Abstract
Background
Ultrasonography (US) still has some limitations in the differentiation of benign and malignant breast masses. Therefore, we introduced new technologies such as S-Detect, microvascular flow imaging (MVFI), and strain elastography (SE) into the examination and compared the multimodal method with Breast Imaging Reporting and Data System (BI-RADS).
Objectives
This prospective study aimed to evaluate the value of multimodal diagnostic methods that add S-Detect, MFI, and SR to US in differentiating benign from malignant breast masses.
Methods
We recruited 186 patients with 189 masses between July 2021 and March 2022. The masses were examined using US, S-Detect, SR, and MFI before biopsy, and the benign and malignant differentiation value of each and their combination were assessed compared with surgical pathology results using the area under the receiver operating characteristic curve (AUC), accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Subgroup analysis by lesion size was also performed.
Results
The respective optimal cutoff values of SR and MFI for differentiating benign from malignant masses were 3.15 and 2.45, respectively, and the sensitivity and specificity were 79.3% and 85.6% and 94.6% and 69.1%, respectively. The multimodal AUC (0.907), sensitivity (97.8%), accuracy (90.5%), PPV (84.9%), and NPV (97.6%) were larger than those of each modality (p < 0.05), regardless of the mass size.
Conclusions
The diagnostic method of S-Detect combined with multimodal ultrasound can effectively improve the diagnostic efficiency of breast masses and is expected to become a routine examination for breast in future for better evaluation the benign and malignancy of breast masses.
Similar content being viewed by others
Background
Breast cancer has recently become the most prevalent malignancy in female individuals [1, 2]. Breast disease has been attracting increasing attention, and it progressively affects younger patients [3, 4]. A study reported that 5-year breast cancer in situ survival rate was 99%, while the 5-year advanced breast cancer survival rate was only 26% [5]. Therefore, early diagnosis and treatment are key for improving patients’ survival rate and quality of life [6].
Ultrasonography (US) is routinely used to examine breast diseases, as it is safe and noninvasive [7]. However, its main limitation is its operator dependency, with low inter-observer consistency and, for some challenging cases, the signs of benign and malignant may be contradictory, which may lead to misdiagnosis [8, 9]. To overcome this problem, researchers have introduced S-Detect, microvascular flow imaging (MVFI), and strain elastography (SE) into the examination to improve the diagnostic efficiency of ultrasound in differentiating benign and malignant breast masses.
S-Detect can effectively improve inter-reader consistency and has good accuracy [9,10,11,12,13,14,15]. Microvascular flow imaging is a new microvascular ultrasound imaging technique, and microvascular flow index (MFI) can automatically be determined by delineating the lesion edges on images from MVFI to quantitatively evaluate the abundance of blood flow [16,17,18,19,20,21,22,23,24,25]. Elastography evaluates tissue deformation by comparing the stiffness between the tumor and nearby healthy tissue with a high spatial resolution [26,27,28,29,30,31,32,33].
Although the potential benefits of each technique have been reported [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33], few studies have investigated the effect of combining them when evaluating breast masses. If the three technologies are added to breast ultrasound examination, it may have a positive impact on the diagnostic efficiency of breast masses.
This prospective study aimed to evaluate the value of multimodal diagnostic methods that add S-Detect, MFI, and SR to US in differentiating benign from malignant breast masses.
Methods
The ethics committee of our hospital approved this prospective study, and informed consent was obtained from all patients.
This study was performed between July 2021 and March 2022. We enrolled patients scheduled to undergo diagnostic US at our institution.
Inclusion criteria were as follows: (a) having a relatively isolated breast mass detect by US (ensuring accurate localization during biopsy); (b) have undergone US, MFI, SR, and S-detect examinations before surgery and have clear images; and (c) patients who was over 18 years old. Exclusion criteria were as follows: (a) having a mass too large to measure the SR and/or MFI in a single section (over 6 cm, the maximum diameter of the probe); (b) not having definite pathological results by biopsy; (c) diagnosed with other malignant tumors previously or with a previous history of cancer/treatment; (d) receiving radiotherapy or chemotherapy in the ipsilateral breast; and (e) being pregnant or lactating.
