Abstract
Background
This study aimed to evaluate the safety and efficacy of the endovascular deconstructive artery technique in the treatment of dissecting posterior cerebral artery (PCA) aneurysms (P1 and P2 segments). We retrospectively analyzed the angiographic characteristics, technical data, and clinical outcomes of nine consecutive patients with dissecting PCA aneurysms treated by our team using the endovascular deconstructive technique between January 2019 and December 2022. The patients consisted of six males and three females ranging in age from 36 to 63 years (mean: 48.1 ± 9). Four patients had a P1 segment dissecting aneurysm, and five patients had a P2 segment dissecting aneurysm. Five patients presented with a headache, one patient presented with epilepsy and headache, and three patients presented with subarachnoid hemorrhage.
Results
A balloon occlusion test (BOT) was performed for one patient. Eight patients were treated by parent artery occlusion (PAO) using micro-coils, whereas one patient was treated by occluding the P2 segment dissecting aneurysm using NBCA glue. There were no cortical infarctions following the occlusion of the parent artery. Two patients with P1 occlusion experienced post-procedural small thalamic infarctions that resolved to mRs:1 at three months of follow-up. P2 segment occlusion was asymptomatic in all patients. One patient showed partial aneurysmal recurrence.
Conclusions
Endovascular parent artery occlusion is an effective and safe treatment approach for the dissecting aneurysms of PCA, even without an occlusion test. In addition, the risk of perforator infarction must be considered in P1 occlusion.
Level of evidence
Level 4, Case Series.
Similar content being viewed by others
Background
Posterior cerebral artery aneurysms are rare, accounting for between 0.7 and 2.3% [1,2,3,4,5]. Typically, they are large or giant, most commonly dissecting non-saccular or fusiform in shape [6,7,8]. The frequency of these aneurysms was not accurately reported [9]. According to some reports, it accounts for less than 1.2% of intracranial aneurysms [10] or 35% of all PCA aneurysms [11, 12]. They commonly occur at P1/P2 segments [1, 13, 14] and commonly manifested by subarachnoid hemorrhage, ischemia, symptoms of mass effect, or headache [1, 15]. They have a high mortality rate reaching up to 80% in non-treated cases as well as a high risk of early rebreeding in ruptured cases [15,16,17]. Therefore, the majority of dissecting PCA aneurysms should be treated.
Surgical treatment was shown to have high morbidity and mortality in the clinical outcome given their location and mostly non-saccular morphology [8, 18,19,20].
Endovascular management consists of deconstructive (artery sacrificing) or reconstructive (artery preserving) techniques [16, 21, 22].
The whole arterial segment is diseased in dissecting aneurysms, and selective aneurysmal coiling is inefficient, even in apparently saccular aneurysms [23, 24]. The challenging morphology of the aneurysm may often prevent the reconstructive approach. In addition, the reconstructive procedure requires a flow-diverter stent or stent-assisted coiling, necessitating double antiplatelet therapy, which can be problematic in the acute phase of ruptured cases [25, 26]. Accordingly, the deconstructive technique (proximal parent artery occlusion, aneurysm, and parent artery occlusion or trapping) may be considered the preferred first-line treatment, despite its associated ischemic risks due to cortical branch hypoperfusion [27,28,29]. Some reports described the pre-treatment balloon occlusion test, but its necessity has not been validated [17, 30]. Moreover, P1 sacrifice carries the risk of perforator occlusion. It is nevertheless mandatory in some aneurysms to occlude the P1 segment despite the associated risk [31, 32]. Few reports studied the endovascular treatment (EVT) of PCA dissecting aneurysms, including P1 occlusion [2, 21]. Therefore, for a better understanding of the associated risk-to-benefit ratio, there is a need for additional research on a larger number of cases treated with the deconstructive technique.
Methods
The Institutional Review Board of the Radiology Department approved the study design and the use of clinical data. All patients or their first-degree relatives provided their written informed consent.
From January 2019 to December 2022, 9 patients with dissecting PCA aneurysms were referred to our team for endovascular embolization.
