Abstract
Background
The aim of this retrospective cross-sectional study was to investigate the relationship between epicardial adipose tissue and coronary artery disease and its severity.
Methods
A total of 344 subjects with clinically suspected coronary artery disease underwent 256-slice multi-detector CT coronary angiography and were categorized as follows: no CAD, non-obstructive CAD (luminal narrowing < 50%) and obstructive CAD (luminal narrowing ≥ 50%). Calcium score was quantified using the Agatston method. Mean Epicardial adipose tissue (EAT) and mean pericoronary fat thickness (PCFT) were measured and utilized for data analysis.
Results
There was a significant association between EAT thickness and PCFT with clinical risk factors of CAD including HTN, DM and obesity (P-value: < 0.001) and dyslipidemia (P-value: 0.003, P-value: 0.008 for EAT and PCFT, respectively). EAT thickness and PCFT were significantly higher in obstructive CAD compared to non-obstructive CAD and no CAD categories (P-value: < 0.001). Also, there is positive strong correlation between EAT thickness, PCFT and Calcium score (P-value: < 0.001). Optimal cut-off point of PCFT for estimating obstructive CAD was > 12.87 mm (sensitivity: 77.06%, specificity: 66.06% and AUC:0.76; 95%CI:0.69–0.81) via receiver operating characteristic curve. On multivariate logistic analysis which included conventional risk factors of CAD, PCFT was an independent predictor of obstructive CAD (Odds ratio:1.55; P-value: < 0.001).
Conclusions
EAT thickness and PCFT were significantly increased in coronary artery disease. PCFT is a simple accessible marker for predicting obstructive CAD with acceptable diagnostic performance.
Similar content being viewed by others
Background
Coronary Artery Disease (CAD) is a frequent health problem and the leading cause of death worldwide [1]. Given CAD’s high impact on mortality and morbidity in populations, early diagnosis and better stratification of patients are completely relevant. There is compelling evidence elucidating visceral fat as an important risk factor for CAD [2]. Epicardial adipose tissue (EAT) which is a type of visceral fat has been demonstrated to be imperatively associated with CAD [3]. Previous studies have reported that EAT is a main source of inflammatory cytokines and bioactive molecules with ultimate direct vasocrine and paracrine effect on coronary arteries with ensuing atherosclerotic disease [4]. Some researchers demonstrated that pericoronary adipose tissue, i.e., a subtype of EAT which is directly surrounding coronary arteries, has a pivotal role in atherogenesis via producing deleterious inflammatory adiopokines [5]. Therefore, the local fat surrounding the coronary artery (as pericoronary fat) might be influencing coronary atherosclerosis more directly and predicted significant CAD more robustly. Notably, correlation of EAT and CAD has been well documented; however, it remains debatable whether EAT can be introduced as an independent marker to predict CAD development and its severity. In recent years, temporal and spatial resolution of Multi-detector CT scan (MDCT) has been markedly improved so that MDCT is widely used as an accurate and reproducible method for measurement of EAT [6].
In this study, we investigated the correlation between EAT thickness and pericoronary fat thickness (PCFT) with the presence and severity of CAD assessed by cardiac MDCT.
Methods
Patients
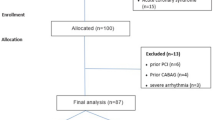
A total of 344 patients with suspected CAD were enrolled in this retrospective cross-sectional study. All subjects included in our study underwent MDCT between April 2019 and February 2022 at Chamran Hospital, Isfahan, Iran. This cross-sectional study was approved by the ethics committee of Isfahan University of Medical Sciences (IR.MUI.MED.REC.1399.625). Exclusion criteria were as follows: those who had history of percutaneous coronary intervention, patients with history of coronary artery bypass graft, renal insufficiency and known history of allergic reaction to iodine-based contrast agents. Information about CAD risk factors was gathered from patient’s medical records including: age, gender, body mass index (BMI) and smoking history and family history of CAD. Hypertension (HTN) was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg, or current use of medication for HTN. Diabetes was defined as Hb1Ac ≥ 6.5%, fasting blood glucose ≥ 126 mg/dl or patients who received glucose-lowering medications. Dyslipidemia was defined according to a widely used guideline [7].
