Abstract
Background
Interval cancers might be divided into true negative interval cancer where a new lesion is detected that no sign of disease could be detected on previous screening mammogram. For false-negative interval cancers, those missed for overt symptoms and those missed for mild or undetectable ones, this includes interpretive error as benign interval cancer (benign mimics), subtle changes, masked carcinoma or slowly growing or patient factors, such as the dense breast parenchyma. Technical failure interval cancer hampered the reader to discover the abnormality. The aim of this study was to relate the risk factors for the development of the interval breast cancer such as breast density, positive family history of breast cancer in Egyptian population. Highlight the causes of missed breast cancer in order to overcome it in the future radiological interpretation.
Methods
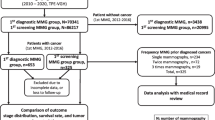
A total of 74,546 screening mammographic examinations were performed in the radiology department at specialized breast cancer center in the period between 2015 and 2021 with about 844 pathologically proved malignant cases. Out of the 844 pathologically proven breast cancer cases, 185 breast cancer patients were interval breast cancer having reported normal examination in the previous year, 88 were true interval breast cancer and 97 were missed on mammography and detected later on. The cases were subjected to full-field digital mammogram (FFDM), complementary ultrasound, contrast-enhanced digital mammography (CEDM) and magnetic resonance imaging (MRI) in some cases, and all cases were histopathologically proven by either fine needle aspiration, core needle biopsy (CNB) or open biopsy.
Results
The mean age of the patients was 53.5 years (range 36–83 years). The overall breast cancer detection rate was 11 per 1000 women. The 185 interval cancers were present at a rate of 3.8 per 1000 women. True negative interval breast cancers where 88 cancers were not present in retrospect on screening mammograms, 17 cases present with benign findings (benign mimics mass or calcifications) and 80 cancers were missed cancers. Analysis of the cause of missed carcinoma revealed patient-related, tumor-related, or provider-related factors. Tumor factors were the most commonly encountered, accounting for 49.5% (48 cases), then provider factors in 25.8% (25 cases) while the patient factors were the least commonly encountered in 24.7% (24 cases). Recorded positive family history found in about 35% (31cases) of the true interval breast cancers.
Conclusions
Although mammography is the standard for detecting early breast cancer, some cancers can be missed due to various causes. Mammographic interpretation must meet high standards to reduce missed cancers. Radiologists should carefully assess screening views and order additional imaging if needed. Palpable lesions and clinical data should be further examined with ultrasound and contrast imaging if necessary. Always compare current images with previous examinations to check for any changes in lesion size. When one pathology is found, search for additional lesions.
Similar content being viewed by others
Background
Interval breast cancer is a term given to cancers detected/presenting within 12 months after a mammographic screening in which findings are considered normal [1].
Awareness about interval cancers is essential, because women diagnosed with interval cancers are more to have poor outcomes. Interval breast cancers typically have a more aggressive nature and are larger than breast tumors that are discovered by screening. Interval cancers account for 20–30% of all breast cancers identified in women who get biannual mammography screenings, with an incidence of 10–20 per 10,000 women [2,3,4,5].
Radiological surveillance, or retrospective mammographic review, of interval breast cancers has methodological limitations, but despite these limitations, the majority of interval cancers are consistently found to represent either true interval or occult cancers that were not visible on the index mammographic screen; roughly 20–25% of interval breast cancers are classified as false negatives, meaning they were missed. Compared to screen-detected tumors, real interval cancers were more likely to have high breast density and the triple negative phenotype [6, 7].
Dense breast parenchyma may obscure a lesion, poor positioning, lesion location outside the field of view, lack of perception of an abnormality that is present, incorrect interpretation of a suspect finding, subtle features of malignancy, or a slowly changing malignancy, all are causes of missed breast cancers. Breast cancers are often overlooked when their morphology suggests a benign etiology (circumscribed masses, e.g., mucinous [colloid] and medullary invasive ductal carcinomas) or when they appear as focal areas of asymmetry or distortion (e.g., invasive lobular carcinoma) [8, 9].
Interval cancers might be divided into true negative interval cancer, retrospectively interval cancer and technical failure interval cancer [10,11,12].
The true negative interval cancer was considered when there was no sign of disease could be detected on previous screening mammogram; the lesion is new [10,11,12].
