Abstract
Background
The chronic nature of ovarian cancer and disease recurrence has a considerable impact on the assessment of follow-up strategies and treatment planning for both oncologists and radiologists. It is imperative to conduct adequate follow-up in ovarian cancer to detect and treat recurrence as early as possible. Presently, surveillance of patients with this malignancy involves the combination of serial CA-125 assay and diverse imaging procedures, yet normal CA-125 levels cannot entirely rule out disease relapse. PET/CT provides whole-body functional imaging that does not necessities contrast injection, and allows for precise diagnosis and restaging of patients with suspected ovarian cancer recurrence, thereby strongly impacting disease management decisions. Our study aims to evaluate the value of FDG-PET/CT as a follow-up imaging tool in detecting and localizing recurrence of ovarian cancer, in conjunction with CA-125 tumor markers.
Results
In our study, it was demonstrated that recurrent disease manifested in FDG-PET/CT in 24 cases, with 9 of those cases exhibiting CA-125 levels within the normal range. There were two instances of false negative results and one instance of false positive results in FDG-PET/CT. Additionally, three cases were found to be free of disease relapse in FDG-PET/CT and exhibited normal CA-125 levels throughout the follow-up period (true negative). The prevalence of disease recurrent sites was 12% for local recurrence, 60% for peritoneal metastasis, 64% for nodal deposits and 28% for distant metastatic disease. The accuracy of FDG-PET/CT was 88.8%, with a sensitivity of 91.3% and specificity of 75%. Furthermore, FDG-PET/CT showed a positive predictive value of 95.5% and a negative predictive value of 60.3%.
Conclusions
PET/CT imaging provides a comprehensive and functional view of the entire body, which can accurately diagnose and restage cases with ovarian cancer recurrence. This approach plays a critical role in identifying peritoneal carcinomatosis and is considered a more dependable method than CA-125 tumor markers for detecting and monitoring ovarian cancer recurrence. Additionally, PET/CT imaging has the potential to decrease the number of second-look laparotomies and can thus significantly impact the management plan.
Similar content being viewed by others
Background
Ovarian cancer is the most lethal of all gynecologic cancers, ranking fourth among all fatal diseases in women. It represents a difficult medical condition that poses significant diagnostic challenges in its early stages and is associated with a high incidence of 2-year relapse of early and advance stages following initial treatment [1].
It has been reported that the main reason for ovarian cancer's high fatality rate is the occurrence of peritoneal recurrence, which occurs frequently because of concealed metastasis [2].
The early detection and accurate localization of ovarian cancer recurrent disease is crucial as it aids in determining the feasibility of secondary surgery distinguishing cases with optimal curative cytoreduction from the palliative approach [3].
The CA-125 transmembrane glycoprotein is crucial for evaluating therapy response and ovarian cancer recurrence. However, its specificity is limited due to increased levels of colorectal cancer and inflammation. Elevated CA-125 levels indicate disease existence, but not the location or quantity of metastatic foci [4, 5].
Computed tomography (CT) and magnetic resonance imaging (MRI) are frequently utilized imaging techniques in the detection of ovarian cancer recurrence. However, a specific challenge arises in traditional diagnostic imaging when these techniques are unable to reveal findings, particularly for small implanted tumors on the visceral surface since the primary way of metastasis is through the peritoneal spread rather than the parenchymal route. However, there is still a suspicion of tumor recurrence due to elevated CA-125 levels. Several reports have then advocated that FDG-PET/CT can reveal lesions otherwise missed on CT alone in recurrent ovarian carcinoma, of particular assistance in identifying possible extra-abdominal metastases [6, 7].
FDG-PET/CT integrates the anatomical architecture of tissues with the metabolic activity of cells, effectively fusing anatomical and functional imaging into a single scan. It possesses a special significance in accurately diagnosing ovarian cancer recurrence. It allows for a precise evaluation of disease recurrence, which in turn enables efficient restaging of the illness optimizing the treatment strategies [8].
The potential risks and limitations of second-look surgery have raised questions about the value of positive FDG-PET as a noninvasive alternative, particularly for peritoneal metastasis evaluation. The baseline PET/CT also plays a crucial role in therapy monitoring that could early implicate neoadjuvant therapy protocols and prevent ineffective therapy in non-responders [9].
Our research was conducted to evaluate the effectiveness of PET/CT as a subsequent imaging modality for identifying and localizing instances of ovarian cancer recurrence, while simultaneously utilizing CA-125 tumor markers as a result aiding in better management aimed to improve quality of life.
