Abstract
Background
Multiple sclerosis (MS) is an autoimmune disease affecting the central nervous system. This study aimed to evaluate the advantages and disadvantages of a positron emission tomography (PET) scan method for diagnosing Alzheimer’s disease (AD) in MS patients with no clinical symptoms or early-onset AD.
Main text
To identify potentially relevant documents, we systematically searched international databases from 2000 to 2021. We abstracted data on article characteristics, ID/country, study, design, population, type of tracer, and outcomes. The primary outcomes were mean amyloid tracer standardized uptake value relative (SUVr), AD diagnosis in MS patients, and the tracer's uptake. Secondary outcomes were the megabecquerel amount of tracer and tracer side effects. Nine studies were finally entered into our research for review. Among the studies included, two studies used 18F-florbetaben, six of these used 11C-Pittsburgh compound B (11C-PiB), and in two studies (18)F‑florbetapir (18F-AV1451) was used for imaging. Data from 236 participants were included in this study (145 MS patients, 17 AD patients, 12 mild cognitive impairment patients, and 62 healthy controls).
Conclusions
PET scan, especially florbetapir-based radio traces in helping to diagnose early AD, is imperative to use an age-specific cutoff in MS patients to support AD diagnosis.
Highlights
-
The years after the first diagnosis and progressive or non-progressive MS are crucial factors in increasing the risk of early AD.
-
The florbetapir-based radio traces in helping to diagnose early AD.
-
Logical to use an age-specific cutoff in MS patients for early AD diagnosis.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS). Demyelination is a hallmark pathologic feature of MS, interrupting the flow of information within the brain and between the brain and organs [1, 2]. The hallmark pathophysiology of MS is an immune response against significant proteins of the myelin sheet mainly operated through T cells and later through the release of cytokines and expression of antibodies by B cells, breakdown of the blood–brain barrier (BBB), and activation of indwelling microglia [3]. Inflammation can potentially decrease the conduction of information between neurons [4, 5]. The soluble factors released impair standard synaptic transmission and precipitate the loss of myelin sheet or even cause direct axonal damage. These and similar processes can precipitate tau accumulation as a neurofibrillary tangle and lead to Alzheimer's disease (AD) [6, 7]. Several inflammatory operations and cytokines may have a role in AD pathology. Inflammation is a standard marker of tissue destruction in any disease and may be secondary to tissue damage in AD or an immunological response marker [8]. There is increasing proof of a strong interplay between the brain's neurons and the immunological mechanisms. Fatness and systemic inflammation may intromit with immunological processes, which promote disease development [9].
Assessment of the cerebrospinal fluid for the presence and levels of amyloid-beta (Aβ) 1–40/1–42 peptides and comparing the level of total and phosphorylated tau proteins in cerebrospinal fluid [10], often in conjunction with positron emission tomography (PET) and magnetic resonance volumetric studies [11], are commonly used modalities to diagnose AD. Definitive diagnosis of AD can now be achieved through PET and via assessment of either cortical tau burden through tau-specific tracers including Fluorine-18-THK 5317, Fluorine-18-THK 5351, 18 F-flortaucipir (AV1451), and 18F-PM-PBB3 (18F-APN-1607) [12, 13, 27], or through demonstration of the presence of cortical beta-amyloid plaques through 11C-Pittsburgh compound B (PiB) or Florbetapir tracers [14, 15].
This technique plays a role in AD research. Scientists in this field can do noninvasive in vivo neuroimaging studies using PET scans in cerebral individuals with different stages of dementia [16]. According to the growing literature on the increased prevalence of AD among patients with MS and shared amyloid and tau-related pathologies, this study aimed to evaluate the advantages and disadvantages of a PET scan method for diagnosing AD in MS patients with no clinical symptoms or early-onset AD (EOAD).