The retrospectively visible interval cancer was considered when a now known lesion was seen on the previous screening mammogram; which included provider, tumor factors and patients factors. The patient factors such as the dense breast parenchyma while provider factors meant that interpretive error on the part of the reader or single reader as a second reader would have discovered the lesion. Tumor factors meant that a lesion that proves to be malignant showed benign morphological characteristics on the previous mammogram or calcific foci not reaching the pathological descriptors (benign mimics), or subtle changes, masked carcinoma or slowly growing [10,11,12].
Technical failure interval cancer where a technically poor image hampered the reader to discover the abnormality; in theory, suboptimal images will not be submitted for interpretation and if they are, should not be read [10,11,12].
The aim of this study was to relate the risk factors for the development of interval breast cancer such as breast density, positive family history of breast cancer in Egyptian population. Highlight the causes of missed breast cancer in order to overcome it in the future radiological interpretation.
Methods
A total of 74,546 screening mammographic examinations were performed in the radiology department at specialized breast cancer center in the period between 2015 and 2021 with about 844 pathologically proved malignant cases.
Out of the 844 pathologically proven breast cancer cases, we included all the pathologically proved malignant breast cancer cases who had previous available mammogram reported as normal or with benign findings within a year; 185 breast cancer patients were interval breast cancer having reported normal examination in the previous examination. Eighty-eight patients were true interval breast cancer, and 97 were missed on mammography and detected on the following follow-up or by symptomatic patient before her next annual mammogram. The mean age of the patients was 53.5 years (range 36 to 83 years).
Excluded cases were the patients skipped the annual screening mammogram with newly developed breast cancer and initial screening detected breast cancers.
The screening process consisted of craniocaudal and mediolateral oblique views. Physical examination was not provided as part of the annual screening mammography examination while the symptomatic patients were referred for the Surgical and Oncology Departments.
Each patient underwent the screening full-field digital mammogram (FFDM) (initial) and the following FFDM with complementary breast ultrasound and in some cases contrast enhanced mammography or MRI breast. All cases were histopathologically proven either by fine needle aspiration cytology (FNAC), core needle biopsy (CNB) or open biopsy.
For all patients, the age group of the patient was recorded; family history and tumor histology (type, tumor size, nodal involvement, and staging) were analyzed and interpreted twice by breast pathologist (ten years’ experience).
Analysis of mammograms included the density of breast parenchyma, skin thickness, nipple areola complex, associated axillary lymph nodes, areas of parenchymal distortion, asymmetrical densities, mass lesions, calcifications, and finally, BIRADS score for detected lesions.
Initial reported studies were done by single reader of different radiology experiences varying from five to twenty years. Retrograde revision of the base line examintions in the current study were done by expert radiologists of more than ten years of experience.
Statistical analysis
Data analysis was performed with commercially available software (IBM SPSS Statistics for Windows version 24.0.2.). Numerical data were expressed as mean, median and range as appropriate. Qualitative data were expressed as frequency and percentage. Comparison of qualitative variables was done using Pearson’s Chi-square test.
Results
The study included 185 women with histopathologically proven breast carcinomas. The overall breast cancer detection rate was 11 per 1000 women. The 185 interval cancers were present at a rate of 3.8 per 1000 women. True negative interval breast cancers where 88 cancers were not present in retrospect on screening mammograms (Figs. 1, 2, and 3). Recorded positive family history in about 35% (31cases) of the true interval breast cancer cases. The rest of the 185 enrolled cases; 97 cases were missed carcinoma. Analysis of the cause of missed carcinoma revealed causative factors: patient, tumor, or provider factors as shown in Table 1. Tumor factors were the most commonly encountered, accounting for 49.5% (48 cases), then provider factors in 25.8% (25 cases) while the patient factors were the least commonly encountered in 24.7% (24 cases).
A 49-year-old female with positive family history, under regular annual mammography. She presented with a right breast newly developed UIQ palpable mass(arrow) (A:CC and B: MLO mammographic views) which was not present in the previous 9 months earlier mammography (C and D). This was a true interval breast cancer revealed mucinous carcinoma, grade 2 by histopathology
A 46-year-old female patient under regular annual follow-up after left breast conservative treatment. After two years of benign postoperative findings, she developed bifocal masses in the UOQ of the right breast (arrows) (A and B; CC view, C and D; MLO view) compared to previous study (E and F, G and H). CEDM of the right breast revealed bifocal UOQ mass enhancement (I and J).Histopathology revealed triple negative, medullary carcinoma, grade 2. This was a true interval breast cancer
An initial BIRADS score was assigned for these 97 missed cases shown in Table 2. BIRADS 0 for (24 cases) 23 cases inherently and 1 acquired dense breast due to postoperative scarring. BIRADS 1 score was assigned for 18 cases with missed subtle areas of asymmetrical densities and parenchymal distortion (Figs. 4 and 5). BIRADS 2 (25 cases) included benign looking calcifications in 7 cases, stability of the changes in 10 cases and 8 carcinomas bad perception. BIRADS 3 (29 cases) included 10 well-circumscribed, 2 masked, 9 misinterpreted carcinomas and 8 single-reader misinterpretation. BIRADS 4 for case initially presented by mild unexplained with diffuse edema pattern later developed pathological lymph nodes detected in her follow-up.
