Abstract
Background
This research aimed at assessing the white matter microstructural (WM) brain changes in tinnitus patients with bilateral normal peripheral hearing using diffuse tensor imaging to test whether, tinnitus alone without hearing loss can cause WM brain changes or not.
Patients and materials
Twenty-eight patients were enrolled in this research, 14 patients with bilateral tinnitus without hearing loss (audiometrically proven) and 14 normal hearing controls. All subjects underwent a full basic audiological evaluation, tinnitus matching, and were asked to fill the Tinnitus Handicap Inventory (THI) questionnaire. They underwent diffusion tensor brain imaging, mean diffusivity (MD) and fractional anisotropy (FA) values were measured at special parts of central auditory pathway, and parts of limbic system. A comparison between study and control groups was held as regards MD and FA at different brain sites using an independent sample Student t-test or Mann–Whitney U test. Furthermore, the relationship between THI scores and the MD/FA measures was examined by correlation tests.
Results
As regards FA values, some regions as [lateral lemniscus (LL), inferior colliculus (IC), frontal arcuate fasciculi (AF) and parahippocampus] showed statistically significant decreased FA values in the tinnitus group compared to Normal Hearing (NH) group (p < 0.05). As regards MD values, tinnitus patients showed significantly higher MD values at (auditory cortex, amygdala, and AF) compared to control group (p < 0.05). As regards correlations, THI scores showed statistically significant positive correlation with MD values measured at Rt Amygdala (r = 0.55, p = 0.04).
Conclusions
The central auditory pathway affection is proved in tinnitus patients with normal hearing (as least as evidenced by conventional audiological examinations) and the auditory-limbic association is proved so far. The involvement of IC confirms the subcortical auditory centres involvement in the generation of the tinnitus. Auditory associations are also significantly impacted by the effect of tinnitus.
Similar content being viewed by others
Background
Tinnitus is an auditory problem characterized by a hissing, tonal, or buzzing sound in the absence of any physical sound source [1].
The usual clinical assessment of tinnitus involves tinnitus matching, measurement of tinnitus distress, assessment of mood (emotional component) as well as assessment of the underlying hearing level, which is a common comorbidity with tinnitus [1, 2].
A few studies using electroencephalogram (EEG), functional magnetic resonance imaging (fMRI), and magnetic encephalogram (MEG) have unanimously agreed on the functional changes in the brain caused by tinnitus and their relation to the types of tinnitus and the degree of hearing loss [2]. Diffusion tensor imaging (DTI) is a recently developed MR acquisition modality that enables the measurement of the structural organization of tissues [3, 4]. As a powerful and non-invasive technique for in vivo exploration of human tissues, DTI is widely used in various medical fields, especially in brain imaging [5, 6]. DTI is based on the diffusion properties of water molecules in the white matter of the brain. The diffusion is limited by the fibrous nature of white matter: the well-organized axon structure, axon membranes, neurofilaments and overall myelin coating surrounding the neurons induce the displacement of water molecules to occur preferentially along the axon fibers rather than perpendicularly to them [7]. This anisotropic diffusion of water molecules can be measured by an MR scanner, allowing us to infer information on white matter connectivity.
In our research, we studied the characteristics of white matter fiber tracts defining the (classical) auditory pathway, especially the pathways from the Lateral Lemniscus (LL) and inferior colliculi (IC) to the medial geniculate body (MGB) up to the auditory cortex. We also investigated the connections between the auditory system and the limbic system (amygdala, hippocampus, and parahippocampus). Furthermore, we measured the intracerebral connection as arcuate fasciculus (AF) and intercerebral connections as corpus callosum (CC). As in most of previous research [8,9,10], the confounding effect of underlying hearing loss contaminated the results, hereby, we focus on tinnitus patients without hearing loss to eradicate the effect of hearing loss.