Scanning method
All patients were imaged with a 256-slice MDCT scanner (Brilliance iCT, Philips Healthcare, Cleveland, OH, USA). For CTA, imaging parameters were set as follows: slice collimation 256 × 0.625 mm, tube voltage 120 kV and 200 mA tube current. At first, non-contrast enhanced scan was taken to measure calcium score according to the standard Agatston method. For coronary CT angiography, ECG-gated acquisition was done after administration of 60–80 ml Iodixanol (Visipaque 320 mg I/ml; GE healthcare, Stockholm, Sweden) directly into antecubital vein with flow rate of 5–6 cc/sec followed by 30–40 ml saline infusion. Imaging data set was transferred to an offline workstation (Philips Medical Systems) for imaging analysis.
Assessment of CAD and epicardial adipose tissue
Presence and severity of CAD were evaluated by an expert radiologist with 12 years’ experience in cardiac imaging using CADRADS guideline [8]. Patients were divided into three categories: no CAD (CADRDADS:0), non-obstructive CAD (CADRADS 1 and 2; luminal narrowing less than 50%) and Obstructive CAD (CADRADS 3,4 and 5; ≥ 50% luminal narrowing).
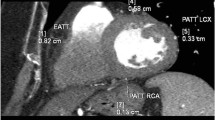
EAT thickness was measured at the basal level of the ventricles on short axis view in the most motionless phase of the cardiac cycle. At the 25%, 50–75% level of the RV and superior LV wall, measurements were made vertically to surface of the heart from visceral pericardium to outer margin of myocardium. Then, mean of these three measurements, namely ‘EAT thickness’ was utilized for analysis. For Pericoronary fat, as previously described [9] assessment was performed on axial view where the maximum fat thickness around LAD, LCX and RCA was measured in perpendicular fashion (in order not to overestimate thickness due to obliquity) from the outer margin of the myocardium to the visceral pericardium as well. Similarly, the mean of the three measurements was applied for data analysis as PCFT (Fig. 1).
Statistical analysis
All data were analyzed using SPSS 26.0 for windows. Relation between EAT thickness and PCFT with continuous variables and categorical variables was determined by the independent t-test and chi-squared test, respectively. One-way analysis of variance (ANOVA) was used in order to compare between three categories based on CAD. Spearman’s correlation coefficient (r) was used for assessment of correlation between EAT thickness, PCFT and Calcium Score.
For determining optimal cut-off value, Receiver Operating Characteristic (ROC) curve analysis was performed. The logistic regression model via multivariate analysis was utilized to determine predictors of CAD presence and its severity considering EAT thickness and PCFT, adjusting for conventional risk factors and Calcium Score.
Results
General features
A total of 344 patients (171 males, 173 female) with a mean age of 57.54 ± 12.32 years were enrolled in this study. Among them, 133 (38.7%) subjects had obesity (BMI ≥ 30). The number of patients suffering from HTN was 178 (51.7%). With respect to DM and dyslipidemia, 87 (25.3) cases had DM and 143 (41.6%) suffered from dyslipidemia. Among the subjects, 75 (21.8%) cases were current or previous smoker and 140 (40.7%) cases had positive family history regarding CAD.
The mean Calcium Score was 153.36 ± 64.52 (ranging from 0 to 736.8). From the total, 132 (38.4%) had no CAD, 103 (29.9%) had non-obstructive CAD and 109 (31.7%) had obstructive CAD. The mean value of EAT thickness was 7.04 ± 1.94 mm while PCFT had a mean value of 12.53 ± 2.17 mm.
EAT thickness, PCFT and clinical CAD risk factors
We found EAT thickness and PCFT were significantly increased in patients with HTN, DM and obesity. There is also a significant association between EAT thickness and PCFT with dyslipidemia. We did not show any significant increase in EAT thickness and PCFT with respect to gender, smoking and family history of CAD (Table 1).
EAT thickness, PCFT and CAD
As shown in Table 2, the mean EAT thickness was significantly increased in obstructive CAD (8.17 ± 1.69 mm) compared to non-obstructive CAD (7.19 ± 1.64 mm) and no CAD (5.98 ± 1.80 mm). Considering PCFT, there was significant difference in obstructive CAD (14.14 ± 1.64 mm) compared to non-obstructive (12.45 ± 1.80 mm) and no CAD (11.25 ± 1.94 mm).
EAT thickness, PCFT and calcium score
We investigated correlation of these three biomarkers obtained from Cardiac MDCT. Calcium Score was found to be significantly correlated with EAT thickness and PCFT. EAT thickness and PCFT showed significant correlation as well (Table 3).