We retrospectively analyzed the angiographic characteristics, technical details, and clinical outcomes of the nine consecutive patients.
The patients included six males and three females, with ages ranging between 36 and 63 years and a mean age of 48.1.
Three patients had hypertensive, and one had liver cirrhosis with elevated liver enzymes. Five of the patients were smokers.
Five patients presented with a headache, one patient presented with epilepsy and headache, and three patients presented with subarachnoid hemorrhage.
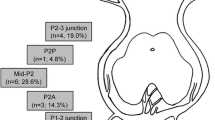
Four patients had dissecting aneurysms initially arising from the P1 segment, and five had dissecting aneurysms of the P2 segment (Figs. 1, 2, 3).
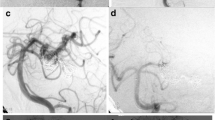
A, B Injection through the left vertebral artery showing giant dissecting aneurysm arising from the very proximal PCA with no filling of the PCA branches. C Selective angiogram with microcatheter tip at the aneurysmal neck, with distal branch filling. D 3D reformatting image of the aneurysm. E, F Deployed small micro-coil occluding the P1 segment with no filling of the aneurysm
A, B Axial and coronal MDCT 3D images showing fusiform dissecting aneurysm at the PCA. C, D DSA, AP, lateral images obtained via left vertebral A injection showing large dissecting fusiform aneurysm involving the PCA. E Aneurysmal packing with neck protection using Eclipse 4 × 20 mm. F, G AP, lateral DSA images with partial aneurysmal neck packing, protecting the thalamoperforators. H Axial FLAIR,MRI demonstrating left thalamic infarction, presented clinically with ataxia and right side weakness. The patient's mRS score improved to 1, at 3 months
Seven of the nine patients we treated had aneurysms exceeding 16 mm in maximum diameter (77.7%).
All embolized cases were treated under general anesthesia with the right trans-femoral arterial approach, followed by the dominant vertebral artery catheterization using a 6-French guiding catheter (Guider soft tip, Boston Scientific, USA). Microcatheterization of the diseased artery was done using Excelsior SL-10 microcatheter (Boston Scientific, USA) over Transcend 0.014" microguidewire (Boston Scientific, USA). Detachable microcoils from different companies were used. In 1 patient, NBCA glue was injected to occlude the P2 segment dissecting aneurysm through Marathon microcatheter (Medtronic, Minnesota, USA), which was diluted 2:1 NBCA: Lipiodol. The glue injection was well controlled, so no distal infiltration of the P2 branches occurred.
In Fig. 2, the wide neck aneurysm coiling was performed under the balloon remodeling technique using Eclipse 4 × 20 mm balloon (Balt Extrusion, France). In Fig. 4, the BOT was performed with Hyper glide 4 × 10 mm balloon (Medtronic, Irvine, California, USA), under general anesthesia with an assessment of the leptomeningeal collaterals coming from the branches of the left middle cerebral artery and left anterior cerebral artery to the branches of the left PCA.
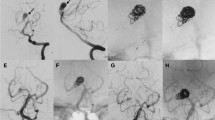
A–C Conventional axial MDCT,MRI images showing vascular lesion at the left crural cistern, with surrounding vasogenic edema. D, E AP, oblique, DSA projections, demonstrating dissecting aneurysm at the left P2 segment with underfilling distal to the aneurysm. F, G AP,LAT, DSA after aneurysmal packing and parent artery occlusion. H, I Six-month follow-up angiogram with partial refilling of the aneurysmal sac through the leptomeningeal collaterals. J, K Follow-up, MRI (showing regression of the aneurysm size and the surrounding edema)
During the first two days following the artery occlusion, the patient's blood pressure was maintained at a normal high level, a prophylactic dose of anticoagulants was administered, and the patient was instructed to stay hydrated and lying down.
Results
A BOT was performed on one patient (Fig. 4). In all other patients, occlusion of the parent artery was not preceded by a BOT.