Methods
Study population
This prospective cross-sectional study has been granted approval by the ethical and scientific committees at our facility. The study involved twenty-seven adult female patients their age above 18 years and had been histopathologically confirmed to have previously managed ovarian cancer. Patients were referred from the oncology department at our institution during the period from December 2020 to December 2022, due to suspicion of recurrence. The initial stage at the time of diagnosis and the result of cytoreductive surgery to the magnitude of residual implants have been known as the most influential prognostic factors for relapse in individuals afflicted with ovarian cancer. Each patient had formally given their written consent after receiving adequate information. All of the patients underwent a PET/CT scan using an 18F-FDG tracer and a 24-slice CT scanner (Discovery IQ 5-ring, GE health care).
Adult female patients who were pathologically proved to have ovarian cancer following initial treatment of either surgery or chemotherapy or a combination of both owing to clinical suspicion of recurrent ovarian malignancy or elevated CA-125 levels were included in our study. Any patient who has been referred for initial staging without having any primary treatment, patients who histopathologically proved to have another malignancy, individuals with uncontrolled blood glucose levels exceeding 150 mg/dl and patients with incomplete clinical data and laboratory result were excluded from our study.
Methodology
Patient preparations
All patients provided relevant clinical history and informed consent after receiving a detailed explanation of the imaging procedure. Patients were asked to bring all related previous investigations, histopathological results and CA-125 levels.
Patients were abstained from consuming food and drinks, except for water, for a minimum of 4–6 h before undergoing examination, they were instructed to stay hydrated and avoid intense physical activity 24 h before the scan, and those with comorbidities may take medications on the day of the scan, except for diabetes medications which must be taken at least 6 hours before the procedure.
Before administering (18F-FDG), the physical mass and blood sugar level were assessed, with a requirement of blood sugar levels below 140 mg/Dl. Diabetic patients with high blood glucose levels may use their insulin medications. If not controlled, they were rescheduled after normalizing their blood sugar with their endocrinologist. In the case of contrast injection, kidney function tests are performed with serum creatinine levels less than 1.5mg/dl and GFR above 45. A large IV cannula was used to deliver the prescribed dosage and probable iodized contrast substance.
FDG-PET/CT image acquisition:
The selected cases were administered 18F-FDG based on their physical mass, with a usual dose of 7.5 MBq/kg. Following the injection, the cases were instructed to remain in quiet resting rooms for approximately 40 minutes and were given oral hydration. PET/CT images were obtained using a GE Discovery IQ machine, with imaging beginning 40 minutes after injection of FDG-radioactive substance and including a low-dose non-contrast CT for attenuation correction and anatomical localization, followed by a CT examination and injection of contrast (if required), and concluding with a 25-minute PET scan.
Axial, sagittal and coronal PET images were created using a standard iterative algorithm with attenuation correction based on CT scan data, and a quantity of nonionic iodinated contrast (Omnipaque 350) was dispensed at a dose of 1 ml/kg in case of contrast administration.
Image analysis
-
1.
Axial and multi-planar images of CT, PET and fused PET/CT images were analyzed for ovarian carcinoma recurrence by an oncology consultant radiologist (more than 10-year experience) and a nuclear medicine consultant (5-year experience in PET/CT), The inter-observer agreement was measured and found to be 0.88, indicating almost perfect agreement, and the final diagnosis was reached by consensus with subsequent anonymization of results.
-
2.
The qualitative analysis involved visual examination of regions with increased FDG uptake in a focused manner. This was to identify local pelvic recurrence, peritoneal metastatic disease, metastatic lymphadenopathy or hematogenous metastatic disease. Lesions with increased FDG uptake were considered abnormal if substantially greater than background soft tissue activity.
-
3.
Semiquantitative analysis was done by recording the maximum SUV of all patients. ROIs were placed over abnormal focal uptake for analysis and compared with soft tissue background. SUV is defined as the radioactive concentration in tissue or lesion in megabecquerels per gram divided by the injected dose in megabecquerels and the patient's body weight in grams. The liver, lung and bone marrow typically have SUVs ranging from 0.5 to 2.5. Neoplastic growths often have a higher SUV than 2.5–3.0.