Diagnostic performance of EAT thickness and PCFT
We used ROC curve in order to determine best cut-off points of EAT thickness and PCFT for predicting obstructive CAD. EAT thickness and PCFT cut-off points for predicting obstructive CAD were > 8.03 mm (sensitivity, 55.05%; specificity, 71.84%) and > 12.87 mm (sensitivity,77.06%; spceficity,66.02%), respectively. Area under the ROC curve (AUC) was 0.66(95%CI:0.59–0.73) for EAT thickness and 0.76 (95%CI:0.69–0.81) for PCFT (Fig. 2), indicating acceptable discriminatory power for PCFT.
Independent predictors of CAD
We evaluated two states by the multivariate logistic regression model: 1. Presence of CAD (no CAD vs all CAD patients) and 2. Obstructive CAD (non-obstructive CAD vs obstructive CAD). Multivariate analysis was performed by including all conventional risk factors and Calcium Score in addition to EAT thickness and PCFT. As shown in Table 4, EAT thickness and PCFT depicted an inconsistent value regarding CAD presence. For second assumption, PCFT was an independent predictor between non-obstructive CAD and obstructive CAD (Odds ratio:1.55; P-value: < 0.001).
Discussion
The current study investigated the possible relation of epicardial adipose tissue thickness, especially in the pericoronary location and its potential diagnostic performance for CAD assessed by cardiac MDCT. The main findings were as follows; (1) EAT thickness and PCFT were significantly higher in obstructive CAD compared to non-obstructive CAD and no CAD groups; (2) HTN, DM, obesity and dyslipidemia were significantly associated with higher EAT thickness and PCFT; (3) PCFT with cut-off value of > 12.87 had acceptable diagnostic performance for obstructive CAD(AUC:0.76); (4) PCFT was an independent predictor of obstructive CAD, hence, the simple quantification of PCFT can be introduced as a surrogate marker of obstructive CAD.
There is growing evidence that epicardial adipose tissue including pericoronary adipose tissue, is related to coronary artery atherosclerosis [10], however, the exact mechanisms are not fully understood. It has been proposed that epicardial adipose tissue can possibly affect CAD via producing cytokines and bioactive molecules with ensuing local inflammation and eventually atherogenesis [11]. In the current study, we found that EAT thickness and PCFT were significantly higher in the obstructive CAD group in comparison with non-obstructive CAD and no CAD groups. Some reports are in line with our results, Xie et al. [12] described that EAT thickness was higher in CAD and significantly increased during progression of CAD severity. In a study conducted by Gać et al. [13], it was indicated that positive correlation was noted between significant CAD, EAT thickness and PCFT in 80 suspected patients by coronary CT angiography. Other studies had similar results as our data regarding correlation of EAT thickness and PCFT and obstructive CAD [14, 15]. However, You et al. [16] reported that there was no correlation between PCFT and CAD severity. A possible explanation for this inconsistency might be a different study design and measurement methods. We found a significant association between EAT thickness and PCFT with HTN, obesity, DM and dyslipidemia but not with gender, smoking and family history. Previous studies evaluating this relationship have yielded conflicting results possibly due to the target study populations [17, 18]. Mohammadzadeh et al. [17] did not show meaningful correlation between DM and dyslipidemia with EAT thickness. Furthermore, they reported that age, male gender, HTN and obesity was correlated with higher rate of EAT thickness in the Iranian population. Any relation of EAT thickness and clinical risk factors of CAD was investigated by Oka et al. [18] who did not show higher values of EAT thickness in male gender consistent with our results. Another prior study showed that PCFT was positively correlated with HTN, high BMI and age [19].
Our analysis shows strong positive correlation between EAT thickness, PCFT and Calcium score. In the study designed by Aydin et al. [20], a total of 150 subjects who underwent cardiac MDCT, larger mean PCFT was significantly associated with Calcium score. Iwasaki et al. [21] tested relation of EAT and calcium score in 651 cases by coronary CTA, and demonstrated a meaningful relationship between them. Contrary to our results, some studies didn’t show any correlation between EAT and calcium score [12, 17].