Although BOT with neurological monitoring can be used to assess collateral flow, its value in the settings of PAO remains controversial. Technically, BOT is sometimes not feasible or risky as the caliber of the vessel may be too small for balloon inflation. Moreover, there is sometimes an inadequate length of the PCA proximal to the aneurysm for balloon inflation. That is why we were not able to perform BOT in all cases.
All patients were treated by endovascular parent artery occlusion. Eight patients received microcoils. However, one patient underwent an NBCA glue injection.
In six of the eight patients treated by microcoils, the coils were packed within the aneurysm, occluding the parent artery and the aneurysm. In one patient (Fig. 5), occlusion of the parent artery was performed by trapping the aneurysm, deploying loops of the coils proximal and loops distal to the dissecting aneurysm. Another patient had a giant aneurysm originating from the most proximal part of the P1 segment with no distal filling of the PCA branches. Proximal occlusion of the parent artery was then achieved using a microcoil. This patient experienced a post-procedural small thalamic infarction manifested by ataxia, which improved over the next few weeks and resulted in an mRs = 1 at three months.
A–C Arterial, parenchymal phases DSA in AP, oblique projections, via left vertebral artery injection, showing dissecting aneurysm at the right P2 segment. D Selective angiographic image with tip of the microcatheter at the P2 segment. E Trapping of the dissecting aneurysm with one coil loop distal and the remaining loops proximal to the aneurysm. Note the stagnant flow within the aneurysmal sac. F, G Final angiogram showing total occlusion of the dissecting aneurysm and right PCA
In one patient (Fig. 2), the aneurysmal neck at the P1 segment was partially coiled to avoid occluding the thalamoperforator branches seen. Nevertheless, the patient had post-procedural small thalamic infarction manifested by ataxia and right-sided weakness, which improved to mRs = 1 at three months of follow-up. This patient was known to have chronic liver disease that was clinically controlled. However, she experienced hepatic decompensation after the procedure, which was medically treated in the following days.
After the PAO, no cortical infarctions were observed. Two patients with P1 occlusion experienced post-procedural small thalamic infarctions that recovered to mRs:1 at 3-month follow-up (Figs. 1, 2). P2 segment occlusion was asymptomatic in all patients.
Three months after aneurysm packing and PAO, minimal aneurysm recurrence was observed in one patient. The aneurysm has been packed by coils. Five months later, the patient complained of the headache. The follow-up angiogram showed minimal refilling of the distal part of the aneurysm by retrograde filling through leptomeningeal collaterals supplying the branches of the occluded artery (Fig. 6).
Discussion
Arterial dissections, either intracranial or extracranial, are a significant cause of stroke in younger patients. The vertebra-basilar system is the most commonly affected, followed by the middle and anterior cerebral arteries [33,34,35,36].
Isolated dissection of the PCA is rare [37,38,39], but accurate diagnosis is essential for clinically appropriate management. The majority of arterial dissections and aneurysms that dissect have no discernible cause. However, a causative relationship has been suggested with conditions such as syphilis, migraine headaches, cystic medial necrosis, Marfan syndrome, mixed connective tissue disease, fibromuscular dysplasia, homocystinuria, polycystic kidney disease, and trauma [40,41,42]. Extracranial dissections are commonly associated with arterial hypertension. Nevertheless, hypertension is uncommon in patients with posterior circulation dissection [40, 43]. Three patients in our series had uncontrolled hypertension, and five were smokers. None of them was diagnosed with collagen vascular disease. None of them reported a history of antecedent previous head trauma. This finding contradicts the association found by Gailloud [44] and is consistent with the results of Wilson and Stryi [40, 43].
In contrast to vertebrobasilar dissections, which are more prevalent in males, isolated PCA dissections are more prevalent in females, with a female-to-male ratio of 3.1:1 [38, 44]. Intracranial arterial dissections typically affect patients in their late third to early fifth decade, whereas PCA primarily affects younger patients [45]. However, the average age in our series was 48.1, which is relatively comparable to Qin et al. [2], who had an average age of 47.5 years and comparable to Hamada et al. [46], who reported an average age of 49.8.
PCA aneurysms are typically large or giant [46, 47], which is consistent with our findings that 77.7% of our patients had large/giant aneurysms.