All ultrasound examinations were conducted using the Samsung RS85A ultrasonographic system (Samsung Madison Co. Ltd., Seoul, the Republic of Korea), with 3–12-MHz linear array probes and included grayscale US, SR, MFI, and analysis with S-Detect software. All data in this study were evaluated by two radiologists with over ten years of experience in breast ultrasound and their results were compared with each other. If there was a disagreement between the two, consulted another senior radiologist with over 15 years of experience. This was a prospective study, all patients underwent histopathological evaluation after ultrasound examination, and all biopsies/surgeries are performed under ultrasound guidance.
The patients were maintained in the supine position, fully exposing the breast.
First, a routine ultrasound examination was performed to assess six US features of breast masses according to the BI-RADS classification system. The features included shape, azimuth, edge, internal echo, internal structure, and calcification. Each feature has a corresponding score based on which the mass was assigned a BI-RADS classification (Figs. 1a, 2a, 3a). Masses identified as 4b to 5 were classified as suspected malignancies.
A 42-year-old female patient with invasive breast ductal carcinoma. The ultrasonographic images of the breast mass long-axis section were assigned a BI-RADS score of 4A (a), a vascular index of 25.5 (b), and a strain ratio of 5.34 (c). The long- and short-axis sections were both judged as malignant by S-Detect (d)
Second, we perform MVFI on breast masses and calculate MFI using images with the most abundant blood flow signal by outlining the lesions’ edges on the images (Figs. 1b, 2b, 3b). Each numerical value was averaged after three measurements.
Third, we switched to the SE mode, keeping the probe perpendicular to the target lesion and applying as little pressure as possible. An appropriate sampling box size was selected, image clarity was adjusted, and stability was maintained. Subsequently, the E-Breast was activated to trace the lesion area and normal breast tissue of the same depth (Figs. 1c, 2c, 3c). The stain radio was automatically calculated as the ratio between them by the software. Measurements were made in triplicate, and the average value was used.
Fourth, we scanned the transverse and longitudinal aspects of the breast masses in 2D mode, considered the vertical section maximum diameter as the standard level, and then switched to the S-Detect mode. The software automatically outlined the breast lesion area, and the lesion nature was quickly and automatically evaluated to determine if it was a benign or malignant lesion (Figs. 1d, 2d, 3d).
The respective optimal cutoff values of SR and MFI were determined by receiver operating characteristic (ROC) analysis and the Youden index. When either value was above the cutoff value, the breast mass was determined to be malignant; if not, it was determined to be benign.
We divided the data into eight groups for analysis and comparison: US, US + S-Detect, US + SR, US + MFI, US + S-Detect + SR, US + S-Detect + MFI, US + MFI + SR, US + S-Detect + SR + MFI (the multimodal diagnosis). The combined analysis of US, SR, MFI, and S-Detect was performed according to the long-axis section of the breast mass. The original BI-RADS classification was based on the US; then SR, MFI, and S-Detect examinations were performed. The new BI-RADS combined with various techniques will be upgraded or downgraded based on the results of S-Detect/MFI/SR, and the specific upgrade/downgrade methods was shown in the flowchart (Fig. 4). The masses originally classified as BI-RADS 3 were no longer down-regulated, while the masses of BI-RADS 5 were no longer up-regulated. Masses with a final BI-RADS score between 3 and 4A were judged benign, and those with a BI-RADS score of 4B and above were considered malignant.