Methods
Subjects
A total of 28 subjects were recruited for our study. Fourteen patients with bilateral untreated persistent idiopathic tinnitus with normal hearing were included as a study group. Fourteen right-handed healthy volunteers were recruited as healthy controls with normal hearing (NH). All the patients met the inclusion criteria: persistent idiopathic tinnitus (≥ 3 months persistently) without any history of associated brain diseases confirmed by conventional MRI, no pre-existing mental illness or cognitive disorder affecting the structural outcome, and no contradictions to MRI. Based on the audiogram results Fig. 1, all the subjects were without hearing loss, which was defined as more than 25 dB hearing loss at frequencies ranging from 0.25 to 8 kHz (0.25, 0.5, 1, 2, 4, and 8 kHz) in a pure tone audiometry (PTA) examination. Patients who met the following criteria weren't included in the study: having [pulsatile tinnitus, hyperacusis, any kind of hearing loss (conductive, sensorineural, or mixed hearing loss) or any other neurological conditions]. All the patients were asked to fill in the tinnitus handicap inventory (THI) to assess the severity of disease at the time of MRI acquisition and tinnitus matching for pitch and loudness was done for all tinnitus patients (Table 1).
Data acquisition
The diffusion images as well as usual brain MRI studies were acquired on a 1.5-T General Electric scanner (GE Medical Systems, Milwaukee).
For DTI performed in the axial plane with the following parameters: field of view = 240 × 240 mm; matrix size = 128 × 128; no. of slices = 50 each 2.2 mm thick without gaps; imaging resolution = 1.85 × 1.85x2 mm3. DTI diffusion was measured along 60 non-collinear directions. For each slice and each gradient direction, two images with no diffusion weighting (b = 0 s/mm2) and diffusion weighting (b = 1000 s/mm2) were acquired. The in-plane resolution was 1 mm. The slice thickness was 4 mm. All our patients were examined in the supine position using the head coil.
For clinical evaluations and for exclusion of any co-incidental brain findings, all patients also had a MRI scan of whole brain that included an axial T1-weighted sequence, an axial and coronal T2-weighted image, an axial and sagittal FLAIR images. The imaging parameters for the spin echo (SE) axial spin echo T2-weighted images was(repetition time [TR] 2000 ms, echo time [TE] 20/100 ms; matrix size 256 × 256; field of view [FOV] 240 mm; slice thickness 5 mm), and T2-FLAIR(TR 8000 ms, TE 50/158 ms; matrix size 256 × 256; FOV 240 mm; slice thickness 5 mm, inversion time, 2200 ms). Axial T1-weighted MR images (TR = 600 ms, TE = 10 ms, axial slices 5 mm).
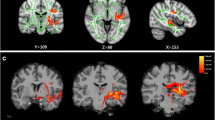
All tensor imaging processing were carried out on independent workstation (DSI studio, version 0.59). The circular (ROI) region of interest was implemented at each anatomical location on both sides and the mean FA and MD values within the ROI were measured (Figs. 2, 3, 4).
3D FIESTA images were used for anatomical reference and ROIs were drawn manually on the axial images for the lateral lemniscus, inferior colliculus (Fig. 2), the medial geniculate body (Fig. 3), amygdala, hippocampus, Para hippocampal region, the auditory cortex, mid splenium (Fig. 4), and mid genu (Fig. 4) of the corpus callosum.
Statistical analysis of the collected data
Results were collected, tabulated, and statistically analysed by Prism 8 (GraphPad software) version 8.0.2 (263).
Two types of statistical analysis were done:
-
a) Descriptive statistics were expressed in Number (No), percentage (%) mean (x̅), standard deviation (SD), median and interquartile range
-
b) Analytic statistics e.g.
-
Unpaired t-test was employed to compare different readings of normally distributed data while Mann Whitney test was used for data that did not follow normal distribution.
-
Fisher's exact test was utilized to compare the sex distribution between distinct groups.
-
p value < 0.05 was considered statistically significant.
-
The normality of distribution parameters was evaluated by Kolmogorov–Smirnov test.
-
Correlation between MD and FA measured values and THI score was applied. Pearson correlation test was used for normally distributed data, spearman test was used when normal distribution not proved.
-
Graphical art was made with Prism 8 (GraphPad software).