The most frequently reported location for PCA aneurysms is the proximal segment, which includes the P1 segment and the proximal P1/P2 junction [3, 47, 48]. In our sample, most aneurysms were found to be at the distal P1, reaching P2 and the P2 segment at 66.6 %, with four ones (44.4%) found at the P2 segment, which aligns with the findings of Hamada et al. [46] and Ferrante et al. [45].
Headache is the most common symptom of vertebrobasilar and PCA dissection, usually occurs in the occipital and posterior cervical regions, and is typically accompanied by large cerebral infarcts that may result in death. However, it may also be accompanied by subarachnoid hemorrhage or both [34, 39, 40]. However, in some studies, their clinical course and prognosis were benign [38, 49]. Headache and subarachnoid hemorrhage were the most prevalent presentations in our sample, similar to Ciceri EF et al. [16].
Histologically, owing to the characteristics of intracranial arteries with the fact of lacking an external elastic membrane, have a thinner adventitia and fewer elastic fibers in the media [40], intracranial dissections typically occur between the intima or internal elastic lamina and the media [40]. Conversely, in extracranial arteries, dissection occurs in the outer layers of the media or between the media and the adventitia [34, 40]. In contrast to the cervical carotid arteries, where these changes occur at the skull base, in the vertebral arteries, these changes occur approximately 1 cm proximal to the dural perforation. This anatomic characteristic correlates with the angiographic appearance of lesions, with focal stenosis in the case of intracranial dissection and pseudoaneurysm formation in the case of extracranial dissection [40].
As the hematoma progresses between the internal elastic membrane and the media, focal stenosis with or without post-stenotic dilatation occurs, resulting in a mass effect on the lumen without affecting the outer media or adventitia. Rarely, intracranial dissections may involve the subadventitia and rupture into the subarachnoid space [34]. Due to the shearing of the vessels, dissecting traumatic aneurysms may occur in the setting of mild head trauma [50, 51].
Angiographically, the appearance of dissecting aneurysms is characterized by a narrowing of the vascular lumen (string sign) caused by a subintimal hematoma; this is likely associated with segmental dilatation either proximal or distal to the stenosis (string and pearl signs) [33, 34, 39].
Different surgical treatment approaches have been suggested for those dissecting aneurysms, including excision, trapping, and reinforcement with or without intra-extra cranial bypass [40, 52, 53].
EVT has been explicitly described for dissecting PCA aneurysms [48, 54, 55], which appears to be a viable alternative to surgery despite being performed on a small number of patients.
The reconstructive technique using stents with or without coils, especially the flow diverter stents, is a valid and effective treatment in many cases. However, in some cases of fusiform or giant serpentine aneurysms, the reconstructive technique is technically challenging or not feasible due to the unfavorable caliber or length of the artery. Moreover, stent deployment necessitates dual antiplatelet administration, which is problematic in the acute settings of subarachnoid hemorrhage. Therefore, PAO offers an alternative endovascular approach.
There are few reports on the EVT for dissecting PCA aneurysms and even fewer discussing the deconstructive technique with PAO.
In one large series by Qin et al. [2], 18 of 32 patients with fusiform/dissecting aneurysms had both the aneurysm and the parent artery completely occluded. Three patients who had complete occlusion developed an infarction. One patient with a left P2 dissecting aneurysm experienced right-limb numbness as a result of a thalamus infarction following the occlusion of the aneurysm and the parent artery. This is comparable to our findings, where two patients have small thalamic infractions when occluding the P1 segment and no cortical infarctions on occlusion of the P2 segment.
Numerous studies have concluded that PAO is safe and effective for P2 or distally located aneurysms due to the presence of abundant collaterals [5, 16, 17, 56, 57]. In our sample, no distal cortical infarctions occurred. This finding can be explained by the hypoperfusion within the PCA distal to the dissecting aneurysm, especially in large and giant aneurysms, which allows the gradual development of lepto-meningeal collaterals.
In their series of patients with P2 segment aneurysms, Hallacq et al. [5] performed the endovascular sacrifice of the PCA; none of the patients experienced complications or visual deficits.