Data analysis
An initial PET/CT assessment and CA-125 levels were done in all patients histopathologically proved to have managed ovarian cancer with clinical suspicion of recurrence. A subsequent follow-up PET/CT and CA-125 tumor marker levels were performed to assess PET/CT observations after treatment. Additional follow-up PET/CT and CA-125 levels were done in 17 cases. Positive cases received the standard chemotherapy regimen (paclitaxel 175 mg/m2 intravenous and carboplatin AUC 5 to AUC 6 intravenous) both on day 1 and the cycle repeats every 21 days and re-assessment done every 3 months. Due to advanced disease stage of our included cases, second-look surgery was only done in seven positive cases, comparing observations to histopathological results. The evaluation of treatment response was additionally carried out in our investigation utilizing PET/CT and CA-125 tumor.
PET/CT imaging follow-up response
Guided by the PERCIST criteria published by the Journal of Nuclear Medicine 2009 [10], the target lesion that was measured for intensity during pre- and post-treatment scans did not necessarily remain the same. In the case of progressive disease, the presence of any new lesion or an increase of greater than 30% in SUVmax of the hottest lesions indicates progression. In contrast, a reduction of 30% or more in SUVmax of the sum of the hottest lesions at baseline and follow-up indicates a partial response. Complete response is defined as the normalization of FDG uptake of all deposits, with FDG uptake similar to that of the surrounding background. Stable disease is characterized by the absence of partial, complete or progressive disease.
CA-125 response
This was achieved as guided by NACB ovarian cancer panel recommendation [11]: CA-125 responders were identified as individuals who had a minimum of a 50% reduction in CA-125 levels in comparison with their initial measurement. Those who did not respond to treatment were classified as non-responders, as indicated by a CA-125 level increase of twofold or more from the baseline value.
The assessment was conducted to evaluate the correlation between 18F-FDG-PET/CT results and CA-125 tumor marker levels. The outcomes of this study were divided into the following categories: If the PET/CT findings in the subsequent PET/CT follow-up exhibited an increase or decrease in morphology/metabolic activity in alignment with the clinical indications of progression or regression, even in the absence of a histological diagnosis, the concordant response of CA-125 levels following the management of ovarian cancer or the findings from histopathology was deemed to be indicative of a true positive outcome. True negative outcomes were established in instances where no recurrence was detected through follow-up PET/CT or clinical monitoring. False negative results were reported when PET/CT results were normal, but relapse was distinguished by tissue biopsy, imaging or clinical follow-up. False positive outcomes were observed when lesions detected by PET/CT were proved to be benign by histopathology or other imaging techniques.
Statistical analysis
The statistical package for the Social Sciences (SPSS) version 28 (IBM Corp., Armonk, NY, USA) was utilized to code and enter data. Quantitative data were summarized using mean, standard deviation, median, minimum and maximum, while categorical data were summarized using frequency (count) and relative frequency (percentage). The nonparametric Mann–Whitney test (Chan, 2003a) was employed for comparisons between quantitative variables, and the Spearman correlation coefficient (Chan, 2003b) was used for correlations between quantitative variables. Standard diagnostic indices, including sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic efficacy, were calculated as described by Galen (1980). Statistical significance was considered at p-values less than 0.05.
Results
A sum of 27 individuals whose ages varied from 20 to 70 years possessing a mean age of 56 years were histopathologically proved as ovarian cancer postoperative. Out of the total, 96% of the patients underwent operative intervention and received chemotherapy, while only 4% underwent surgical treatment solely as a primary approach. Epithelial serous carcinoma was the main pathological subtype of our cases (Table 1).
They were directed to our institution and experienced an FDG-PET/CT scan for suspected recurrence on clinical grounds or for routine monitoring. For every focal abnormal uptake of radiotracer, qualitative and semiquantitative SUVmax was assessed. Guided by CT the localization of focal FDG uptake was ascertained in the following manner: 12% of our cases exhibited local/operative bed recurrence, 60% had peritoneal metastasis, 64% of cases demonstrated nodal deposits, and 28% of cases presented with distant metastatic disease with overlapping of findings. Estimated SUVmax showed significant values compared to the physiological uptake rates observed at various sites of recurrent disease (Table 2).
Cancer antigen 125 (CA-125) levels of included cases showed a range of 2–1700 U/ml with a mean value of 150.59 U/ml, and nine of our included cases showed CA-125 levels below normal range (normal level 35 U/ml) Table 3.