We determined the best cut-off points of EAT thickness and PCFT for predicting obstructive CAD and their diagnostic performance. On AUC for estimating obstructive CAD, PCFT (AUC: 0.76) had larger area under the curve compared with EAT thickness (AUC:0.66). This finding showed PCFT has acceptable diagnostic value for predicting obstructive CAD. Further studies with larger sample sizes can improve the discriminatory power and better clarify optimal cut-off points of PCFT. In a multivariate regression model, when conventional risk factors of CAD and Calcium Score added to EAT thickness and PCFT in order to evaluate independent predictors of CAD, results showed PCFT as an independent predictor of obstructive CAD (Odds ratio:1.55; P-value: < 0.001). This finding provides additional support to the notion that local epicardial adipose tissue surrounding coronary arteries possibly has more potent impact on atherosclerosis than global cardiac adiposity. Maimaituxun et al. [22] evaluated PCFT in 197 subjects with obstructive CAD versust non-CAD groups, and demonstrated higher value of PCFT around the left anterior descending artery (LAD) in obstructive CAD. Furthermore, this report introduced LAD-PCFT as a strong independent predictor of obstructive CAD. In the EVASCAN study which enrolled 970 patients, EAT was introduced as low power predictor of CAD with unacceptable diagnostic performance which is consistent with our results [23]. Another investigation showed that EAT volume had inconsistent predictive value for obstructive CAD [24]. On the other hand, Wu et al. [25] depicted excessive left AV groove EAT as an independent important risk factor of obstructive CAD. According to our results, PCFT can be a reliable predictor of obstructive CAD with acceptable diagnostic performance, hence, further studies with larger sample sizes with multi-center and improved methodology could help radiologists and clinicians to better stratify CAD subjects.
It is important to note that there are several limitations in our study. First, this study was performed in a single center thereby selection bias was inevitable and the sample size was relatively small. Second, it was not possible to include medications and life style interventional details of patients in our analysis. Third, our study was a retrospective cohort, so prognostic value of EAT thickness and PCFT needs to be investigated in future studies with a prospective design.
Conclusions
This study demonstrated that EAT thickness and PCFT significantly increased in obstructive CAD compared with non-obstructive CAD and no CAD subjects. Positive strong correlation was shown between EAT thickness and PCFT and Calcium score. Importantly, PCFT had acceptable diagnostic performance in order to estimate obstructive CAD and our results indicated that PCFT was an independent predictor of obstructive CAD. Therefore, measurement of PCFT may be viewed as an indicator of obstructive CAD in patients undergoing calcium scoring and consequently could add diagnostic value of calcium score.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CAD:
-
Coronary artery disease
- EAT:
-
Epicardial adipose tissue
- PCFT:
-
Pericoronary fat thickness
References
Malakar AK, Choudhury D, Halder B, Paul P, Uddin A, Chakraborty S (2019) A review on coronary artery disease, its risk factors, and therapeutics. J Cell Physiol 234(10):16812–16823
Monti CB, Codari M, De Cecco CN, Secchi F, Sardanelli F, Stillman AE (2020) Novel imaging biomarkers: epicardial adipose tissue evaluation. Br J Radiol 93(1113):20190770
Liu Z, Wang S, Wang Y, Zhou N, Shu J, Stamm C et al (2019) Association of epicardial adipose tissue attenuation with coronary atherosclerosis in patients with a high risk of coronary artery disease. Atherosclerosis 284:230–236
Mancio J, Barros AS, Conceicao G, Pessoa-Amorim G, Santa C, Bartosch C et al (2020) Epicardial adipose tissue volume and annexin A2/fetuin-A signalling are linked to coronary calcification in advanced coronary artery disease: computed tomography and proteomic biomarkers from the EPICHEART study. Atherosclerosis 1(292):75–83
Baker AR, da Silva NF, Quinn DW, Harte AL, Pagano D, Bonser RS et al (2006) Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol 5(1):1
De Vos AM, Prokop M, Roos CJ, Meijs MFL, Van Der Schouw YT, Rutten A et al (2008) Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart 29(6):777–783
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS et al (2019) 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 139(25):E1082–E1143
Cury RC, Abbara S, Achenbach S, Agatston A, Berman DS, Budoff MJ et al (2016) CAD-RADSTM: coronary artery disease—reporting and data system: an expert consensus document of the society of cardiovascular computed tomography (SCCT), the American college of radiology (ACR) and the North American society for cardiovascular imaging (NASCI). Endorsed by the American college of cardiology. J Am Coll Radiol 13(12):1458-1466.e9