A 64-year-old female coming for her regular annual screening with no palpable lesions. Right breast views (A and B) show lower inner quadrant non-circumscribed dense mass(circle), which was present, retrograde in the previous examination (C and D) as a smaller benign mimic nodule (1 year earlier). Associated pleomorphic grouped microcalcifcations are seen at the present study (arrow and magnification) with stable other several macro-calcifications. Biopsy revealed IDC, grade 2
A 50-year—old female on her regular annual screening mammography. CC and MLO views of the left breast (A and B) revealed irregular non-circumscribed dense mass at the UOQ (circle), which was present as subtle focal asymmetry in the previous annual mammography (C and D). This case was missed carcinoma due to subtle mammographic changes. Biopsy revealed IDC, grade 2
The mean age of the patients was 53.5 years (ranges from 36 to 83 years). Table 3 lists the true interval and missed cancers by age group. It shows the incidence of interval, and the missed carcinoma is higher above 50 years of age.
The mammographic densities with age correlate are shown in Table 4.
Revising the pathological specimens of these 185 cases yielded 24 carcinomas in situ, 22 invasive lobular carcinoma, 91 invasive ductal carcinomas, 13 mixed IDC and ILC, 2 medullary, 4 papillary carcinomas, 5 mucinous, 21 invasive tubular/commedu carcinoma, and 3 cases with metastatic adenocarcinoma. Table 5 lists the histologic types and tumor grade.
Double reading aids in the cancer detection, post-processing capabilities available on digital mammography like using the magnifying lens and the inverted images, ultrasound, CEDM and MRI examinations. Complementary ultrasound examination was performed for all 185 cases (100%) and showed a higher sensitivity than mammography in carcinoma detection. It was diagnostic in 163 (88%) cases only. (Table 6). In the remaining 22 cases that have been not detected adequately (12 from true interval cancer and 10 cases of missed cancers), further CEDM or MRI were performed and confirmed by biopsy. These cases included two multi-centric DCIS, three multifocal DCIS, five single DCIS, five multifocal IDC, one multi-centric ILC, three metastatic adenocarcinoma and three cases of DCIS were diagnosed by stereotactic biopsy without need for contrast study.
Discussion
Interval breast cancer is a term given to cancers detected/presenting within 12 months after a mammographic screening in which findings were considered normal [1]. Women diagnosed with interval cancers have more aggressive nature. Interval cancers account for 20–30% of all breast cancers identified in women who get biannual mammography screenings, with an incidence of 10–20 per 10,000 women [2,3,4,5].
We aimed to relate the risk factors for the development of the interval breast cancer and highlighted the causes of missed breast cancer.
In the current study, the overall breast cancer detection rate was 11 per 1000 women. The 185 interval cancers were present at a rate of 3.8 per 1000 women screened which is comparable to Burhenne et al. [12]. In the prospective population-based Malmö breast tomosynthesis screening trial, the interval cancer rate of 1.6 per 1000 women screened [13]. In the study conducted for women diagnosed with breast cancer between January 2004 and June 2010 in Manitoba, Canada, the total of 69 025 women aged 50–64 years had 212 579 screening mammograms. Out of which 1687 diagnoses of invasive breast cancer and 206 were interval breast cancers [14, 15].
The Canadian National Breast Screening Study found 108 interval cancers in 44,925 women, at an interval cancer rate of 2.4 per 1000 women [16].
For false-negative interval cancers, authors have separated cases into two categories: those missed for overt symptoms and those missed for mild or undetectable ones [17].
In a review of interval cancers in the Malmo screening trial, they found that 10 of 94 cases were missed due to observer error and 21 of 94 showed subtle signs of malignancy [17].