The initial PET/CT and CA-125 levels were correlated with follow-up, and PET/CT scans were found to be true positive in 77.7% of cases; the PET/CT findings were in accordance with the follow-up CA-125 tumor markers and subsequent follow-up PET/CT results, with seven cases being confirmed through histopathology. True negative results were detected in 11.7% of cases that remained disease-free during follow-up. A false positive result was observed in 3.7% of cases, where one patient showed a healed stress fracture with complete metabolic remission, initially regarded as a metastatic deposit. False negative results were found in 7.4% of cases, with PET/CT scan missed small peritoneal lesions in one case that were later detected through a second-look surgery. In another case, non-metabolically active pulmonary nodules were missed by PET/CT but detected through conventional CT and were regressed following chemotherapy treatment (considered metastatic).
The statistical analysis has indicated that FDG-PET/CT possesses sensitivity, specificity, NPV, PPV and accuracy of 91.3%, 75%, 60.3%, 95.4% and 88.89%, respectively (Table 4). Among the 24 positive cases that were diagnosed with recurrent disease on PET/CT, 9 of them exhibited CA-125 levels within the normal range of <35 U/mL. CA-125 tumor markers have a sensitivity of 62.5% and an accuracy of 64%.
Following the administration of chemotherapy to patients with positive PET/CT results, subsequent evaluation of treatment response was carried out through follow-up PET/CT and CA-125 tumor markers at 6 months for all cases and 1 year for 17 cases (table 5).
Correlation between follow-up semiquantitative PET parameters and CA-125 levels was done to detect treatment response. The results showed that a significant association was only observed between CA-125 values and semiquantitative PET parameters (P < 0.05) in the third follow-up. Figure 1 provides a visual representation of these findings.
The PERCIST criteria were utilized to classify patients as either responders (partial metabolic response and complete metabolic response) or non-responders (stable metabolic disease and progressive metabolic disease) with a split of 22.2% and 66.6%, respectively, as illustrated in Table 6 and Figure 2. While results for CA-125 tumor marker responders were only 12.0% and non-responders were 28%, the rest was nonsignificant value change.
FDG-PET/CT has proved to be effective in detecting and assessing treatment responses for patients with peritoneal metastasis, thereby guiding their management. This is supported by a significant P-value of 0.002, as depicted in Figures 3, 4, 5, 6.
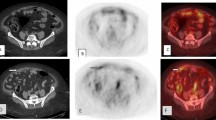
A 66-year-old female patient, having a history of ovarian mucinous carcinoma with positive peritoneal metastasis underwent TAH and BSO 2 years ago. CA-125 tumor marker level was within the normal range of about 16.9 U/ml. PET/CT was requested on a clinical base which revealed low-grade metabolically active abdomino-pelvic peritoneal and omental sheets and soft tissue lesions; associated with mild ascites. Non-contrast CT, fused PET/CT and PET images from up to down SUVmax of liver and of mediastinal blood pool = 2.5 and 2, respectively. a Large peritoneal lesion was seen over the left lumbar measuring 6.5 × 2.9 with SUVmax was 4.7 . b Other sizable deep pelvic hypodense peritoneal lesion SUVmax was 3.4
Follow-up PET/CT for the same case was done 6 months later after receiving chemotherapy showed a favorable therapeutic response, tumor marker was also declining compared to her baseline level CA-125 = 7.2 U/ml. Non-contrast CT, fused PET/CT and PET images from up to down with SUVmax of liver and of mediastinal blood pool = 2.5 and 2, respectively, showed morphological and metabolic regressive course of abdomino-pelvic peritoneal and omental sheets and soft tissue lesions with 38.3% SUVmax decline of the hottest lesion, a the left lumbar lesion measured 3.9 × 2.2 cm with estimated SUVmax = 2.9 b while SUVmax on deep pelvic lesion 2.9
A 62-year-old female patient, having a history of TAH and BSO 3 years ago for a pathologically proven case of ovarian endometrioid grade II carcinoma with positive peritoneal metastasis. The patient presented with an elevated CA-125 tumor marker 150.4 U/ml. A total body PET/CT was requested. Contrast CT, fused PET/CT and PET images from up to down SUVmax of liver and mediastinal blood pool = 3 and 2.5, respectively, revealed metabolically active abdominal abdomino-pelvic peritoneal and omental sheets and nodules associated with moderate ascites. a Largest omento-peritoneal lesion seen deep to the anterior abdominal wall measuring 2.7cm with SUVmax = 6.1 and b other one seen along peritoneal reflection of the right side of pelvis measuring 19mm with SUVmax = 6.7
Follow-up PET/CT was done 6 months after receiving chemotherapy and showed poor therapeutic response. Tumor markers showed further increased levels of CA-125 by 205 U/ml. Non-contrast CT, fused PET/CT and PET images with SUVmax of liver and mediastinal blood pool = 3 and 2.5 showed morphological and metabolic progressive course marked by an increase of 38.8% of the hottest lesion. a Peri-hepatic abdomino-pelvic peritoneal (b) and omental nodules and sheets; associated with moderate ascites; c the most sizable lesion seen underneath the anterior abdominal wall at the level of iliac crest measuring 3.8 cm with SUVmax = 9.3
FDG-PET/CT was also able to detect, follow up and evaluate the treatment response guiding the patient management in cases with nodal and distant metastasis with significant P values =0.012 and 0.025 for nodal and distant metastatic disease, respectively (Figures 7, 8, 9, 10, 11).