We separated the masses by size into masses ≤ 20 mm or > 20 mm. The size of the breast cancer at the time of diagnosis is generally considered as the basic and key factor to determine the clinical outcome [34]. In the tumor–node–metastasis-based staging of breast cancer proposed by the International Union for Cancer Control and the American Joint Commission on Cancer, a tumor with a diameter of less than 20 mm is defined as belonging to stage T1 and a tumor with a diameter of more than 20 mm belongs in stages T2 and above [35, 36]. Therefore, a significant cutoff value of breast mass is 20 mm. The accuracy, sensitivity, specificity, AUC, PPV, and NPV of the US and multimodal diagnostic approaches were analyzed by group. Differences in diagnostic efficiency were compared between the mass size groups and the entire cohort.
Quantitative parameters, such as age and size, were represented by mean and standard deviation and compared using the t test. Chi-square test was used to compare the categorical variables (BI-RADS and S-Detect), while the t test was used to compare the continuous variables (SR and MFI) between the groups. The Z test compared the AUC values. Two-sided statistical significance was set at P < 0.05. A Chi-square test was used to compare the size of benign and malignant masses against the mean values of SR and MFI in each group, and the diagnostic efficacy of each group and the US group. Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 25.0 (IBM Corp., Armonk, NY, USA).
Results
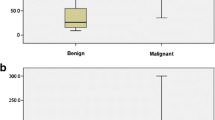
Based on the inclusion and exclusion criteria, this study recruited 186 patients (mean age, 47.2 ± 12.7 years) with 189 breast masses (Fig. 4), including 92 malignant (48.7%) and 97 benign (51.3%) masses; the diameters were ≤ 20 and > 20 mm in 98 and 91 masses, respectively. Invasive ductal carcinoma was the most common malignant breast tumor accounting for 79.3% of the malignancies (73/92). Fibroadenoma was the most prevalent benign lesion (49.5%, 48/97; Table 1). The patients with malignant tumors were older than those with benign masses (53.3 ± 11.9 and 41.5 ± 10.6 years; P < 0.01) and had larger lesions (28.0 ± 13.8 and 17.8 ± 8.9 mm; P < 0.01).
The mean values of the quantitative parameters were all significantly higher in malignant lesions than in benign lesions: MFI (mean, 12.6 ± 11.4 and 5.8 ± 8.6; P < 0.01); SR (mean, 4.1 ± 2.2 and 2.4 ± 1.5; P < 0.01). The optimal MFI and SR cutoff values for differentiating malignant from benign breast masses were 2.45 and 3.15, respectively, and their AUCs were 0.818 and 0.825, respectively (Fig. 5). The diagnostic sensitivity, specificity, accuracy, PPV, and NPV of US + MFI were 94.6%, 69.1%, 81.5%, 74.4%, and 93.1%, respectively, and those of US + SR were 79.3%, 85.6%, 82.5%, 83.9%, and 81.4%, respectively (Table 2). Most MFI and SR values increased with the lesion’s BI-RADS score. The benign/malignant lesion ratio and mean values of MFI and SR for each category are shown in Table 3. Using the pathology examination results as the standard, the combined US and S-Detect assessment had higher sensitivity, accuracy, and NPV (93.5%, 85.2%, and 92.6%, respectively) and a larger AUC (0.911 and 0.862) for differentiating benign from malignant breast masses than US alone (Fig. 5). However, the specificity and PPV of the combined US and S-Detect approach were lower than that of US alone (77.3% and 83.5%; 79.6% and 82.0%, respectively).
The receiver operating characteristic curve. ROC of MFI and SR in distinguish between benign and malignant masses (A), ROC of US, US + S-Detect, US + MFI, US + SR, and the multimodal diagnostic approach (B), and ROC of US, US + S-Detect + MFI, US + S-Detect + SR, US + MFI + SR, and the multimodal diagnostic approach (C). US: ultrasonography; MFI: microvascular flow index; SR: strain ratio
The accuracy, specificity, AUC, PPV, and NPV of the US + S-Detect + MFI group increased compared with those of the US group (0.922, 88.4%, 96.7%, 82.4%, 96.3%, respectively); however, the sensitivity decreased (80.4% and 83.5%). The accuracy, sensitivity, PPV, and NPV of the US + S-Detect + SR group compared with the US group increased (86.8%, 90.2%, 83.8%, 90.0%, respectively), and the specificity did not change significantly (both were 83.5%), while the AUC also increased from 0.862 to 0.914. For the US + MFI + SR group, the accuracy, sensitivity, and NPV increased compared with those of the US group (85.2%, 91.3%, and 90.6%, respectively), while the specificity and PPV decreased (79.4% and 80.8%, respectively), and the AUC increased to 0.923.