-
Results
Twenty-eight subjects were enrolled at this study, 14 tinnitus patients with normal hearing and 14 normal hearing controls. The group of healthy volunteers consisted of 9 male and 5 females, their age varied from 18 to 55 years, with a mean ± SD (37 ± 9). The tinnitus patients were (4 men and 10 women) with ages varying from 18 to 53 years, with a mean ± SD (38 ± 13). All the subjects were right-handed. All our patients suffered from bilateral tinnitus. Both groups were matched for age and gender. Tinnitus description, laterality, and tinnitus matched frequency are illustrated in Table 1. The frequency and loudness level of tinnitus were determined by a matching procedure. Figure 1 shows the hearing levels of the tinnitus patients and control NH at different frequencies.
White matter changes in Tinnitus Patients compared to healthy control group
As regard FA
Statistically significant decreased values in the tinnitus group compared to NH group were detected at the following regions: Left LL and right IC (p values = 0.0381 and 0.0213 respectively), right AF and left AF with (p value = 0.0016 and p value = 0.0057 respectively). There was also statistically significant difference measured at left para hippocampus with reduced FA level in tinnitus patients compared to control with good level of significance (p value = 0.0185) (Table 2, Fig. 5).
Comparison between control and tinnitus as regards FA and MD values measured at different anatomical brain regions. A student t test for normally distributed data (data are expressed as a mean ± SD). B Mann Whitney U test for data did not follow normal distribution (data are expressed as a Median & IQ range). (Statistical significance if p < 0.05). AF: Arcuate Fasciculus, AMG: amygdala, C: control, IC: inferior colliculus, FA: fractional anisotropy, LL: lateral lemniscus, Lt left, MD: mean diffusity, Para Hippo: Para hippocampus, Rt: right, SD: standard deviation, T: tinnitus
As regard MD
Tinnitus patients showed significantly higher MD values in (Rt Auditory cortex, right amygdala, and right AF) compared to control group with (p values levels = 0.018, 0.0325 and 0.0113 respectively) (Table 3, Fig. 5).
Correlation
THI scores showed a strong positive correlation with MD values measured at Rt Amygdala (r = 0.55, p = 0.04) (Fig. 6).
Discussion
“Tinnitus is the conscious awareness of a tonal or composite noise for which there is no identifiable corresponding external acoustic source" [11]. Although most incidences of tinnitus are temporary, chronic subjective tinnitus occurs in 4–15% of the general population, with an increasing prevalence in populations with an age more than 50 years to almost 20% [12]. Although tinnitus is usually linked to presence of hearing loss, 8% of patients with tinnitus had normal hearing thresholds (defined as better than or equal to 20 dB HL) up to 8000 Hz [13] and others reported that more than 60% of people with normal hearing (based on tonal audiometry) have tinnitus [14, 15]. Norena [16] proposed three distinct subtypes of tinnitus: cochlear tinnitus, peripheral dependent central tinnitus, and peripheral-independent central tinnitus [16]. Cochlear tinnitus refers to the tinnitus that originates from aberrant inner ear activity [16]. Peripheral dependent central tinnitus refers to a tinnitus associated with cochlear spontaneous activity, while peripheral independent central tinnitus refers to a tinnitus that is independent from cochlear spontaneous activity [16].
The auditory nerve (AN), which transmits action potentials in response to stimulation of the hair cells in the cochlea, is where the central auditory system begins. The auditory system's cochlear nucleus (CN), which receives information from the ipsilateral cochlea, is the first auditory system nucleus. The auditory message then travels from LL to IC before being projected to (MGB) of the thalamus. MGB is a relay station of several types of information of which the auditory pathway is only one. Fibers leaving the MGB project to the primary auditory cortex (AC). In addition to this classical auditory pathway, connections between the auditory system and the limbic system are also found [12]. The limbic system is involved in motivation, mood, and emotion [17], and consists of many subsystems, including the hippocampus, the amygloid complex [18]. Typical complaints attached to tinnitus such as anxiety, depression, and emotions such as fear indicate the association of the limbic system with tinnitus.