A 59-year-old female patient, presented with a history of ovarian cancer treated with surgery pathologically proven high-grade serous carcinoma followed by postoperative chemotherapy remained disease free for 3 years. Suspected local recurrence was detected by conventional imaging despite a normal CA-125 tumor marker level (13.5 U/ml). A second-look laparotomy and excision was done for multiple deposits at the anterior abdominal wall scar, vagina, lymph nodes, serosal deposits allover bowel and ileocecal; all confirmed by histopathology. A whole-body PET/CT was requested for re-evaluation, SUVmax of liver and mediastinal blood pool = 2.3 and 1.9, respectively. a Non-contrast CT, fused PET/CT and PET images showed residual multiple abdomino-pelvic serosal and peritoneal deposits. b PET images showed a metabolically active most sizable peritoneal lesion at the gastro splenic region measuring 5 cm achieving SUVmax of 11.8. c Metabolically active left inguinal LN measuring 1.7 cm achieving SUVmax of 2.9
The patient started chemotherapy and a further follow-up PET/CT assessment was done 6 months later and showed poor therapeutic response with increased metabolic activity by 37.2% of the hottest lesion. A low-dose CT, fused PET/CT and PET images with SUVmax of liver and mediastinal blood pool = 2.3 and 1.9, respectively. a Newly developed multiple metabolically active pelvic peritoneal metastatic nodules. b Increased number and metabolic activity of metastatic pelvic lymph nodes largest right external iliac 3.7 cm with SUVmax 16.2. c and d Patient also developed supra-diaphragmatic metabolically active mediastinal nodal lesions and low-grade metabolically active pulmonary nodules, note also peri-hepatic peritoneal nodule at last PET image
The patient changed the line of chemotherapy treatment and a further follow-up PET/CT was done which showed a good response to therapy. Coronal PET images showed a pre-treatment multiple supra- and infra-diaphragmatic metabolically active metastatic lesions and b a remarkable regressive course of supra- and infra-diaphragmatic metabolically active metastatic lesions
A 64-year-old female patient, presented with a history of ovarian cancer treated with surgery pathologically proven high-grade serous carcinoma followed by postoperative chemotherapy remained disease free for 1 year. Axillary lump and excisional biopsy were done one year later pathologically proven metastatic carcinoma of ovarian origin. Persistent mildly elevated CA-125 tumor marker level (47.9 U/ml) was detected, so PET/CT was requested for re-assessment. Non-contrast CT, fused PET/CT and PET images with SUVmax of liver and of mediastinal blood pool = 2.9 and 2, respectively, showed metabolically active metastatic supra- and infra-diaphragmatic nodal metastatic lesions together with moderate ascites and low-grade metabolically active peritoneal, mesenteric and omental nodules and sheets. a Metabolically active right axillary lymph node. b Metabolically active upper abdominal lymph nodes. c Metabolically active omental soft tissue nodules underneath the anterior abdominal wall SUVmax = 4.7. d Metabolically active pelvic external iliac lymph node
A 60-year-old female patient, presented with a history of ovarian cancer treated with surgery pathologically proven ovarian serous adenocarcinoma followed by postoperative chemotherapy remained disease free for 2 years. A recent elevated CA-125 tumor marker level (660U/ml) was detected, so PET/CT was requested for re-assessment. Contrast CT, fused PET/CT and PET images SUVmax of liver and of mediastinal blood pool = 2.7 and 2.2, respectively, showed multiple metabolically active metastatic lymph nodes in the following groups. a Bilateral supra- and infra-clavicular lymph node groups. b Left presternal, multiple superior mediastinal, carinal, infra-carinal and right hilar lymph nodes. The largest and most active one measures 3 × 3.5 cm with SUVmax = 11.8. c Multiple abdominal lymph nodes the most sizable one seen at porto-caval lymph node group measures 6.7 × 5.2cm with SUVmax = 9.6. d Moderate ascites with metabolically active herniated omental soft tissue sheets
Discussion
Around 70% to 80% of people who have been diagnosed with epithelial ovarian cancer will encounter a recurrence of the disease, even after undergoing the most effective surgical procedures and initial treatment [12]. Traditionally, the CA-125 has been examined at regular intervals, although there has been controversy about the clinical efficacy of utilizing CA-125 progression alone as a trigger for beginning second-line chemotherapy. It is prudent to postpone treatment in asymptomatic patients with CA-125 advancement and small volume disease or no radiological indications of recurrence [13, 14].