Kamal et al. [18] classified the causes of missed breast cancers to various factors, including those related to the patient, to the nature of the tumor itself, to technical factors and to provider factor.
In the current study 'Patient factors,' accounted for 24.7% of cases (24 cases) mainly attributed to increased breast parenchyma density whether inherent (23 cases) or acquired dense parenchyma following surgery (1 case). An equal percentage (24%) was recorded in previous studies performed by Bird et al. [19] in a screening population and found that 77 of 320 cancers (24%) in a screening population were missed, primarily due to dense breasts and a developing density that was not identified by the radiologist. Another study held by Kamal et al. [18] found nearly equal percentage of 22.4% (34 patients).
Screening mammography is utilized to discover clinically occult breast carcinomas, while diagnostic mammography is used to investigate an abnormality found on screening mammography or to assess symptomatic individuals [10]. Naturally dense breast parenchyma makes it more difficult to find a mass, particularly a non-calcified, non-distorting lesion. Looking for areas of architectural distortion or subtle microcalcifcations requires a radiologist to pay close attention. Magnification views are employed to assess the morphologic characteristics of microcalcifcations that is faint or suspicious [11].
For additional assessment and characterization of palpable masses in dense tissue and confined iso-dense masses, as well as for the examination of asymmetric densities observed at mammography, any patient with dense breast parenchyma, a palpable mass, and negative mammographic results should undergo ultrasound examination [11].
'Tumor factor' was the most common factor responsible for missed breast carcinoma in our study accounting for 49.5% of cases (48 patients) compared to 44.1% of cases (67 patients) in Kamal et al. study [18]. According to Majid et al. [20], the most difficult cancers to diagnose are those with subtle or indistinct features of malignancy. These features include areas of architectural distortion, small groups of amorphous or punctate microcalcifcations, focal asymmetrical densities, dilated ducts and well-circumscribed masses. Missed tumors were observed on just one of two views more frequently than identified malignancies and were statistically significant to have a lower density. We encountered 18 (9.7%) subtle carcinomas that were missed in mammography including asymmetry and parenchymal destortion, 10 well-circumscribed masses (5 infiltrating duct, 3 ILC and 2 intra-ductal papillary carcinomas), 5 cases DCIS with stationary course, 5 cases with slowly growing nodule,7 cases benign mimic calcifications, one case of diffuse breast edema and 2 masked carcinomas.
Asymmetric breast densities are commonly observed at mammography. According to Majid et al. [20], these findings in isolation have a low positive predictive value for malignancy; however, when they are associated with microcalcifcations or architectural distortion, the risk of malignancy is increased. They suggested that the workup of areas of asymmetric density should include clinical examination, additional mammography views and ultrasound examination. The clinical assessment, ultrasound examination and post-processing manipulation (magnification, inversion and density control) were the main methods used in our investigation [20].
A circumscribed carcinoma should always be considered in menopausal women who present with a circumscribed solid mass, since fibroadenomas are uncommon at this age [20]. In the current study, we found 10 well-circumscribed masses falsely diagnosed as benign that turned to be malignant circumscribed lesions as follows (5 IDC, 3 ILC and 2 intra-ductal papillary carcinomas).
In one instance, diffuse edema pattern was found, even though hindered the detection of underlying breast masses but associated with suspicious segmental amorphous microcalcific foci. There was underlying infiltrating duct cancer found by further contrast MRI.
When a radiologist notices a clear finding—whether benign or malignant—it could lead to 'happy eye syndrome,' which prevents them from closely inspecting for other abnormalities or lesions [20]. In the present study, two cancers were missed due to overt benign masses, on inversion images of digital mammography, one of which showed subtle microcalcific cluster.
In our investigation, provider factors were responsible for 25 (25.8%) of missed carcinomas. Out of 97 cases of missed malignancies in the current study, eight cases were related to single reader interval cancer. These cases were considered when carcinomas were detected on double reading by a more experienced senior radiologist in eight cases. Hofvind et al. [21] retrospectively examine whether various review designs have an impact on the estimate of missed interval cancer. Of the 231 interval cancers, 46 (19.9%) were reclassified as missed cancers using the mixed blinded individual review, and 54 (23.4%) were classified as missed cancers using the mixed blinded paired review. Eighty-three cancers (35.9%) were classified as missed cancers with individual informed review, and 78 (33.8%) were classified as missed cancers with consensus informed review. Thirty-nine cancers (16.8%) were reclassified as missed when four or more radiologists assigned a score of two or more (probably benign or more suspicious); three cancers (1.3%) were reclassified as missed when a score of four or more (probably malignant or more suspicious) was assigned.