In the series by Arat et al. [56], PAO was performed on eight patients with giant fusiform distal PCA aneurysms, and only one patient developed occipital infarcts resulting in permanent homonymous hemianopia. In addition, Lv et al. [17] reported PAO in eight patients with P2 dissecting aneurysms with no neurological deficits.
In a review by Xu et al. [58], of 98 cases of P2-segment and distal aneurysms reported between 2001 and 2012 and treated with PAO, 13 patients experienced new visual field deficits. However, Xu et al. concluded that endovascular PCA sacrifice is a safe method for managing P2-segment and distal aneurysms.
Qin et al. [2] treated 28 of 34 fusiform/dissecting aneurysms located at the P2 or distal segments in his series with PAO with or without coiling of the aneurysm. Although BOT with neurological monitoring can be used to assess collateral flow, its value in the settings of PAO remains controversial. Technically, BOT is sometimes not feasible or risky as the caliber of the vessel may be too small for balloon inflation. Moreover, there is sometimes an inadequate length of the PCA proximal to the aneurysm for balloon inflation. In our series, we performed BOT in one case. Nevertheless, the occlusion was uneventful in all cases, even without BOT.
EVT is difficult for proximal PCA fusiform/dissecting aneurysms due to the perforators that supply the brain stem and thalamus. Posterior thalamoperforators and long circumflex arteries arise from the P1 segment, while the peduncle, thalamogeniculate perforators, and short circumflex arteries arise from the P2 segment [56]. Occlusion of these perforators or endovascular occlusion of the parent artery with the aneurysm frequently results in severe ischemic complications, particularly in patients with fetal-type PCA aneurysms because of the insufficient collateral flow [17, 58]. Qin et al. [2] stated that gradual thrombosis of these aneurysms provides a time window for collateral development. In his study, five patients had partial coiling of the aneurysm and PAO. The smallest length of the parent artery was nearly completely occluded, and none of these patients had any postoperative complications. However, there was still minimal flow at the distal PCA, and follow-up angiography showed complete occlusion of the aneurysm and parent artery. Nonetheless, recanalization or even rupture of such ones is not uncommon after partial coiling, necessitating typically more definitive treatment.
Four patients were treated for P1 segment occlusion in our series. Consequently, two patients experienced ischemic complications in the form of small thalamic infarcts, which improved to mRS=1 at three months. In two patients, the P1 occlusion had no clinical consequences. Occlusion of the P1 segment poses a risk for perforator infarction. However, it may be necessary to occlude the P1 segment whenever it is involved in a giant dissecting aneurysm, as the risk of its rupture is higher.
Conclusions
For dissecting PCA aneurysms, endovascular PAO is an effective and safe approach; even without BOT, the risk of cortical infarction is low due to the lepto-meningeal collaterals. The risk of perforator infarction should be considered in P1 occlusion.