The sensitivity, accuracy, PPV, and NPV of the multimodal diagnostic approach (97.8%, 90.5%, 84.9%, and 97.6%, respectively) were significantly higher and the AUC significantly larger (0.939, P < 0.001) than those of the other seven groups. The specificity of the multimodal diagnostic was second only to that of the US + SR group; but this finding was not significant (85.6% and 83.5%; P = 0.69; Table 2). Using the multimodal diagnostic approach, the score of 61 lesions was downgraded from the original BI-RADS grade, including two incorrectly downgraded malignant lesions; biopsy was correctly avoided for 55 masses; and 106 lesions were upgraded, including 16 incorrectly upgraded benign masses (Table 4).
The AUC between various diagnostic methods showed significant statistical significance (P < 0.05). The mean was statistically significant only between the US + MFI and US + SR groups (P = 0.025), while there was no significant difference between the other groups (P > 0.05).
The multimodal diagnostic AUC, accuracy, sensitivity, and NPV were greater than the corresponding values of the US group in both tumor-size subgroups, particularly in the small mass group that had an AUC of 0.955. The multimodal diagnostic specificity and PPV were slightly lower than in US group (Table 2).
Discussion
Ultrasonography is an important method for distinguishing between benign and malignant breast masses [7]. Currently, there are some new ultrasound techniques such as S-Detect, MVFI, and SE [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Would combining them be more helpful for diagnosing breast masses?
This study explored the application of the S-Detect program and the quantitative parameters of MFI and SR on the basis of US and compared their diagnostic value in differentiating benign from malignant breast lesions.
Our study was performed on 189 patients (92 malignant and 97 benign), unlike Seo et al. [28], who performed their study on 45 patients (20 malignant and 25 benign). And our study was a prospective study, similar to Lee et al. [30], unlike Lee et al. [27] and Zhi et al. [37], whose study was retrospective.
In this study, the sensitivity in the US + S-Detect and US + MFI groups increased, but the specificity and PPV decreased. Our analysis found that this may be due to the inconspicuous malignant characteristics of small tumors, which are often misdiagnosed as benign by conventional ultrasound. However, the addition of S-Detect reduced human error in judging small tumors, and after the addition of MFI, further judgment can be made based on the richness of internal blood flow. Both can avoid the omission of some small malignant lesions, thereby improving sensitivity. However, some large tumors with rapid growth, such as phyllodes tumors and larger fibroadenomas [38, 39], may also have rich internal blood flow signals and irregular morphology, leading to misdiagnosis by MFI and S-Detect, ultimately resulting in lower specificity and positive predictive value.
Wang et al. [13] studied that S-Detect can effectively improve inter-reader consistency and has good accuracy.
Cai et al. [20] studied that the vascular index can be used to appropriately downgrade benign lesions classified as BI-RADS 4, which can improve the diagnostic accuracy and PPV and reduce unnecessary biopsies.
At the same time, this study found that the specificity of the US + SR group was the highest, yet the sensitivity was lower, and the NPV was not significantly increased. The reasons may be that when the malignant mass was small in size, the interstitial reaction was not obvious, the fibrous component arrangement was loose, and the cancer tissue did not infiltrate into the surrounding area. Therefore, the hardness was lower, the elasticity score was lower, and false negatives were prone to occur. Therefore, the sensitivity was lower, and the negative predictive value was not high, even though the specificity was high. As shown in the study by Song et al. [40], it is believed that the hardness of large masses is higher than that in small masses.