Radiologists errors were mainly attributed to two major factors, namely perception and interpretation problems [18]. In perception mistakes, which accounted 4.3% of cases of missed carcinomas in our study (eight patients), the lesions were included within the field of view and was evident, yet the radiologists failed to interpret it. Subtle, small, non-calcified and non-distorting carcinomas are responsible for these cases. Interpretation errors by the radiologists occur when a lesion with worrisome characteristics was misinterpreted as a benign or probably benign lesion (BIRADS 2 and 3). A number of things can affect how something is perceived and interpreted, such as inadequate training, inexperience, subtle indicators of malignancy, the presence of an evident finding, exhaustion, haste, poor viewing conditions, and distractions [10]. Numerous studies, including our own, have shown that years of experience and training have a significant impact on radiologists' accuracy in interpreting mammograms. According to their research, radiologists' years of experience had the biggest bearing on their effectiveness; those with fewer years of experience had higher specificity but lower sensitivity [18, 22]. When combined with training, direct feedback on performance traits could be more beneficial than experience gained in isolation. Discussing misinterpreted mammograms openly could be helpful [23]. A view box with sufficient luminance, less outside light, and low ambient room light are all ideal viewing circumstances as well as minimized distractions [18].
Limitations of the study included being a single institute study and variable experience of the initial examination reporting radiologists.
Conclusions
Although mammography is the standard for detecting early breast cancer, some cancers can be missed due to various causes. Mammographic interpretation must meet high standards to reduce missed cancers. Radiologists should carefully assess screening views and order additional imaging if needed. Palpable lesions and clinical data should be further examined with ultrasound and contrast imaging if necessary. Always compare current images with previous examinations to check for any changes in lesion size. When one pathology is found, search for additional lesions.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACR:
-
American college of radiology
- BIRADS:
-
Breast imaging-reporting and data system
- CC:
-
Craniocaudal
- CEDM:
-
Contrast enhanced digital mammography
- CNB:
-
Core needle biopsy
- DCIS:
-
Ductal carcinoma in situ
- FFDM:
-
Full field digital mammogram
- FNAC:
-
Fine needle aspiration cytology
- IDC:
-
Invasive ductal carcinoma
- ILC:
-
Invasive lobular carcinoma
- MLO:
-
Mediolateral oblique
- MRI:
-
Magnetic resonance imaging
- US:
-
Ultrasound
References
Byng D, Strauch B, Gnas L, Leibig C, Stephan O, Bunk S, Hecht G (2022) AI-based prevention of interval cancers in a national mammography screening program. Eur J Radiol 152:110321
McCarthy AM, Ehsan S, Appel S, Welch M, He W, Bahl M, Chen J, Lehman CD, Armstrong K (2021) Risk factors for an advanced breast cancer diagnosis within 2 years of a negative mammogram. Cancer 127(18):3334–3342
Holm J, Humphreys K, Li J, Ploner A, Cheddad A, Eriksson M, Törnberg S, Hall P, Czene K (2020) Risk factors and tumor characteristics of interval cancers by mammographic density. J Clin Oncol 33(9):1030–1037
Houssami N, Hofvind S, Soerensen AL, Robledo KP, Hunter K, Bernardi D, Lång K, Johnson K, Aglen CF, Zackrisson S (2021) Interval breast cancer rates for digital breast tomosynthesis versus digital mammography population screening: an individual participant data meta-analysis. EClinicalMedicine. 1:34
Kerlikowske K, Zhu W, Tosteson AN, Sprague BL, Tice JA, Lehman CD, Miglioretti DL (2015) Breast cancer surveillance consortium. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Inter Med 162(10):673–681
Houssami N, Hunter K (2017) The epidemiology, radiology and biological characteristics of interval breast cancers in population mammography screening. NPJ Breast Cancer. 3(1):12
Domingo L, Sala M, Servitja S, Corominas JM, Ferrer F, Martínez J, Macià F, Quintana MJ, Albanell J, Castells X (2010) Phenotypic characterization and risk factors for interval breast cancers in a population-based breast cancer screening program in Barcelona, Spain. Cancer Causes Control 21:1155–1164