Previous research in this issue dealt with people complaining of combined tinnitus and hearing loss as Aldhafeeri et al. [8] & Lee et al. [9] and Ahmed et al. [19], so, the results of DTI studies were contaminated by the presence of hearing loss. So, the significant findings in those works were not accounted for tinnitus alone in the presence of the other confounding factor i.e., hearing loss.
In this research we studied the characteristics of WM fiber tracts defining the (classical) auditory pathway, especially the pathways from the Lateral Lemniscus (LL) and Inferior Colliculi (IC) to the Medial Geniculate Body (MGB) up to the auditory cortex. We also investigate the connections between the auditory system and the limbic system (amygdala, hippocampus, and parahippocampus). Furthermore, we measured the intracerebral connection as Arcuate Fasciculus (AF) and intercerebral connections as Corpus Callosum (CC). As most of previous research as that held by Aldhafeeri et al. [8] & Lee et al. [9] and Crippa et al. [10] the confounding effect of underlying hearing loss contaminated the results, hence, we focued on tinnitus patients without hearing loss (audiometric basis) to eradicate the effect of hearing loss.
Our results revealed significant decrease in the FA values in study group compared to normal healthy pears in the following regions (Left Lateral Lemniscus, Rt inferior colliculus, Rt Frontal arcuate Fasciculus, Left Frontal arcuate fasciculus, and left Para hippocampus). While there was significant increase in MD values in the study group compared to the control group in the following areas (Rt Broadman area, Rt amygdala and Rt frontal arcuate fasciculus). Moreover, we found statistically significant positive correlation between THI scores and the MD values at (Rt amygdala).
Our results agreed with Aldhafeeri et al.[8], Lee et al. [9], Ahmed et al. [19] & Chen et al. [20]who found decreased FA in AF in the tinnitus group. However, in the 1st three studies, the tinnitus was contaminated with HL While the latter conducted their studies on tinnitus with normal hearing. On concordance with our study, Gunbey et al. [21], found statistically significant decrease in FA at IC and Amygdala in tinnitus compared to control group Also, FA significantly decreased in LL in tinnitus with normal hearing compared to control group. According to Ryu et al. [22] research the tinnitus group with hearing loss also exhibited increased MD in WM under the auditory cortex and limbic system. Hereby, we also found significant increase in the MD in the Rt AC and Rt Amygdala.
On the other hand, other studies as Schmidt et al. [23] found no differences in diffusion indices in patients when compared with controls, and the severity and duration did not affect FA values as well. They considered this due to the differences in imaging parameters, which did not have the sensitivity to detect the changes. Most recently Khan et al. [24] who had their studies on tinnitus group with normal hearing and tinnitus with hearing loss and normal hearing control, found no significant alteration in FA values in tinnitus group with normal hearing compared to normal hearing controls.
According to the earlier tinnitus model, the tinnitus signal is generated at the periphery (linked to the periphery of auditory system) and is detected and processed by the subconscious centers of the auditory pathways. Finally, it is interpreted at the highest level of the auditory system (the secondary auditory cortices). The person's reaction and thoughts determine the tinnitus signal spread. The tinnitus signal may be constrained inside the auditory pathways if a person only detects tinnitus without experiencing any unpleasant effects from it. However, if this activity spreads to the limbic and autonomic nervous systems by specifically activating the sympathetic part of the autonomic system, it elicits several unfavourable responses, such as irritation, anxiety and panic, and triggers the survival reflexes, which results in a decreased ability to enjoy life's activities [1].