In this study, PET/CT was found to be a precise method for detecting suspected ovarian cancer recurrence, with positive scans observed in all cases with elevated CA-125 levels, and even in most patients with normal CA-125 levels.
In our study PET/CT performed efficiently in very low-level change of CA-125, Additionally, FDG-PET/CT proved to be a much more sensitive and accurate method in the early detection of ovarian cancer recurrence compared to serum CA-125 tumor marker levels. FDG-PET/CT had a sensitivity of 91% and an accuracy of 88.8%, whereas the serum CA-125 tumor marker had a sensitivity and accuracy of only 63% and 64%, respectively. These findings are supported by similar studies conducted by Dragosavac [15], Gu [16], Khiewvan [17] and colleagues, which all demonstrate the superior sensitivity of PET/CT over serum CA-125 (91.3% vs. 62%, 99% vs. 72% and 80% vs. 64%, respectively).
In contrast to previous research findings, they noted that PET/CT demonstrated a lower level of sensitivity, measuring 58.2%. Furthermore, their study did not identify any statistically significant disparities in the diagnostic precision of FDG-PET, CT and the combined FDG-PET and CT modalities [18].
Our study found that even though nine individuals had CA-125 levels within the normal range, disease recurrence was still present due to clinical suspicion, which was revealed by FDG-PET/CT. This led us to conclude that a normal CA-125 level should not be used as evidence to rule out tumor recurrence. Sun et al have mentioned that combining FDG-PET/CT imaging with serum CA-125 can complement and validate each other, significantly improving the diagnostic efficacy of ovarian cancer recurrence and metastasis [19]. This provides a crucial reference for the early identification of ovarian cancer recurrence and metastasis.
It has been stated that the transcoelomic route is the most common way of spreading and is responsible for peritoneal metastatic spread in ovarian cancer [20]. Conversely, our study revealed that nodal recurrence, predominantly in the abdomino-pelvic region, was the most frequently encountered site of disease relapse, with a prevalence of 64%. The observations of Dragosavac and colleagues are consistent with our findings, as they also reported that lymph nodes were the primary site of recurrent disease in their study [15].
The average SUVmax of the metastatic lesions was notably higher than the physiological uptake in various areas of recurrent disease at the initial assessment PET/CT scans. Through our research, we discovered that PET/CT can successfully detect metastatic lesions, even in small-sized lymph nodes guided by elevation of their metabolic activity, surpassing other imaging methods. This corresponds with the findings of Yuan Y et al, who highlighted that FDG-PET/CT is more precise than CT and MR imaging in detecting lymph node metastasis in ovarian cancer patients [21]. Manganaro and colleagues have also reported that PET/CT imaging has demonstrated superior accuracy than other imaging techniques in the identification of small lymph node deposits [22].
In our study, the peritoneal metastatic deposits were found to be the subsequent most common sites for recurrent illness with 60% prevalence. It was found that FDG-PET/CT can effectively evaluate peritoneal sheets and ascites, thereby distinguishing nodular peritoneal lesions from the gut. This, in turn, can prove beneficial in reducing the frequency of laparotomies, as pointed out by Rubini et al [23]. PET/CT has been shown to have an increased likelihood of detecting metastatic lesions in the mesentery and peritoneum as previously reported [17]. However, it is important to acknowledge that the presence of minute peritoneal seeding, measuring less than 5 mm, has been recognized as a significant contributing factor to the occurrence of false negative outcomes, as shown in our study. This highlights the importance of considering the limitations of imaging techniques, as peritoneal tumor spread may not always be detectable with these methods.
