998/wjcc.v7.i23.3980
Mousa AY, Coyle BW, Affuso J, Haser PB, Vogel TR, Graham AM (2009) Nonoperative management of isolated celiac and superior mesenteric artery dissection: case report and review of the literature. Vascular 17(6):359–364. https://doi.org/10.2310/6670.2009.00053
Takayama T, Miyata T, Shirakawa M, Nagawa H (2008) Isolated spontaneous dissection of the splanchnic arteries. J Vasc Surg 48(2):329–333. https://doi.org/10.1016/j.jvs.2008.03.002
Osman AM, Abdrabou A (2016) Celiac trunk and hepatic artery variants: a retrospective preliminary MSCT report among Egyptian patients. Egypt J Radiol Nucl 47(4):1451–1451. https://doi.org/10.1016/j.ejrnm.2016.09.011
Uflacker R (2007) Abdominal aorta and branches in atlas of vascular anatomy: an angiographic approach, 2nd edn. Lippincott Williams & Wilkins, Baltimore
Gourley EJ, Gering SA (2005) The meandering mesenteric artery: a historic review and surgical implications. Dis Colon Rectum 48(5):996–1000. https://doi.org/10.1007/s10350-004-0890-7
Lin PH, Chaikof EL (2000) Embryology, anatomy, and surgical exposure of the great abdominal vessels. Surg Clin North Am 80(1):417–433. https://doi.org/10.1016/s0039-6109(05)70413-8
Rosenblum JD, Boyle CM, Schwartz LB (1997) The mesenteric circulation. Anatomy and physiology. Surg Clin N Am 77(2):289–306. https://doi.org/10.1016/s0039-6109(05)70549-1
Lovisetto F, Finocchiaro De Lorenzi G, Stancampiano P, Corradini C, De Cesare F, Geraci O, Manzi M, Arceci F (2012) Thrombosis of celiacomesenteric trunk: report of a case. World J Gastroenterol 18(29):3917–3920. https://doi.org/10.3748/wjg.v18.i29.3917
Ailawadi G, Cowles RA, Stanley JC, Eliason JL, Williams DM, Colletti LM, Henke PK, Upchurch GR Jr (2004) Common celiacomesenteric trunk: aneurysmal and occlusive disease. J Vasc Surg 40(5):1040–1043. https://doi.org/10.1016/j.jvs.2004.08.028
Bauersfeld SR (1947) Dissecting aneurysm of the aorta; a presentation of fifteen cases and a review of the recent literature. Ann Intern Med 26(6):873–889. https://doi.org/10.7326/0003-4819-26-6-873
Eldine RN, Dehaini H, Hoballah J, Haddad F (2022) Isolated superior mesenteric artery dissection: a novel etiology and a review. Ann Vasc Dis 15(1):1–7. https://doi.org/10.3400/avd.ra.21-00055
Daoud H, Abugroun A, Subahi A, Khalaf H (2018) Isolated superior mesenteric artery dissection: a case report and literature review. Gastroenterol Res 11(5):374–378. https://doi.org/10.14740/gr1056w
Alam W, Kamareddine MH, Geahchan A, Ghosn Y, Feghaly M, Chamseddine A, Bou Khalil R, Farhat S (2020) Celiacomesenteric trunk associated with superior mesenteric artery aneurysm: a case report and review of literature. SAGE Open Med Case Rep. 8:2050313X20938243. https://doi.org/10.1177/2050313X20938243
Sangster G, Ramirez S, Previgliano C, Al Asfari A, Hamidian Jahromi A, Simoncini A (2014) Celiacomesenteric trunk: a rare anatomical variation with potential clinical and surgical implications. J La State Med Soc 166(2):53–55
Jesrani A, Sethar S (2020) Common origin of celiac and superior mesenteric arteries as celiacomesenteric trunk: case report of rare vascular anatomic variation. OSP J Cancer Biol Clin Oncol. 1:1–2
Gouëffic Y, Costargent A, Dupas B, Heymann MF, Chaillou P, Patra P (2002) Superior mesenteric artery dissection: case report. J Vasc Surg 35(5):1003–1005. https://doi.org/10.1067/mva.2002.122152
Subhas G, Gupta A, Nawalany M, Oppat WF (2009) Spontaneous isolated superior mesenteric artery dissection: a case report and literature review with management algorithm. Ann Vasc Surg 23(6):788–798. https://doi.org/10.1016/j.avsg.2008.12.006
Park SY, Jeong WJ (2022) Diagnosing isolated superior mesenteric artery dissection and thrombosis using point-of-care ultrasonography: a case series. World J Emerg Med 13(3):239–241. https://doi.org/10.5847/wjem.j.1920-8642.2022.037
Wadhwani R, Modhe J, Pandey K, Gujar S, Sukthankar R (2001) Color Doppler sonographic diagnosis of dissecting aneurysm of the superior mesenteric artery. J Clin Ultrasound 29(4):247–249. https://doi.org/10.1002/jcu.1028
Morris JT, Guerriero J, Sage JG, Mansour MA (2008) Three isolated superior mesenteric artery dissections: update of previous case reports, diagnostics, and treatment options. J Vasc Surg 47(3):649–653. https://doi.org/10.1016/j.jvs.2007.08.052
Casella IB, Bosch MA, Sousa WO Jr (2008) Isolated spontaneous dissection of the superior mesenteric artery treated by percutaneous stent placement: case report. J Vasc Surg 47(1):197–200. https://doi.org/10.1016/j.jvs.2007.07.051
Sakamoto I, Ogawa Y, Sueyoshi E et al (2007) Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur J Radiol 64:103–110. https://doi.org/10.1016/j.ejrad.2007.05.027
Zerbib P, Perot C, Lambert M, Seblini M, Pruvot FR, Chambon JP (2010) Management of isolated spontaneous dissection of superior mesenteric artery. Langenbeck’s Arch Surg 395(4):437–443. https://doi.org/10.1007/s00423-009-0537-1
Satokawa H, Takase S, Seto Y, Yokoyama H, Gotoh M, Kogure M, Midorikawa H, Saito T, Maehara K (2014) Management strategy of isolated spontaneous dissection of the superior mesenteric artery. Ann Vasc Dis 7(3):232–238. https://doi.org/10.3400/avd.oa.14-00071
Ullah W, Mukhtar M, Abdullah HM, Ur Rashid M, Ahmad A, Hurairah A, Sarwar U, Figueredo VM (2019) Diagnosis and management of isolated superior mesenteric artery dissection: a systematic review and meta-analysis. Korean Circ J 49(5):400–418. https://doi.org/10.4070/kcj.2018.0429
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
VR and RK analyzed and interpreted the patient data regarding the clinical history and of the imaging modality. VR, RK, DK and ED were major contributors in data collection, literature search and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our institution does not require ethics approval for reporting individual case reports.
Consent for publication
Written informed consent was obtained from the patient for procedure, publication of this case report and accompanying images. Patient identity is not disclosed.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rangankar, V., Kapoor, R., Kumar, D. et al. Spontaneous dissection of celiacomesenteric trunk with isolated extension and thrombosis in the superior mesenteric artery: a case report and literature review. Egypt J Radiol Nucl Med 55, 32 (2024). https://doi.org/10.1186/s43055-024-01202-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01202-5