Validation of the durability and efficacy of this treatment approach requires additional research involving larger case series.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BOT:
-
Balloon occlusion test
- PAO:
-
Parent artery occlusion
- PCA:
-
Posterior cerebral artery
- EVT:
-
Endovascular treatment
- NBCA:
-
N-acetyl cyanoacrylate glue
References
Mehrotra M, Mehrotra A, Nair A, Srivastava A, Sahu R, Pradhan M et al (2017) Dissecting intracranial aneurysm in pregnancy: a rare association. Asian J Neurosurg 12(01):127–130
Qin X, Xu F, Maimaiti Y, Zheng Y, Xu B, Leng B et al (2017) Endovascular treatment of posterior cerebral artery aneurysms: a single center’s experience of 55 cases. J Neurosurg 126(4):1094–1105
Goehre F, Jahromi BR, Hernesniemi J, Elsharkawy A, Kivisaari R, von und zu Fraunberg M et al (2014) Characteristics of posterior cerebral artery aneurysms: an angiographic analysis of 93 aneurysms in 81 patients. Neurosurgery 75(2):134–144
Vakharia K, Munich SA, Waqas M, Nagesh SVS, Levy EI (2019) Deployment of distal posterior cerebral artery flow diverter in tortuous anatomy. Neurosurg Focus 46(Suppl_1):V9
Hallacq P, Piotin M, Moret J (2002) Endovascular occlusion of the posterior cerebral artery for the treatment of p2 segment aneurysms: retrospective review of a 10-year series. Am J Neuroradiol 23(7):1128–1136
Kitazawa K, Tanaka Y, Muraoka S, Tada T, Okudera H, Orz Y et al (2001) Specific characteristics and management strategies of posterior cerebral artery aneurysms: report of eleven cases. J Clin Neurosci 8(1):23–26
Kim YB, Lee JW, Huh S-K, Kim BM, Kim DJ (2013) Outcomes of multidisciplinary treatment for posterior cerebral artery aneurysms. Clin Neurol Neurosurg 115(10):2062–2068
Essibayi MA, Oushy SH, Keser Z, Lanzino G (2022) Natural history and management of posterior cerebral artery aneurysms: a systematic review and meta-analysis of individual patient data. Neurosurg Rev 25:1–14
Park W, Kwon DH, Ahn JS, Lee SH, Park JC, Kwun BD (2015) Treatment strategies for dissecting aneurysms of the posterior cerebral artery. Acta Neurochir 157(10):1633–1643
Rodríguez-Hernández A, Zador Z, Rodríguez-Mena R, Lawton MT (2013) Distal aneurysms of intracranial arteries: application of numerical nomenclature, predilection for cerebellar arteries, and results of surgical management. World Neurosurg 80(1–2):103–112
Kwon JY, Kim N-Y, Suh DC, Kang D-W, Kwon SU, Kim JS (2015) Intracranial and extracranial arterial dissection presenting with ischemic stroke: lesion location and stroke mechanism. J Neurol Sci 358(1–2):371–376
Göhre F (2016) Posterior cerebral artery aneurysms
Wang H, Du R, Stary J, Gkogkas C, Kim D, Day A et al (2012) Dissecting aneurysms of the posterior cerebral artery: current endovascular/surgical evaluation and treatment strategies. Neurosurgery 70(6):1581–1588
Taqi MA, Lazzaro MA, Pandya DJ, Badruddin A, Zaidat OO (2011) Dissecting aneurysms of posterior cerebral artery: clinical presentation, angiographic findings, treatment, and outcome. Front Neurol 2:38
Coert BA, Chang SD, Do HM, Marks MP, Steinberg GK (2007) Surgical and endovascular management of symptomatic posterior circulation fusiform aneurysms. J Neurosurg 106(5):855–865
Ciceri EF, Klucznik RP, Grossman RG, Rose JE, Mawad ME (2001) Aneurysms of the posterior cerebral artery: classification and endovascular treatment. Am J Neuroradiol 22(1):27–34
Lv X, Li Y, Jiang C, Yang X, Wu Z (2009) Parent vessel occlusion for P2 dissecting aneurysms of the posterior cerebral artery. Surg Neurol 71(3):319–325
Primiani CT, Ren Z, Kan P, Hanel R, Pereira VM, Lui WM et al (2019) A2, M2, P2 aneurysms and beyond: results of treatment with pipeline embolization device in 65 patients. J Neurointervent Surg 11(9):903–907
Sanai N, Zador Z, Lawton MT (2009) Bypass surgery for complex brain aneurysms: an assessment of intracranial-intracranial bypass. Neurosurgery 65(4):670–683