Lee et al. [27] studied that the addition of SR in ultrasound improved the accuracy and specificity significantly, while increased the sensitivity, this is similar to our research findings.
This study showed that the highest diagnostic efficacy was achieved when the MFI cutoff value was 2.45. This cutoff value was lower than previously reported values of 2.95 [16] and 3.35 [24], possibly due to differences in the included subjects or the instruments. Chae et al. [16] and Lee et al. [24] used instruments from Aplio, Japan, while we used a diagnostic US instrument from Samsung, Korea, with each instrument coming with measurement software produced by a different company (SMI technology in their case, MVFI technology in ours).
Our study showed that the SR value of malignant masses was larger than that of benign masses, which is similar to the study by Suvannarerg et al. [41], Seo et al. [28], Lee et al. [27] and Moon, et al., and our cutoff value of SR was 3.15, while the cutoff of SR in Suvannarerg et al. was 2.78, Seo et al. was 2.63, Lee et al. was 3.57 and Moon et al. [42] was 5.16.
To the best of our knowledge, there are currently few studies that combine these four techniques (US, SR, MFI, and S-Detect) for the diagnosis of breast masses.
Leng et al. [43] combined routine ultrasonography, elastography, contrast-enhanced ultrasonography and color Doppler, and they showed that multimodal ultrasound is more effective in diagnosing breast lesions.
Lee et al. [27] combined all quantitative values of shear wave elastography and superb microvascular imaging with B-mode US, they showed the combination improved the diagnostic performance in differentiating between benign and malignant breast lesions.
Lee et al. [30] showed that addition of elastography and color Doppler US to B-mode US can increase the PPV of screening US in women with dense breasts while reducing the number of false-positive findings without missing cancers.
Our results indicated that the multimodal diagnostic approach that combined all four techniques had higher AUC, sensitivity, accuracy, PPV, and NPV values than the other seven groups without a loss of specificity (only second to the US + SR group).
All lesions with a BI-RADS score of 3 based on the multimodal diagnostic approach were judged benign using S-Detect, and their SR and MFI were below the respective cutoff values. The BI-RADS score for 61 cases was downgraded, including 55 benign masses being correctly downgraded from BI-RADS 4A to 3. These masses were a mixed variety of benign lesions, so their US images were atypical, resulting in misjudging them as BI-RADS 4A. The multimodal diagnosis could assess these lesions more objectively than US alone, resulting in downgrading these masses, avoiding unnecessary biopsies, reducing the patients’ pain and stress, and decreasing overtreatment. Two malignant masses were incorrectly downgraded. One was a mucinous carcinoma, possibly misjudged due to its unique pathological type. Many studies have reported that mucinous carcinoma usually has poor blood supply and because there is more internal mucus, its hardness is also lower [44], resulting in a lower MFI and SR. The other case was an invasive ductal carcinoma, possibly misjudged because of its small size, only 4 mm in diameter, leading to large MFI and SR measurement errors.
Among the 106 upgraded cases, there were 90 malignant lesions correctly upgraded. The ultrasound images of most of these upgraded masses showed insignificant malignant characteristics as the lesions were relatively small. The calculated MFI and SR indicated a high blood flow signal and hard texture, leading to an upgrade of their BI-RADS scores. Multimodal diagnosis can reduce the missed diagnosis rate of smaller breast masses. There were 16 benign masses were incorrectly upgraded, four from BI-RADS 4A to 4B and 12 from BI-RADS 4B to 4C. These lesions included 12 adenopathies with fibrous adenomas, two interstitial fibroses, and two inflammatory lesions. Active hyperplasia of the surrounding tissue may have led to their irregular growth and a strong blood flow signal.