The advantage provided by PET/CT is the ability to conduct functional imaging of the entire body in a single session. This capability enables the detection of concealed lesion sites. In our study, PET/CT was able to detect unexpected lesion sites like supra-diaphragmatic lymph node metastases, with a P value of 0.001 and accurately identified distant metastases in various organs such as the liver, spleen, lungs, pleura and bone marrow.
In the context of post-chemotherapy management of recurrent cases, FDG-PET/CT was found to be effective in monitoring and assessing the response of different sites of disease relapse with a special emphasis on peritoneal metastasis, with a significant P value of 0.002. In alignment with the findings of previous research which stated that PET/CT is a better choice with the added benefit of being able to guide future treatments by using metabolic response data derived from SUV change of 20 and 55% from baseline after the first and third cycles, respectively, making FDG-PET an early predictor of treatment response [1]. We found that FDG-PET/CT is more sensitive and accurate than serum CA-125 in monitoring the treatment response in the follow-up of recurrent cases. CA-125 is only closely related to PET/CT findings at the third follow-up. This supports previous research that reported a gap between PET response and CA-125 response [24]. Highlighting the potential importance of PET as an early scanning tool for evaluating tumor response.
The limitation of our study is the quite small size of the sample. Additionally, some of our cases were not confirmed through a second-look laparotomy and pathological examination owing to the advanced stage of the disease.
Conclusions
In our study, all cases of patients with elevated CA-125 levels displayed positive PET/CT findings. Furthermore, a majority of patients with normal CA-125 levels exhibited a positive PET/CT finding. As a result, we emphasize that PET/CT provides a comprehensive functional imaging approach that does not necessitate contrast injection, enabling accurate diagnosis and restaging of ovarian cancer recurrence, potentially influencing patient management. Additionally, PET/CT serves as a more dependable tool for detecting and monitoring therapy response than CA-125 tumor markers.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 18F:
-
18 Fluorine
- AUC:
-
Area under the curve
- eGFR:
-
Estimated glomerular filtration rate
- FDG:
-
Fluorodeoxyglucose
- NACB:
-
National Academy of Clinical Biochemistry
- NCCN:
-
The National Comprehensive Cancer Network
- PET/ CT:
-
Positron emission tomography/computed tomography
- SD:
-
Standard deviation
- SUVmax:
-
Maximum standardized uptake value
References
Kemppainen J, Hynninen J, Virtanen J, Seppänen M (2019) PET/CT for evaluation of ovarian cancer. Seminars in nuclear medicine. WB Saunders, pp 484–492
Nakamura K, Yoshikawa N, Mizuno Y, Ito M, Tanaka H, Mizuno M, Kajiyama H (2021) Preclinical verification of the efficacy and safety of aqueous plasma for ovarian cancer therapy. Cancers 13(5):1141
Nougaret S, Addley HC, Colombo PE, Fujii S, Al Sharif SS, Tirumani SH, Reinhold C (2012) Ovarian carcinomatosis: how the radiologist can help plan the surgical approach. Radiographics 32(6):1775–1800
Parkash J, Bansro V, Chhabra GS, Mujahid Z (2023) Rising CA-125 (cancer antigen 125) levels: Cancer recurrence or a vaccine reaction? Cureus. https://doi.org/10.7759/cureus.34534
Rubello D, Marzola MC, Colletti PM (2018) The prognostic value of 18F-FDG PET/CT in monitoring chemotherapy in ovarian cancer both at initial diagnosis and at recurrent disease. Clin Nucl Med 43(10):735–738
Gouhar GK, Siam S, Sadek SM, Ahmed RA (2013) Prospective assessment of 18F-FDG PET/CT in detection of recurrent ovarian cancer. Egypt J Radiol Nucl Med 44(4):913–922
Low RN, Barone RM (2018) Imaging for peritoneal metastases. Surg Oncol Clin 27(3):425–442
Suppiah S, Chang W, Hassan H, Kaewput C, Asri A, Saad F, Vinjamuri S (2017) Systematic review on the accuracy of positron emission tomography/computed tomography and positron emission tomography/magnetic resonance imaging in the management of ovarian cancer: is functional information really needed? World J Nucl Med 16(03):176–185
Dhingra VK, Kand P, Basu S (2012) Impact of FDG-PET and-PET/CT imaging in the clinical decision-making of ovarian carcinoma: an evidence-based approach. Women’s Health 8(2):191–203