Terasaka S, Sawamura Y, Kamiyama H, Fukushima T (2000) Surgical approaches for the treatment of aneurysms on the P2 segment of the posterior cerebral artery. Neurosurgery 47(2):359–366
Van Rooij W, Sluzewski M, Beute G (2006) Endovascular treatment of posterior cerebral artery aneurysms. Am J Neuroradiol 27(2):300–305
Mohammadian R, Taheraghdam AA, Sharifipour E, Mansourizadeh R, Pashapour A, Shimia M et al (2013) Endovascular treatment of intracranial artery dissection: clinical and angiographic follow-up. Neurol Res Int 2013
Bond KM, Krings T, Lanzino G, Brinjikji W (2021) Intracranial dissections: A pictorial review of pathophysiology, imaging features, and natural history. J Neuroradiol 48(3):176–188
Qian Z, Feng X, Kang H, Wen X, Xu W, Li Y et al (2017) Dissecting aneurysms of the distal segment of the posterior cerebral artery: clinical presentation and endovascular management. Chin Neurosurg J 3(02):74–82
Meckel S, Singh T, Undrén P, Ramgren B, Nilsson O, Phatouros C et al (2011) Endovascular treatment using predominantly stent-assisted coil embolization and antiplatelet and anticoagulation management of ruptured blood blister-like aneurysms. Am J Neuroradiol 32(4):764–771
Maybaum J, Henkes H, Aguilar-Pérez M, Hellstern V, Gihr GA, Härtig W et al (2021) Flow diversion for reconstruction of intradural vertebral artery dissecting aneurysms causing subarachnoid hemorrhage—a retrospective study from four neurovascular centers. Front Neurol 947:164
Barest GD, Mian AZ, Nadgir RN, Sakai O (2009) Traumatic and non-traumatic emergencies of the brain, head and neck. Emerg Radiol Requisities 1–60
Rahme R, Alimi M, Langer DJ (2019) Complex intracranial aneurysms: strategies for surgical trapping and cerebral revascularization. In: Management of cerebrovascular disorders. Springer, pp 113–129
Matouk C, Kaderali Z, Willinsky R (2012) Long-term clinical and imaging follow-up of complex intracranial aneurysms treated by endovascular parent vessel occlusion. Am J Neuroradiol 33(10):1991–1997
Briganti F, Cicala D, Tortora F, Leone G, Napoli M, Maiuri F (2012) Endovascular treatment of a giant dissecting aneurysm of the posterior cerebral artery: a case report and literature review. Neuroradiol J 25(6):695–701
Liu L, He H, Jiang C, Lv X, Li Y, Wu Z (2011) Deliberate parent artery occlusion for non-saccular posterior cerebral artery aneurysms. Interv Neuroradiol 17(2):159–168
Surdell DL, Hage ZA, Eddleman CS, Gupta DK, Bendok BR, Batjer HH (2008) Revascularization for complex intracranial aneurysms. Neurosurg Focus 24(2):E21
Scott GE, Neubuerger KT, Denst JJN (1960) Dissecting aneurysms of intracranial arteries. Neurology 10(1):22
Yonas H, Agamanolis D, Takaoka Y, White RJ (1977) Dissecting intracranial aneurysms. Surg Neurol 8(6):407–415
Fisher CM, Ojemann RG, Roberson GH (1978) Spontaneous dissection of cervico-cerebral arteries. Can J Neurol Sci 5(1):9–19
Pozzati E, Padovani R, Fabrizi A, Sabattini L, Gaist G (1991) Benign arterial dissections of the posterior circulation. J Neurol 75(1):69–72
Fullerton HJ, Johnston SC, Smith WS (2001) Arterial dissection and stroke in children. Neurology 57(7):1155–1160
Kawahara I, Hiu T, Onizuka M, Toda K, Baba H, Yonekura M (2003) Isolated posterior cerebral artery dissection–case report and review of the literature. Neurol Surg 31(6):671–675
Maillo A, Diaz P, Morales F (1991) Dissecting aneurysm of the posterior cerebral artery: spontaneous resolution. Neurosurgery 29(2):291–294
Berger MS, Wilson CB (1984) Intracranial dissecting aneurysms of the posterior circulation: report of six cases and review of the literature. J Neurosurg 61(5):882–894
Le Tu P, Zuber M, Meder J, Mas J (1996) Isolated dissection of the posterior cerebral artery. Revue Neurol 152(8–9):542–547
Berthier E, Bourrat C (1993) Dissecting aneurysm of the posterior cerebral artery: case report and review of the literature. Cerebrovasc Dis 3(1):56–59