Main text
Protocol and registration
Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR) checklist and explanation [17], which was developed prospectively by Peters et al. [18], was used for this study, which was revised by the Alzheimer's research team and members of Health Iran. On December 6, 2020, the Open Science Platform (OSP) (https://osf.io/xhqeg/).
Eligibility criteria
A protocol should be developed a priori, and it is essential to include the protocol information in the scoping review [18]. To ensure transparency and reduce work duplication, the protocol was registered by the OSP [19].
Inclusion criteria
Articles written in English from 2000 to 2021 that include human participants (female, male), a minimum age of 18 years, and using PET scan evaluation of AD in MS patients were evaluated in this study.
Exclusion criteria
Papers were excluded if they did not fit into the study's conceptual framework and focused on other neurodegenerative diseases or different types of dementia. Articles about AD diagnosis in patients with MS have also included letters to the editor without original data, commentaries, and editorials.
Information sources
To identify potentially relevant documents, we systematically searched international databases from 2000 to 2021: PubMed, Scopus, Cochrane Library, CINAHL, ISI Web of Science, Science Direct, PROSPERO, EMBASE, ALZFORUM, and PsycINFO. We also manually searched through the reference lists to select relevant papers. Two experienced librarians [K.S.H, P.J] drafted search strategies and refined them through team discussion. The final search strategy for MEDLINE is available in Additional file 1: Table S1.
The final search results were exported into EndNote, and a library technician removed duplicates. The electronic database search was supplemented by searching the Canadian Medical Protective Association website (https://www.cmpa-acpm.ca/en) and scanning-related reviews [20]. A comprehensive literature search should be done for a scoping study, and it may include both published and difficult-to-locate or gray literature [18].
Search
The literature search strategy was peer-reviewed by (M.KH.) using the Peer Review of Electronic Search Strategies (PRESS) checklist, a set of recommendations for librarians and other information specialists to use to evaluate electronic search strategies [21], using medical subject headings (MeSH) terms (Additional file 1: Table S1).
Selection of sources of evidence
All reviewers screened the same publications, discussed the results, and revised the screening and data extraction manual before screening to increase consistency among reviewers. Four reviewers worked in pairs and evaluated the titles, abstracts, and full text of all publications identified by our searches for potentially relevant publications. We settled disagreements on study selection and data extraction by agreement and discussion with other reviewers if needed.
Data charting process
Data from eligible studies used a standardized data abstraction tool designed for this study. Two reviewers [K.S.H., P.J.] independently extracted data from each relevant article. Some inconsistencies were settled by consultation between the two reviewers or a third reviewer's further adjudication. REDCap, a customizable Web program based on informatics systems, was used to implement results [17].
Data items
We abstracted data on article characteristics, ID/Country, study design, population, type of tracer, and outcomes. Mean tracer standardized uptake value relative (SUVr), AD diagnosis in MS patients, and uptake of the tracers were primary outcomes. Secondary outcomes were the megabecquerel (MBq) amount of tracer and tracer side effects.
Critical appraisal of individual sources of evidence
In addition to this, a quality assessment of the selected studies was individually performed by two researchers using the modified Jadad scale for randomized controlled trial [22] Methodological Index for Non-randomized Studies (MINORS) tool for non-randomized interventional study [23].
A workshop on the tool was held with the team to ensure high inter-rater agreement, and two pilot tests were conducted on a random sample. Each pilot test consisted of a facilitated team meeting for feedback and discussion on discrepant items. Upon completing the pilot tests, pairs of reviewers [K.SH., M.KH., P.J., P.P, A.M.R.R., H.K.SH, H.S, and F.R] assessed the first included articles independently. One reviewer (P.J.) considered the remaining papers and verified them by a second reviewer [K.SH., M.KH.]. All inconsistencies were revised by a third reviewer [A.M.R.R.].
Synthesis of results
We grouped the studies by the types of behavior they analyzed and summarized the settings, populations, and study designs for each group, along with the measures used and broad findings. We identified a systematic review, counted the number of studies included in the thought that potentially met our inclusion criteria, and remarked on how many studies had been missed by our search [17].