Ian TW, Tan EY, Chotai N (2021) Role of mammogram and ultrasound imaging in predicting breast cancer subtypes in screening and symptomatic patients. World J Clin Oncol 12(9):808
Lim ZL, Ho PJ, Khng AJ, Yeoh YS, Ong AT, Tan BK, Tan EY, Tan SM, Lim GH, Lee JA, Tan VK (2022) Mammography screening is associated with more favourable breast cancer tumour characteristics and better overall survival: case-only analysis of 3739 Asian breast cancer patients. BMC Med 20(1):1–9
Muttarak M, Pojchamarnwiputh S, Chaiwun B (2006) Breast carcinomas: why are they missed? Singap Med J 47(10):851
Lamb LR, Mohallem Fonseca M, Verma R, Seely JM (2020) Missed breast cancer: effects of subconscious bias and lesion characteristics. Radiographics 40(4):941–960
Burhenne HJ, Burhenne LW, Goldberg F, Hislop TG, Worth AJ, Rebbeck PM, Kan L (1994) Interval breast cancers in the Screening Mammography Program of British Columbia: analysis and classification. AJR Am J Roentgenol 162(5):1067–1071
Johnson K, Lång K, Ikeda DM, Åkesson A, Andersson I, Zackrisson S (2021) Interval breast cancer rates and tumor characteristics in the prospective population-based Malmö Breast Tomosynthesis Screening Trial. Radiology 299(3):559–567
Ellison J, Wu X, McLaughlin C, Lake A, Firth R (2003) Data quality indicators by year and registry. North American Association of Central Cancer Registries APPENDIX C. 2006;Cancer In North America: 1999-Volume One: Incidence: North American Association of Cancer Registries Inc:II-325.
Niraula S, Biswanger N, Hu P, Lambert P, Decker K (2020) Incidence, characteristics, and outcomes of interval breast cancers compared with screening-detected breast cancers. JAMA Netw Open 3(9):e2018179
Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA (2014) Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ 11:348
Hovda T, Hoff SR, Larsen M, Romundstad L, Sahlberg KK, Hofvind S (2022) True and missed interval cancer in organized mammographic screening: a retrospective review study of diagnostic and prior screening mammograms. Acad Radiol 29:S180–S191
Kamal RM et al (2007) Missed breast carcinoma; why and how to avoid? J Egypt Natl Cancer Inst 19(3):178–194
Yeom YK, Chae EY, Kim HH, Cha JH, Shin HJ, Choi WJ (2019) Screening mammography for second breast cancers in women with history of early-stage breast cancer: factors and causes associated with non-detection. BMC Med Imag 19(1):1–9
Majid AS, de Paredes ES, Doherty RD, Sharma NR, Salvador X (2003) Missed breast carcinoma: pitfalls and pearls. Radiographics 23(4):881–895
Hofvind S, Skaane P, Vitak B, Wang H, Thoresen S, Eriksen L, Bjørndal H, Braaten A, Bjurstam N (2005) Influence of review design on percentages of missed interval breast cancers: retrospective study of interval cancers in a population-based screening program. Radiology 237(2):437–443
Barlow WE, Chi C, Carney PA, Taplin SH, D’Orsi C, Cutter G, Hendrick RE, Elmore JG (2004) Accuracy of screening mammography interpretation by characteristics of radiologists. J Natl Cancer Inst 96(24):1840–1850
Mann RM, Athanasiou A, Baltzer PA, Camps-Herrero J, Clauser P, Fallenberg EM, Forrai G, Fuchsjäger MH, Helbich TH, Killburn-Toppin F, Lesaru M (2022) Breast cancer screening in women with extremely dense breasts recommendations of the European Society of Breast Imaging (EUSOBI). Eur Radiol 32(6):4036–4045
Acknowledgements
No Acknowledgements. All authors have read and approved the manuscript.
Funding
This research did not receive specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
OS wrote the manuscript, performed the statistical analysis and is responsible for correspondence to journal. MA and DH collected patient data and was responsible for image processing and collection of patient’s images. EK and MF participated in the design of the study and was responsible for the review of the draft from a clinical point of view.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board (IRB) of Baheya foundation for early detection and treatment of breast cancer, with IRB no.202106210010. Informed written consent was taken from all subjects.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study.
Competing interests
No financial or non-financial competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shetat, O.M.M., Abdelaal, M.M.A., Hussein, D. et al. Interval breast cancer: radiological surveillance in screening Egyptian population. Egypt J Radiol Nucl Med 55, 20 (2024). https://doi.org/10.1186/s43055-024-01193-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01193-3