Nelson J (1968) Dissecting subintimal hematomas of the intracranial arteries: report of a case. J Am Osteop Assoc 67:512–517
Sherman P, Oka M, Aldrich E, Jordan L, Gailloud P (2006) Isolated posterior cerebral artery dissection: report of three cases. Am J Neuroradiol 27(3):648–652
Ferrante L, Acqui M, Trillo G, Lunardi P, Fortuna A (1996) Aneurysms of the posterior cerebral artery: do they present specific characteristics? Acta Neurochirurg 138:840–852
Hamada J-I, Morioka M, Yano S, Todaka T, Kai Y, Kuratsu J (2005) Clinical features of aneurysms of the posterior cerebral artery: a 15-year experience with 21 cases. Neurosurgery 56(4):662–670
Drake CG, Peerless SJ, Hernesniemi JA, Drake CG, Peerless SJ, Hernesniemi JA, Ontario Experience on Patients (1996) Giant posterior cerebral aneurysms: 66 patients, pp 230–248
Lazinski D, Willinsky R, TerBrugge K, Montanera W (2000) Dissecting aneurysms of the posterior cerebral artery: angioarchitecture and a review of the literature. Neuroradiology 42(2):128–133
Ohki M, Nakajima M, Sato K, Kondo R, Saito S, Nakai O et al (1994) A case of dissecting aneurysm associated with mixed connective tissue disease. No to Shinkei Brain Nerve 46(9):855–858
Johnson A, Graves V, Pfaff J Jr (1977) Dissecting aneurysm of intracranial arteries. Surg Neurol 7(1):49–52
Batjer HH, Chandler J, Giller C (2000) Intracranial and cervical vascular injuries. In: Head injury, vol 4. Williams and Wilkins Publishers
Sasaki O, Koike T, Takeuchi S, Tanaka R (1991) Serial angiography in a spontaneous dissecting anterior cerebral artery aneurysm. Surg Neurolo 36(1):49–53
Sasaki O, Koizumi T, Ito Y, Sorimachi T, Koike T, Tanaka R (1992) Dissecting aneurysm of the posterior cerebral artery treated with proximal ligation. Surg Neurol 37(5):394–401
Leibowitz R, Do H, Marcellus M, Chang S, Steinberg G, Marks M (2003) Parent vessel occlusion for vertebrobasilar fusiform and dissecting aneurysms. Am J Neuroradiol 24(5):902–907
Yamaura I, Tani E, Yokota M, Nakano A, Fukami M, Kaba K et al (1999) Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J Neurosurg 90(5):853–856
Arat A, Islak C, Saatci I, Kocer N, Cekirge S (2002) Endovascular parent artery occlusion in large-giant or fusiform distal posterior cerebral artery aneurysms. Neuroradiology 44(8):700
Chang SW, Abla AA, Kakarla UK, Sauvageau E, Dashti SR, Nakaji P et al (2010) Treatment of distal posterior cerebral artery aneurysms: a critical appraisal of the occipital artery-to-posterior cerebral artery bypass. Neurosurgery 67(1):16–26
Xu J, Xu L, Wu Z, Chen X, Yu J, Zhang J (2015) Fetal-type posterior cerebral artery: the pitfall of parent artery occlusion for ruptured P2 segment and distal aneurysms. J Neurosurg 123(4):906–914
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
All authors participated in the study design, material preparation, and collection of data was done by ''FH.'' The first draft of manuscript was written by ''AI.'' The statistical results and final manuscript were revised by ''AO.'' All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was done in line with Declaration of Helsinki. Approval was granted by Ethical Committee of Faculty of Medicine, Minia University No. 656:2/2023 ''Retrospective registered'' and faculty of medicine, Minia University. All participants and authors approve the publication of the work; written consent was obtained from all patients or their first-degree relatives.
Consent for publication
Not applicable.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, F., Osman, A.A.L. & Issa, A.S. Deconstructive endovascular technique for dissecting posterior cerebral artery aneurysms: a single-center case series study. Egypt J Radiol Nucl Med 55, 14 (2024). https://doi.org/10.1186/s43055-024-01186-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-024-01186-2