While all our cases were without hearing loss (audiometric basis), the central auditory system was significantly affected (LL and IC). This usually happened in cases of the peripheral theory of tinnitus i.e., cochlear pathology. Our explanation for this entity is that, (1) it might be peripheral independent central tinnitus as described earlier by Noreña [16] (2) Or, possibly, these parts might be affected because they part of descending auditory pathway (the noise reduction pathway). The top-down noise suppressing system may not be working properly, which can result in the perception of sound. An imbalance between the bottom-up ascending auditory pathways and the noise suppressing descending pathways can generate tinnitus [25,26,27]. Specifically, that, inferior colliculus is an obligatory synaptic station in the descending auditory pathways [28] and according to our results the FA was significantly affected at this part of the auditory pathway. However, as we didn’t perform any audiological tests to assess the concomitant affection of the descending pathway, so, it is hard to confirm this theory 3) The third explanation is the deafferentation theory. Deafferentations of the cochlea yielded hyperexcitability in the ascending auditory pathway thus being consistent with the idea that central changes and tinnitus may be initially triggered by peripheral auditory system deficits. Although our study was done on tinnitus patients with normal hearing, the presence of hidden hearing loss is still a factor. Before pure tone audiometry can detect the hearing loss, the OHCs can be damaged by up to 30% [29]. A state that is recently described as hidden hearing loss (HHL). Such hearing loss that is not detectable with conventional audiometric tests (pure tone audiometry) [30]. The damage to the synaptic ribbons involving pre and post dendritic synapses is another possible pathophysiology of HHL [31, 32]. Recently, Wan and Corfas [33] reported the third mechanism for underlying HHL. The authors discovered that temporary Schwann cell demyelination causes a persistent disruption of the cochlear hemi nodal, which produces in long-term auditory impairments that are indicative of HHL. Taken together, these findings suggest that deafferentation-induced reorganization model is still a suggested theory.
It is well known that the limbic and paralimbic systems serve as a crucial link between the auditory system and the emotional processing system [22]. The limbic system's emotional and cognitive abilities may be impacted by tinnitus, and the limbic system itself may play a role in the emergence and maintenance of tinnitus. The amygdala, one of the major components of the limbic system, has the capacity to modulate the activity and plasticity of the AC with direct neural input received from the MGB, as it also receives direct input from the AC [34]. FMRI research has provided evidence of altered interactions between multiple auditory and limbic related brain structures in tinnitus [35,36,37,38]. Contrary to those using BOLD fMRI, only a few studies as Aldhafeeri et al. [8], Lee et al., [9], Seydell-Greenwald et al. [39] and Gunbey et al. [21] have examined structural changes in the brain in tinnitus. The structural changes that take place as part of the pathophysiological mechanism of tinnitus have been usefully described by these structural studies. Functional network studies in tinnitus patients have revealed the greater interaction of auditory network and limbic systems [38, 40]. Volumetric imaging studies such those held by Chen et al. [41], Schlee et al. [42], Maudoux et al. [43], have demonstrated that tinnitus patients had structural abnormalities in both the central auditory and limbic systems. In the current study we found a significant decrease of FA at the left parahippocampus and a significant increase in MD at the Rt amygdala which confirms the limbic system affection. Furthermore, a positive correlation was found between the MD values at the Amygdala and THI scores which in turn confirms the theory. However, in this study, the involvement of the hippocampus, which is the second major area of the limbic system, was not documented. Crippa et al. [10] demonstrated that the tracts connecting the AC, the amygdala and the MGB to each other belong to the non-classical auditory pathways and showed the connection between hearing and the limbic system. According to Gunbey et al. [21], FA reduced in the amygdala, which may be a sign of early DTI findings in tinnitus sufferers. They also found a significant correlation between FA values at amygdala and THI scores. Also, Ryu et al. [22] found a significant alteration in WM in both auditory cortex and limbic system in tinnitus patients. The Para hippocampal area was hypothesized to play a vital role in transporting information to the areas of the hippocampus associated with remembering, and a dysfunction of this mechanism was presumed to be an explanation of complex auditory imaginary perceptions, such as auditory hallucinations [44]. The Para hippocampal area is involved in tinnitus and tinnitus-related stress [45]. Together with some previous research, all these findings support the hypotheses that the limbic system plays a significant role in the generation of tinnitus, and that tinnitus can stress and change neuronal activity and plasticity in the limbic system [20].