During the subgroup study, we found that multimodal diagnosis had a higher diagnostic efficiency in the small tumor group, with an AUC of 0.955. As shown in the above analysis, small tumors with insignificant malignant characteristics are prone to missed diagnosis by conventional ultrasound, and combining multimodal diagnosis can more effectively evaluate their benign and malignant characteristics. However, regardless of whether it is a large mass group or a small mass group, the accuracy, sensitivity, AUC, and NPV of multimodal diagnosis are greater than the corresponding values of the US group. The specificity of the large mass group is low, and the reason is as shown above. Benign lesions with rapid growth are easily misdiagnosed as malignant.
Zhi et al. [37] only studied masses with a diameter less than 20 mm, and they only discussed the role of elastography in BI-RADS. They showed that the combination of elastography and BI-RADS could give BI-RADS some help in the differentiation of benign and malignant breast small lesions.
This study had several limitations. First, it was a single-center study with a small sample, possibly resulting in some bias. Second, we did not perform a subgroup analysis for the association between various pathological types and MFI or SR. Given the few cases available for each subgroup, a larger sample is needed for such analysis. Third, as the same instrument acquired all US images used in this study, the adaptability of our findings to other devices could not be confirmed.
Conclusions
The multimodal diagnostic method combining US, S-Detect, MFI, and SR can effectively improve the diagnostic efficiency of breast masses, reduce unnecessary biopsies of BI-RADS 4A masses. And for small masses with a diameter less than 20 mm, malignant signs can be detected earlier to avoid delaying treatment. The multimodal diagnostic is recommended to be used as a routine examination for breast in future for better evaluation the benign and malignancy of breast masses.
Availability of data and materials
The authors can provide all raw data that support the conclusions of this paper.
Abbreviations
- US:
-
Ultrasonography
- SR:
-
Strain ratio
- MFI:
-
Microvascular flow index
- BI-RADS:
-
Breast Imaging Reporting and Data System
- ROC:
-
Receiver operating characteristic curve
- AUC:
-
Area under the curve
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- MVFI:
-
Microvascular flow imaging
- SE:
-
Strain elastography
References
Sung H, Ferlay J, Siegel RL et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249
Anastasiadi Z, Lianos GD, Ignatiadou E et al (2017) Breast cancer in young women: an overview. Updates Surg 69(3):313–317
Radecka B, Litwiniuk M (2016) Breast cancer in young women. Ginekol Pol 87(9):659–663
Alonso Roca S, Delgado Laguna AB et al (2020) Screening in patients with increased risk of breast cancer (part 1): pros and cons of MRI screening. Radiologia (Engl Ed) 62(4):252–265
Chang JM, Cha JH, Park JS et al (2015) Automated breast ultrasound system (ABUS): reproducibility of mass localization, size measurement, and characterization on serial examinations. Acta Radiol 56(10):1163–1170
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30
Singh R, Deo SVS, Dhamija E et al (2020) To evaluate the accuracy of axillary staging using ultrasound and ultrasound-guided fine-needle aspiration cytology (USG-FNAC) in early breast cancer patients-a prospective study. Indian J Surg Oncol 11(4):726–734
Gonzalez-Huebra I, Elizalde A, Garcia-Baizan A et al (2019) Is it worth to perform preoperative MRI for breast cancer after mammography, tomosynthesis and ultrasound? Magn Reson Imaging 57:317–322
Kim K, Song MK, Kim EK et al (2017) Clinical application of S-Detect to breast masses on ultrasonography: a study evaluating the diagnostic performance and agreement with a dedicated breast radiologist. Ultrasonography 36(1):3–9
Bartolotta TV, Orlando AAM, Spatafora L et al (2020) S-Detect characterization of focal breast lesions according to the US BI RADS lexicon: a pictorial essay. J Ultrasound 23(2):207–215