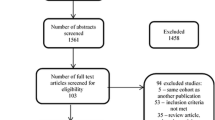
Following the search, 508 related studies were found, and after reviewing the title and abstract of the studies, 499 studies were excluded. Our review included nine studies [24,25,26,27,28,29,30,31,32] (Fig. 1). The studies included; two studies that had used 18F-florbetaben [26, 31], six studies that had used [11C] PiB [24, 25, 27,28,29, 32], and two studies (18)F‑florbetapir (18F-AV1451) [30, 32] were used for imaging. In total, 236 participants were included in this study (145 MS patients, 17 AD patients, 12 mild cognitive impairment (MCI) patients, and 62 healthy controls) (Table 1).
18F-florbetaben tracer
Forty-one patients in both studies underwent PET imaging through an 18F-florbetaben tracer [26, 31]. SUVr was higher in normal-appearing white matter (NAWM) (1.51 ± 0.12) than in damaged white matter (DWM) (1.24 ± 0.12; P = 0.002) [26], and also, a lower SUV ratio in NAWM and lower thalamic volume [31]. A decrease in 18F-florbetaben uptake in the damaged brain tissue was visible after imaging (Table 1).
[11C] PiB tracer
Six of the ten studies were related to the [11C] PiB PET-Scan [24, 25, 27,28,29, 32]. In the study by Schubert et al., 11 AD patients, 12 patients with MCI, and 20 MS patients were included [29]. In these patients, after [11C] PiB PET scan, the amount of absorption in the lateral ventricles of AD and MS was significantly reduced compared to healthy individuals. In two similar studies aimed at evaluating the [11C] PiB's uptake in white matter (WM) after imaging in patients with advanced MS, a decrease in WM uptake was observed [27, 28]. However, Kim et al. explicated that pathological relevance is obscure [24]. In another study of 16 MS patients, older patients with MS, cortical beta-amyloid deposition was decreased compared to healthy control. In contrast, tau deposition increased [32]. Reduced memory and language of MS patients significantly reduced memory, language, and dysfunction due to decreased volume of the thalamus, frontal cortex, and temporal lobe [32] (Table 1).
(18)F‑florbetapir (18F-AV1451) tracer
Among three MS patients with an early cognitive impairment studied by Kolanko et al., two indicated AD through undergoing (18)F‑florbetapir PET scan [30].
In another study, the frequency of elevated AD signature AV1451 SUVr was different (OR [95% CI] = 10.65 [1.10–103.35], p = 0.04) between patients with MS (n = 4, 33%) and controls (n = 6, 10%) [32] (Table 1).
MCI, AD, frontotemporal dementia, and dementia of Lewy body PET scan patterns
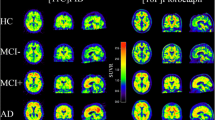
18F-florbetaben PET transaxial images of healthy control (HC), patients with Parkinson's disease (PD), dementia with Lewy bodies (DLB), MCI, AD, frontotemporal lobar degeneration (FTLD), and vascular dementia (VaD) were analyzed. AD patients show cortical 18F-florbetaben uptake and non-specific white matter uptake visible in negative scans of HC, PD, FTLD, and VaD. Sagittal and coronal amyloid PET images in AD patients demonstrate generalized increased activity within the cortical gray matter in all lobes with complete loss of gray-white differentiation consistent with widespread amyloid deposition (Fig. 2).