Finally, the prefrontal cortex is a critical area that is responsible for attention, speech in noise listening and the cocktail party effect (noise reduction) [21]. Arcuate fasciculi are an important connection between this critical area and the auditory areas. With affection of these important connections, a suggested cognitive affection will be postulated. In the current study we found a significant decrease of FA in both Rt and Left frontal arcuate fasciculi.
Knowing the exact underlaying pathology or affected sites of tinnitus determine the future therapeutic modality for tinnitus management.
Conclusions
From this study we conclude that tinnitus alone might be responsible for WM microstructure abnormalities. The auditory-limbic association of the tinnitus pathway in patients without HL (audiometric basis) has been proved so far. The deafferentation theory of tinnitus is still questionable, as hidden hearing loss is still postulated as a peripheral cause of the tinnitus. The involvement of IC confirms the subcortical auditory centers involvement in generation of tinnitus and/or the descending pathway affection. Secondary auditory cortices and auditory associations are both significantly impacted by the effect of tinnitus.
Limitations and recommendations
Several issues should be noticed in the future. First, the sample size was relatively small. Second, as we could not exclude the effect of hidden hearing loss as a possible cause, and we rely on conventional hearing tests, in the future research other tests to detect the hidden hearing loss should be applied. Lastly, we did not perform any audiological tests to assess cognitive dysfunction in this group of patients.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due [ethical/privacy issues] but are available from the corresponding author on reasonable request.
Abbreviations
- AC:
-
Auditory cortex
- AF:
-
Arcuate fasciculus
- AMG:
-
Amygdala
- AN:
-
Auditory nerve
- CAP:
-
Central auditory pathway
- CC:
-
Corpus callosum
- CN:
-
The cochlear nucleus
- DTI:
-
Diffusion tensor image
- EMG:
-
Elecrtoencephalogram
- FA:
-
Fraction anisotropy
- FMRI:
-
Functional MRI.
- IC:
-
Inferior colliculus.
- LL:
-
Lateral lemniscus.
- MD:
-
Mean diffusity
- MEG:
-
Magnetic encephalogram
- MGB:
-
Medial geniculate body
- NH:
-
Normal hearing.
- PTA:
-
Pure tone audiometry.
- ROI:
-
Region of interest
- THI:
-
Tinnitus handicap inventory
- VAS:
-
Visual analogue scale
- WM:
-
White matter
References
Jastreboff PJ (1990) Phantom auditory perception (tinnitus): mechanisms of generation and perception. Neurosci Res 8:221–254. https://doi.org/10.1016/0168-0102(90)90031-9
Lau P, Wollbrink A, Wunderlich R et al (2018) Targeting heterogeneous findings in neuronal oscillations in tinnitus: analyzing meg novices and mental health comorbidities. Front Psychol 9:235. https://doi.org/10.3389/fpsyg.2018.00235
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Resonan Ser B. 103(3):247–254. https://doi.org/10.1006/jmrb.1994.1037
Pierpaoli C, Jezzard P, Basser PJ et al (1996) Diffusion tensor MR imaging of the human brain. Radiology 201(3):637–648. https://doi.org/10.1148/radiology.201.3.8939209
Werring DJ, Clark CA, Barker GJ et al (1999) Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 52:1626–1626. https://doi.org/10.1212/wnl.52.8.1626
Werring DJ (2000) Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiat 69:269–272. https://doi.org/10.1136/jnnp.69.2.269
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15:435–455. https://doi.org/10.1002/nbm.782
Aldhafeeri FM, Mackenzie I, Kay T et al (2012) Neuroanatomical correlates of tinnitus revealed by cortical thickness analysis and diffusion tensor imaging. Neuroradiology 54:883–892. https://doi.org/10.1007/s00234-012-1044-6
Lee Y-J, Bae S-J, Lee S-H et al (2007) Evaluation of white matter structures in patients with tinnitus using diffusion tensor imaging. J Clin Neurosci 14:515–519. https://doi.org/10.1016/j.jocn.2006.10.002