Gorter PM, de Vos AM, van der Graaf Y, Stella PR, Doevendans PA, Meijs MFL et al (2008) Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am J Cardiol 102(4):380–385
Wang Q, Chi J, Wang C, Yang Y, Tian R, Chen X (2022) Epicardial adipose tissue in patients with coronary artery disease: a meta-analysis. J Cardiovasc Dev Dis 9(8):253
Shimabukuro M, Hirata Y, Tabata M, Dagvasumberel M, Sato H, Kurobe H et al (2013) Epicardial adipose tissue volume and adipocytokine imbalance are strongly linked to human coronary atherosclerosis. Arterioscler Thromb Vasc Biol 33(5):1077–1084
Xie Z, Zhu J, Li W, Liu L, Zhuo K, Yang R et al (2021) Relationship of epicardial fat volume with coronary plaque characteristics, coronary artery calcification score, coronary stenosis, and CT-FFR for lesion-specific ischemia in patients with known or suspected coronary artery disease. Int J Cardiol 332:8–14
Gać P, Macek P, Poręba M, Kornafel-Flak O, Mazur G, Poręba R (2021) Thickness of epicardial and pericoronary adipose tissue measured using 128-slice MSCT as predictors for risk of significant coronary artery diseases. Ir J Med Sci 190(2):555–566
Samy NI, Fakhry M, Farid W (2020) Relation between epicardial adipose tissue thickness assessed by multidetector computed tomography and significance of coronary artery disease. World J Cardiovasc Dis 10(02):91–101
Demircelik MB, Yilmaz OC, Gurel OM, Selcoki Y, Atar IA, Bozkurt A et al (2014) Epicardial adipose tissue and pericoronary fat thickness measured with 64-multidetector computed tomography: potential predictors of the severity of coronary artery disease. Clinics 69(6):388
You D, Yu H, Wang Z, Wei X, Wu X, Pan C (2022) The correlation of pericoronary adipose tissue with coronary artery disease and left ventricular function. BMC Cardiovasc Disord 22(1):398
Mohammadzadeh M, Mohammadzadeh V, Shakiba M, Motevalli M, Abedini A, Kadivar S et al (2018) Assessing the relation of epicardial fat thickness and volume, quantified by 256-slice computed tomography scan, with coronary artery disease and cardiovascular risk factors. Arch Iran Med 21(3):95–100
Oka T, Yamamoto H, Ohashi N, Kitagawa T, Kunita E, Utsunomiya H et al (2012) Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int J Cardiol 161(1):45–49
Chang S, Lee W, Kwag B, Chung YH, Kang IS (2017) Maximal pericoronary adipose tissue thickness is associated with hypertension in nonobese patients with acute or chronic illness. Korean J Intern Med 32(4):668–674
Aydın AM, Kayalı A, Poyraz AK, Aydın K (2015) The relationship between coronary artery disease and pericoronary epicardial adipose tissue thickness. J Int Med Res 43(1):17–25
Iwasaki K, Matsumoto T, Aono H, Furukawa H, Samukawa M (2011) Relationship between epicardial fat measured by 64-multidetector computed tomography and coronary artery disease. Clin Cardiol 34(3):166–171
Maimaituxun G, Shimabukuro M, Fukuda D, Yagi S, Hirata Y, Iwase T et al (2018) Local thickness of epicardial adipose tissue surrounding the left anterior descending artery is a simple predictor of coronary artery disease—new prediction model in combination with framingham risk score. Circ J82(5):1369–1378
Picard FA, Gueret P, Laissy JP, Champagne S, Leclercq F, Carrie D et al (2014) Epicardial adipose tissue thickness correlates with the presence and severity of angiographic coronary artery disease in stable patients with chest pain. PLoS ONE 9(10):e110005
Milanese G, Silva M, Ledda RE, Goldoni M, Nayak S, Bruno L et al (2020) Validity of epicardial fat volume as biomarker of coronary artery disease in symptomatic individuals: results from the ALTER-BIO registry. Int J Cardiol 314:20–24
Wu FZ, Chou KJ, Huang YL, Wu MT (2014) The relation of location-specific epicardial adipose tissue thickness and obstructive coronary artery disease: systemic review and meta-analysis of observational studies. BMC Cardiovasc Disord 14:1–10
Acknowledgements
Not applicable
Funding
No funding was received to assist with preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
VT and MM proposed initial idea and study design. Material preparation and data collection were performed by MM and VT. The data analysis was performed by VT. The first draft of the Manuscript was written by VT. All authors commented on previous version of the manuscript and approved the last manuscript. Both authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the research ethics committee of Kerman University of Medical Sciences. This investigation used available data from patient’s medical records. Considering the retrospective nature, the requirement for individual patient consent was waived by the research committee as a data protection safeguard was in place.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moradi, M., Talebi, V. Evaluation of epicardial adipose tissue by coronary multi-detector computed tomography: an independent predictor of obstructive coronary artery disease. Egypt J Radiol Nucl Med 54, 68 (2023). https://doi.org/10.1186/s43055-023-01018-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01018-9