A 18F-florbetaben PET transaxial images of healthy control (HC), patients with Parkinson's disease (PD), dementia with Lewy bodies (DLB), mild cognitive impairment (MCI), Alzheimer's disease (AD), frontotemporal lobar degeneration (FTLD), and vascular dementia (VaD). AD patients show cortical 18F-florbetaben uptake and non-specific white matter uptake visible in negative scans of HC, PD, FTLD, and VaD. All images are scaled to the same SUVR maximum. This research was initially published in JNM. Villemagne et al. B, 2011 [58]. B She has a family history of dementia in her sister's late 60s. Difficulty completing the MMSE. She had been diagnosed with MS, and MRI imaging had been consistent with demyelination Brain MRI showed several inflammatory demyelinating lesions. API was positive. Axial T2-weighted C Coronal FLAIR images through the brain showing typical MS lesions in the periventricular white matter perpendicular to the ependymal surface. Note generalized neuroparenchyma loss, with relative sparing of the temporal lobe white matter (left mesial temporal atrophy score is 2; correct mesial temporal atrophy score is 1). D Sagittal and coronal amyloid PET images demonstrating generalized increased activity within the cortical gray matter in all lobes with complete loss of gray-white differentiation consistent with widespread amyloid deposition. This research was initially published in J. Neurol. M Kolanko et al. 2020 [30]
MS is a multifactorial demyelinating disease that is more common in women than men [33, 34]. On the other hand, immune cell function, especially microglia, causes myelin degeneration, which causes inflammation and damage to neural cells; several pathological pathways play a role in MS [2, 35]. A decrease in interleukin (IL)-10 activity as a preventive factor is an excellent cause of Th-0 dysregulation, leading to an autoimmune condition by overstimulating microglia activities [36]. In AD patients, inflammation is caused by Tau and amyloid-beta proteins [37, 38]. Overall, Inflammation in either MS and AD patients in an overlapping pathway leads to decreased cerebral blood flow (CBF), and extrinsic caspase pathway activation leads to apoptosis [4, 39] (Fig. 3). Furthermore, in the research by Campaholo et al., 51 MS patients were split into PMS and RRMS groups; as a result, the study revealed that in RRMS division, cognition and psychomotor speed were related to decreasing uptake. In another way, lower myelin content was connected to poor cognitive status [40].
Therefore, atrophic areas in the brain tissue enhance amyloid-beta and tau protein deposition probability more than in healthy individuals [41, 42]. Tau proteins have a structural role in microtubules. These proteins are separated from the microtubules following phosphorylation, disrupting the microtubules on the one hand and tangles of tau protein [43]. As though the levels of intermediate products of proteolysis of the amyloid precursor protein (APP) are decreased in patients with MS. APP has a crucially significant role in MS; APP proteolytic processing happens regarding demyelination because of the presence of myelin protein, and it also has a role in remyelination to a greater or lesser degree [44]. Following inefficient remyelination due to the function of Aβ plaques [45] and glial scars because of astrocytes [46], these plaques intensify the abnormal function of phosphorylated cyclin-dependent kinase 5 (CDK5) proteins by IL-6 and IL-18. Moreover, the effect of microglia on increasing the abnormal activity of CDK5 by IL-18 [47,48,49,50] results in the formation of hyperphosphorylated tau tangles following increases in the phosphorylation activity of phosphorylated collapsing response mediator protein 2 (pCRMP) [51, 52] (Fig. 3).
In that case, where MS shows itself at a younger age than AD and the possibility of increased apoptosis due to the simultaneous presence of MS and AD, a reliable diagnostic method can be helpful in the screening and prevention of AD [53]. An amyloid PET scan can recognize amyloid-beta deposits as a valuable modality in detecting demyelination and remyelination in MS [26]. The affinity of amyloid tracers for myelin appears to be described by the β-sheet formation of both β-amyloid and myelin essential proteins [54]. In addition, this mechanism happens in other proteins, such as pathological prion protein, which is rich in β-sheet [44]. After 300 MBq of 18F-florbetaben, amyloid PET images showed decreased absorption in demyelinated areas. The pioneering research in this regard was done by Stankoff et al. [55]. In the study, Jordi et al. stated that the decrease in SUVr in the affected regions was quite apparent after MRI and PET images. It is more critical in patients with progressive MS, both primary and secondary, than in relapsing–remitting MS (RRMS) patients, showing a SUVr decrease in demyelinated areas [26, 31]. Moreover, the absorption reduction in images obtained from the amyloid PET scan, especially in primary progressive MS (PPMS) and secondary progressive MS (SPMS) compared to RRMS, could indicate a higher chance of depositing amyloid peptides in the coming years. Thus, in such cases, the 18F-florbetaben amyloid PET scan can play an essential role in diagnosing early AD in MS patients [16, 26].