Xia Q, Cheng Y, Hu J et al (2021) Differential diagnosis of breast cancer assisted by S-Detect artificial intelligence system. Math Biosci Eng 18(4):3680–3689
Bartolotta TV, Orlando AAM, Di Vittorio ML et al (2021) S-Detect characterization of focal solid breast lesions: a prospective analysis of inter-reader agreement for US BI-RADS descriptors. J Ultrasound 24(2):143–150
Wang X, Meng S (2022) Diagnostic accuracy of S-Detect to breast cancer on ultrasonography: a meta-analysis (PRISMA). Medicine 101(34):E30359
Xing B, Chen X, Wang Y et al (2022) Evaluating breast ultrasound S-detect image analysis for small focal breast lesions. Front Oncol 12:1030624
Chen P, Tong J, Lin T et al (2022) The added value of S-detect in the diagnostic accuracy of breast masses by senior and junior radiologist groups: a systematic review and meta-analysis. Gland Surg 11(12):1946–1960
Chae EY, Yoon GY, Cha JH et al (2021) Added value of the vascular index on superb microvascular imaging for the evaluation of breast masses comparison with grayscale ultrasound. J Ultrasound Med 40(4):715–723
Diao X, Zhan J, Chen L et al (2020) Role of superb microvascular imaging in differentiating between malignant and benign solid breast masses. Clin Breast Cancer 20(6):E786–E793
Zhu YC, Zu DM, Zhang Y et al (2019) A comparative study on superb microvascular imaging and conventional ultrasonography in differentiating BI-RADS 4 breast lesions. Oncol Lett 18(3):3202–3210
Zhu YC, Zhang Y, Deng SH et al (2018) Diagnostic forirformance of superb microvascular imaging (SMI) combined with shear-wave elastography in evaluating breast lesions. Med Sci Monit 24:5935–5942
Cai SM, Wang HY, Zhang XY et al (2020) The vascular index of superb microvascular imaging can improve the diagnostic accuracy for breast imaging reporting and data system category 4 breast lesions. Cancer Manag Res 12:1819–1826
Park AY, Seo BK (2018) Up-to-date Doppler techniques for breast tumor vascularity: superb microvascular imaging and contrast-enhanced ultrasound. Ultrasonography 37(2):98–106
Park AY, Seo BK, Woo OH et al (2018) The utility of ultrasound superb microvascular imaging for evaluation of breast tumour vascularity: comparison with colour and power Doppler imaging regarding diagnostic performance. Clin Radiol 73(3):304–311
Cai S, Wang H, Zhang X et al (2021) Superb microvascular imaging technology can improve the diagnostic efficiency of the BI-RADS system. Front Oncol 11:634752
Lee EJ, Chang YW, Oh E et al (2021) Reproducibility and diagnostic performance of the vascular index of superb microvascular imaging in real-time breast ultrasonography for evaluating breast masses. Ultrasonography 40(3):398–406
Ding Z, Liu W, He N et al (2021) Value of ultrasound elastography combined with contrast-enhanced ultrasound and micro-flow imaging in differential diagnosis of benign and malignant breast lesions. Am J Transl Res 13(12):13941–13949
Shiina T, Nightingale KR, Palmeri ML et al (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 1: basic principles and terminology. Ultrasound Med Biol 41(5):1126–1147
Lee EJ, Chang YW (2020) Combination of quantitative parameters of shear wave elastography and superb microvascular imaging to evaluate breast masses. Korean J Radiol 21(9):1045–1054
Seo M, Ahn HS, Park SH et al (2018) Comparison and combination of strain and shear wave elastography of breast masses for differentiation of benign and malignant lesions by quantitative assessment: preliminary study. J Ultrasound Med 37(1):99–109
Youk JH, Gweon HM, Son EJ (2017) Shear-wave elastography in breast ultrasonography: the state of the art. Ultrasonography 36(4):300–309
Lee SH, Chung J, Choi HY et al (2017) Evaluation of screening US-detected breast masses by combined use of elastography and color Doppler US with B-mode US in women with dense breasts: a multicenter prospective study. Radiology 285(2):660–669
Barr RG, Nakashima K, Amy D et al (2015) WFUMB guidelines and recommendations for clinical use of ultrasound elastography: part 2: breast. Ultrasound Med Biol 41(5):1148–1160