Crippa A, Lanting CP, van Dijk P, Roerdink JBTM (2010) A diffusion tensor imaging study on the auditory system and tinnitus. Open Neuroimag J 4:16–25. https://doi.org/10.2174/1874440001004010016
De Ridder D, Schlee W, Vanneste S, et al (2021) Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). 1–25. https://doi.org/10.1016/bs.pbr.2020.12.002
Møller AR (2007) Tinnitus: presence and future. Progress Brain Res. https://doi.org/10.1016/s0079-6123(07)66001-4
Barnea G, Attias J, Gold S, Shahar A (1990) Tinnitus with Normal hearing sensitivity: extended high-frequency audiometry and auditory-nerve brain-stem-evoked responses. Int J Audiol 29:36–45. https://doi.org/10.3109/00206099009081644
Heller MF, Bergman M (1953) VII tinnitus aurium in normally hearing persons. Ann Otol Rhinol Laryngol 62:73–83. https://doi.org/10.1177/000348945306200107
Tucker DA, Phillips SL, Ruth RA et al (2005) The effect of silence on tinnitus perception. Otolaryngol Head Neck Surg 132:20–24. https://doi.org/10.1016/j.otohns.2005.08.016
Noreña AJ (2015) Revisiting the cochlear and central mechanisms of tinnitus and therapeutic approaches. Audiol Neurotol 20:53–59. https://doi.org/10.1159/000380749
Dalgleish T (2004) The emotional brain. Nat Rev Neurosci 5:583–589. https://doi.org/10.1038/nrn1432
Morgane PJ, Mokler DJ (2006) The limbic brain: continuing resolution. Neurosci Biobehav Rev 30:119–125. https://doi.org/10.1016/j.neubiorev.2005.04.020
Ahmed S, Mohan A, Yoo HB et al (2021) Structural correlates of the audiological and emotional components of chronic tinnitus. Progress Brain Res. https://doi.org/10.1016/bs.pbr.2021.01.030
Chen Q, Wang Z, Lv H et al (2020) Reorganization of brain white matter in persistent idiopathic tinnitus patients without hearing loss: evidence from baseline data. Front Neurosci 14:591. https://doi.org/10.3389/fnins.2020
Gunbey H, Gunbey E, Aslan K, Bulut T, Unal A (2017) Incesu LJCn Limbic-auditory interactions of tinnitus: an evaluation using diffusion tensor imaging. Clin Neuroradiol 27(2):221–230
Ryu C-W, Park MS, Byun JY et al (2016) White matter integrity associated with clinical symptoms in tinnitus patients: a tract-based spatial statistics study. Eur Radiol 26:2223–2232
Schmidt SA, Zimmerman B, Bido Medina RO et al (2018) Changes in gray and white matter in subgroups within the tinnitus population. Brain Res 1679:64–74. https://doi.org/10.1016/j.brainres.2017.11.012
Khan RA, Sutton BP, Tai Y et al (2021) A large-scale diffusion imaging study of tinnitus and hearing loss. Sci Rep. https://doi.org/10.1038/s41598-021-02908-6
Hong SK, Park S, Ahn M-H, Min B-K (2016) Top-down and bottom-up neurodynamic evidence in patients with tinnitus. Hear Res 342:86–100. https://doi.org/10.1016/j.heares.2016.10.002
De Ridder D, Adhia D, Langguth B (2021) Tinnitus and brain stimulation. 249–293. https://doi.org/10.1007/7854_2021_219
Vanneste S, Alsalman O, De Ridder D (2019) Top-down and bottom-up regulated auditory phantom perception. J Neurosci 39:364–378. https://doi.org/10.1523/jneurosci.0966-18.2018
Shulman A, Strashun A (1999) Descending auditory system/cerebellum/tinnitus. Int Tinnitus J 5(2):92–106
Chen G-D, Fechter LD (2003) The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res 177:81–90. https://doi.org/10.1016/s0378-5955(02)00802-x
Haider HF, Bojić T, Ribeiro SF et al (2018) Pathophysiology of subjective tinnitus: triggers and maintenance. Front Neurosci. https://doi.org/10.3389/fnins.2018.00866
Rüttiger L, Singer W, Panford-Walsh R et al (2013) The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PLoS ONE 8:e57247. https://doi.org/10.1371/journal.pone.0057247
Kujawa SG, Liberman MC (2015) Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 330:191–199. https://doi.org/10.1016/j.heares.2015.02.009