N-methyl-[11C]2-(4′-methylaminophenyl)-6-hydroxybenzothiazole ([11C] PIB) due to the high bonding capability to AB plaques is useful for the detection of AD [56]. A study aimed at imaging 12 late MS patients showed decreased uptake in the white matter and frontal and temporal cortex, while uptake in other cortex areas was like healthy individuals [28]. The accurate mechanism of the physiological uptake for white matter is not well-developed, even though demyelination is the possible mechanism describing the decreased uptake in MS [54]. Similarly, Zeydan et al., AB, showed that plaque deposition decreased compared with peers in the control group by age. However, the enhancement of tau protein deposition, especially in progressive MS patients compared to the healthy control group, was significant [32]. Two studies with an almost similar design after [11C] PIB imaging reported a SUVr reduction in areas affected by MS lesions [27, 29]. In contrast, the 18F-florbetaben amyloid PET scan can detect amyloid-beta plaques in MS patients for early AD diagnosis; the white matter uptake in [11C] PiB is lower than in flora-based radiotracers such as Florbetapir and Florbetaben [15]. Similarly, [11C] PiB PET scan was shown less effective in early AD diagnosis in MS patients and more useful in identifying the cause of memory loss according to functional changes and uptake reduction in demyelinated areas [28, 32]. (18)F‑florbetapir (18F-AV1451) amyloid PET scan was used by Kolanko et al. in three MS patients at least 20 years after the diagnosis; images showed a SUVr reduction and increased possibility of AD in these three patients [30]. The presence of atrophic MS lesions and a greater tendency to form accumulation in these areas automatically lead to the suspicion that the risk of AD in these patients increases due to the increased risk of AB and tau accumulation.
The amyloid brain PET imaging value is wholly based on the scan's quality and the evaluation's accuracy. This evolving modality challenges image evaluation criteria, positive and negative clinical scans, and technical imaging attention. Dependence on the Accreditation Council for Graduate Medical Education (ACGME) for safe performance of amyloid brain PET scan needs training teams that consist of physicians, expert supervision, and technician that are certified by nuclear radiology organizations and familiar with brain anatomy, metabolism, and amyloid PET tracers mechanisms [57].
Conclusions
In conclusion, MS, due to the nature of the disease and the progression of brain lesions to atrophic areas, shows that years after the first diagnosis and progressive or non-progressive MS are crucial factors in increasing the risk of early AD. With the valuable results obtained from PET scan, especially florbetapir-based radio traces, in helping to diagnose early AD, it sounds logical to use an age-specific cutoff in MS patients for early AD diagnosis.