Wahl RL, Jacene H, Kasamon Y, Lodge MA (2009) From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 50(Suppl 1):122S-150S
Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, Diamandis EP (2008) National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers
Pignata S, Cecere SC, Du Bois A, Harter P, Heitz F (2017) Treatment of recurrent ovarian cancer. Ann Oncol 28:viii51–viii56
Berek JS, Kehoe ST, Kumar L, Friedlander M (2018) Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynecol Obst 143:59–78
Dragosavac S, Derchain S, Caserta NM (2013) GDES. Staging recurrent ovarian cancer with (18)FDG PET/CT. Oncol Lett 5:593–597. https://doi.org/10.3892/ol.2012.1075
Dragosavac S, Derchain S, Caserta NM, De Souza G (2013) Staging recurrent ovarian cancer with 18FDG PET/CT. Oncol Lett 5(2):593–597
Gu P, Pan LL, Wu SQ, Sun L, Huang G (2009) CA 125, PET alone, PET–CT, CT and MRI in diagnosing recurrent ovarian carcinoma: a systematic review and meta-analysis. Eur J Radiol 71(1):164–174
Khiewvan B, Torigian DA, Emamzadehfard S, Paydary K, Salavati A, Houshmand S, Alavi A (2017) An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur J Nucl Med Mol Imaging 44:1079–1091
Cho SM, Ha HK, Byun JY, Lee JM, Kim CJ, Nam-Koong SE, Lee JM (2002) Usefulness of FDG PET for assessment of early recurrent epithelial ovarian cancer. Am J Roentgenol 179(2):391–395
Sun J, Cui XW, Li YS, Wang SY, Yin Q, Wang XN, Gu L (2020) The value of 18 F-FDG PET/CT imaging combined with detection of CA125 and HE4 in the diagnosis of recurrence and metastasis of ovarian cancer. Eur Rev Med Pharmacol Sci 24(13):7276–7283
Rizvi I, Gurkan UA, Tasoglu S, Alagic N, Celli JP, Mensah LB, Hasan T (2013) Flow induces epithelial-mesenchymal transition, cellular heterogeneity and biomarker modulation in 3D ovarian cancer nodules. Proc Natl Acad Sci 110(22):E1974–E1983
Yuan Y, Gu ZX, Tao XF, Liu SY (2012) Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: a meta-analysis. Eur J Radiol 81(5):1002–1006
Manganaro L, Gigli S, Antonelli A, Saldari M, Tomao F, Marchetti C, Laghi A (2019) Imaging strategy in recurrent ovarian cancer: a practical review. Abdom Radiol 44:1091–1102
Rubini G, Altini C, Notaristefano A, Merenda N, Rubini D, Ianora AS, Asabella AN (2014) Role of 18F-FDG PET/CT in diagnosing peritoneal carcinomatosis in the restaging of patient with ovarian cancer as compared to contrast enhanced CT and tumor marker Ca-125. Revista Española de Medicina Nuclear e Imagen Molecular (English Edition) 33(1):22–27
Aide N, Fauchille P, Coquan E, Ferron G, Combe P, Meunier J, Joly F (2021) Predicting tumor response and outcome of second-look surgery with 18 F-FDG PET/CT: insights from the GINECO CHIVA phase II trial of neoadjuvant chemotherapy plus nintedanib in stage IIIc-IV FIGO ovarian cancer. Eur J Nucl Med Mol Imaging 48:1998–2008
Acknowledgements
We thank Dr. Mohamed Samy (associate professor of radiology at national cancer institute) for interpretation of FDG-PET/CT images.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
RZE and RHA reviewed the images. GAE and KSA analyzed and interpreted the patient data. GAE wrote the manuscript and RHA reviewed it. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent for participation
Approval of the ethical committee of the ‘Radiology department, Faculty of Medicine, Ein-Shams University’ was granted before conducting this prospective study; Reference number: not applicable. Local institutional review board approval was granted before conducting this prospective study, and written informed consent was obtained from all patients.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. If the patients were less than 16 years old, deceased or unconscious when consent for publication was requested, written informed consent for the publication of these data was given by their parents or legal guardians.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsayed, G.A., Abdullah, R.H., Elia, R.Z. et al. Role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the detection of recurrence and peritoneal metastasis from ovarian cancer in correlation with cancer antigen-125 tumor marker levels. Egypt J Radiol Nucl Med 55, 9 (2024). https://doi.org/10.1186/s43055-023-01153-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01153-3