Wei Q, Yan YJ, Wu GG et al (2021) Added value of a new strain elastography technique in conventional ultrasound for the diagnosis of breast masses: a prospective multicenter study. Front Oncol 11:779612
Mercado CL (2014) BI-RADS update. Radiol Clin North Am 52(3):481–489
Foulkes WD, Reis-Filho JS, Narod SA (2010) Tumor size and survival in breast cancer-a reappraisal. Nat Rev Clin Oncol 7(6):348–353
Bertero L, Massa F, Metovic J et al (2018) Eighth edition of the UICC classification of malignant tumours: an overview of the changes in the pathological TNM classification criteria-What has changed and why? Virchows Arch 472(4):519–531
Sawaki M, Shien T, Iwata H (2019) TNM classification of malignant tumors (breast cancer study group). Jpn J Clin Oncol 49(3):228–231
Zhi H, Xiao XY, Ou B et al (2012) Could ultrasonic elastography help the diagnosis of small (≤ 2 cm) breast cancer with the usage of sonographic BI-RADS classification? Eur J Radiol 81(11):3216–3221
Yeo SH, Kim GR, Lee SH et al (2018) Comparison of ultrasound elastography and color Doppler ultrasonography for distinguishing small triple-negative breast cancer from fibroadenoma. J Ultrasound Med 37(9):2135–2146
Bernardi G, Cavallaro G, Indinnimeo M et al (2012) Usefulness of ultrasounds in the management of breast phyllodes tumors. G Chir 33(3):81–85
Song EJ, Sohn YM, Seo M (2018) Tumor stiffness measured by quantitative and qualitative shear wave elastography of breast cancer. Br J Radiol 91(1086):20170830
Suvannarerg V, Chitchumnong P, Apiwat W et al (2019) Diagnostic performance of qualitative and quantitative shear wave elastography in differentiating malignant from benign breast masses, and association with the histological prognostic factors. Quant Imaging Med Surg 9(3):386–398
Moon JH, Koh SH, Park SY et al (2019) Comparison of the SRmax, SRave, and color map of strain-elastography in differentiating malignant from benign breast lesions. Acta Radiol 60(1):28–34
Leng X, Huang G, Yao L et al (2015) Role of multi-mode ultrasound in the diagnosis of level 4 BI-RADS breast lesions and Logistic regression model. Int J Clin Exp Med 8(9):15889–15899
Zhang H, Qiu L, Peng Y (2018) The sonographic findings of micropapillary pattern in pure mucinous carcinoma of the breast. World J Surg Oncol 16(1):151–157
Acknowledgements
Not applicable.
Funding
This work was supported by University Research Project of Anhui Province, major project (No. 2023AH040373) and the Clinical Research Cultivation Program of the Second Affiliated Hospital of Anhui Medical University (No. 2021LCZD06).
Author information
Authors and Affiliations
Contributions
MP and NL contributed to conceptualization; MP, NL, and WLL provided methodology; NL performed writing—original draft preparation; MP, WLL, YYZ, YB, and XBW performed writing review and editing; and MP performed supervision. All authors have read and agreed to the published version of the manuscript. All authors have agreed both to be personally accountable for own contributions and ensured that questions related to the accuracy or integrity of any part of the work, even ones in which one was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In this study, all procedures abided by the protocol of The Second Affiliated Hospital of Anhui Medical University. The institutional review board approved this study. Ethical approval was approved by the Ethical Committee of the hospital, and obtained ethics approval in written (SL-YX2022-015). Written informed consent to participate was obtained from all the patients for publication of this study.
Consent for publication
All patients included in this research were above 18 years of age and gave written consent to publish the data included in the study.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, N., Liu, W., Zhan, Y. et al. Value of S-Detect combined with multimodal ultrasound in differentiating malignant from benign breast masses. Egypt J Radiol Nucl Med 55, 22 (2024). https://doi.org/10.1186/s43055-023-01183-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01183-x