Wan G, Corfas G (2017) Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nat Commun. https://doi.org/10.1038/ncomms14487
De Ridder D, Fransen H, Francois O et al (2006) Amygdalohippocampal involvement in tinnitus and auditory memory. Acta Oto-Laryngol 126:50–53. https://doi.org/10.1080/03655230600895580
Lanting CP, de Kleine E, van Dijk P (2009) Neural activity underlying tinnitus generation: Results from PET and fMRI. Hear Res 255:1–13. https://doi.org/10.1016/j.heares.2009.06.009
Burton H, Wineland A, Bhattacharya M et al (2012) Altered networks in bothersome tinnitus: a functional connectivity study. BMC Neurosci. https://doi.org/10.1186/1471-2202-13-3
Kim J, Kim Y, Lee S et al (2012) Alteration of functional connectivity in tinnitus brain revealed by resting-state fMRI? A pilot study. Int J Audiol 51:413–417. https://doi.org/10.3109/14992027.2011.652677
Maudoux A, Lefebvre P, Cabay J-E et al (2012) Auditory resting-state network connectivity in tinnitus: a functional MRI study. PLoS ONE 7:e36222. https://doi.org/10.1371/journal.pone.0036222
Seydell-Greenwald A, Raven EP, Leaver AM et al (2014) Diffusion imaging of auditory and auditory-limbic connectivity in tinnitus: preliminary evidence and methodological challenges. Neural Plast 2014:1–16. https://doi.org/10.1155/2014/145943
Landgrebe M, Langguth B, Rosengarth K et al (2009) Structural brain changes in tinnitus: grey matter decrease in auditory and non-auditory brain areas. Neuroimage 46:213–218. https://doi.org/10.1016/j.neuroimage.2009.01.069
Chen Y-C, Li X, Liu L et al (2015) Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife. https://doi.org/10.7554/elife.06576
Schlee W, Weisz N, Bertrand O et al (2008) Using auditory steady state responses to outline the functional connectivity in the tinnitus brain. PLoS ONE 3:e3720. https://doi.org/10.1371/journal.pone.0003720
Maudoux A, Lefebvre Ph, Cabay J-E et al (2012) Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res 1485:10–21. https://doi.org/10.1016/j.brainres.2012.05.006
Diederen KMJ, Neggers SFW, Daalman K et al (2010) Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. AJP 167:427–435. https://doi.org/10.1176/appi.ajp.2009.09040456
Vanneste S, Plazier M, der Loo E, van, et al (2010) The neural correlates of tinnitus-related distress. Neuroimage 52:470–480. https://doi.org/10.1016/j.neuroimage.2010.04.029
Melcher JR, Knudson IM, Levine RA (2013) Subcallosal brain structure: correlation with hearing threshold at supra-clinical frequencies (>8 kHz), but not with tinnitus. Hear Res 295:79–86
Acknowledgements
None.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
NN proposed the idea, collected and interpreted the data, referred the patients from the audio vestibular unit and contributed to writing the original draft. AE made the radiological test analysis and contributed to writing and reviewing the article. Both authors have read approved the final manuscript.
Corresponding author
Ethics declarations
Ethical consent and approval to participate
This is a prospective observational study comprising 28 subjects that was conducted at a Tanta university hospital, between April 2022 to September 2022. Tanta university research ethics committee review board has approved the study with approval report code 35755. This study conforms to the Declaration of Helsinki. Informed consents were obtained from all participants in this study.
Consent for publication
All participants included in the research gave written consent to publish the data included in the study. Authors accepted to publish the paper.
Competing interests
Authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eltabbakh, A., Nada, N. Quantitative analysis of white matter brain changes in tinnitus patients with normal hearing: a case-controlled study with diffusion tensor imaging. Egypt J Radiol Nucl Med 54, 101 (2023). https://doi.org/10.1186/s43055-023-01024-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01024-x