Availability of data and materials
All data generated or analyzed during this study are included in the supplementary material files in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- MS:
-
Multiple sclerosis
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- BBB:
-
Blood–brain barrier
- MRI:
-
Magnetic resonance imaging
- Aβ:
-
Amyloid-beta
- AD:
-
Alzheimer's disease
- PET:
-
Positron emission tomography
- EOFAD:
-
Early-onset familial Alzheimer's disease
- [11C] PiB:
-
11C- Pittsburgh compound B
- MeSH:
-
Medical subject headings
- SUVr:
-
Standardized uptake value relative
- PRISMA-ScR:
-
Systematic reviews and Meta-Analyses extension for Scoping Reviews
- OSP:
-
Open Science Platform
- PRESS:
-
Peer Review of Electronic Search Strategies
- MINORS:
-
Methodological Index for Non-randomized Studies
- MCI:
-
Mild cognitive impairment
- IV:
-
Intravenously
- MBq:
-
Megabecquerel
- NAWM:
-
Normal-appearing white matter
- DWM:
-
Damaged white matter
- WM:
-
White Matter
- IL:
-
Interleukin
- CDK5:
-
Cyclin-dependent kinase 5
- pCRMP:
-
Phosphorylated collapsing response mediator protein
- RRMS:
-
Relapsing–Remitting MS
- PPMS:
-
Primary progressive MS
- CBF:
-
Cerebral blood flow
References
Miller DH, Leary SM (2007) Primary-progressive multiple sclerosis. Lancet Neurol 6(10):903–912
Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O (2018) Multiple sclerosis. The Lancet 391(10130):1622–1636
Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M et al (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132(Pt 5):1175–1189
Peterson JW, Bo L, Mork S, Chang A, Trapp BD (2001) Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol 50(3):389–400
Cai Z, Qiao PF, Wan CQ, Cai M, Zhou NK, Li Q (2018) Role of blood-brain barrier in Alzheimer’s disease. J Alzheimers Dis 63(4):1223–1234
Alzheimer’s A (2016) 2016 Alzheimer’s disease facts and figures. Alzheimers Dement 12(4):459–509
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14(1):32
Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V et al (2018) High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 554(7691):249–254
Calsolaro V, Edison P (2016) Neuroinflammation in Alzheimer’s disease: current evidence and future directions. Alzheimers Dement 12(6):719–732
Lyons B, Friedrich M, Raftery M, Truscott R (2016) Amyloid plaque in the human brain can decompose from Abeta (1–40/1-42) by spontaneous nonenzymatic processes. Anal Chem 88(5):2675–2684
Hooker JM, Carson RE (2019) Human positron emission tomography neuroimaging. Annu Rev Biomed Eng 4(21):551–581
Sun A, Liu X, Tang G (2018) Carbon-11 and fluorine-18 labeled amino acid tracers for positron emission tomography imaging of tumors. Front Chem 5:124
Su Y, Fu J, Yu J, Zhao Q, Guan Y, Zuo C et al (2020) Tau PET imaging with [18F]PM-PBB3 in frontotemporal dementia with MAPT mutation. J Alzheimers Dis 76(1):149–157
Jia J, Sun B, Guo Z, Zhang J, Tian J, Tang H et al (2011) Positron emission tomography with Pittsburgh compound B in diagnosis of early stage Alzheimer’s disease. Cell Biochem Biophys 59(1):57–62
Auvity S, Tonietto M, Caille F, Bodini B, Bottlaender M, Tournier N et al (2020) Repurposing radiotracers for myelin imaging: a study comparing 18F-florbetaben, 18F-florbetapir, 18F-flutemetamol,11C-MeDAS, and 11C-PiB. Eur J Nucl Med Mol Imaging 47(2):490–501
Takenoshita N, Fukasawa R, Ogawa Y, Shimizu S, Umahara T, Ishii K et al (2018) Amyloid and tau positron emission tomography in suggested diabetesrelated dementia. Curr Alzheimer Res 15(11):1062–1069
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169(7):467–473
Peters MD, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB (2015) Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 13(3):141–146
Foster MED, Deardorff MA (2017) Open science framework (OSF). J Med Libr Assoc 105(2):203–206
Cardoso R, Zarin W, Nincic V, Barber SL, Gulmezoglu AM, Wilson C et al (2017) Evaluative reports on medical malpractice policies in obstetrics: a rapid scoping review. Syst Rev 6(1):181
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C (2016) PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75:40–46
Oremus M, Wolfson C, Perrault A, Demers L, Momoli F, Moride Y (2001) Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials. Dementia Geriatric Cognitive Disorders 12(3):232–236
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73(9):712–716
Kim HJ, Jeon BS, Kim YE, Kim JY, Kim YK, Sohn CH et al (2013) Clinical and imaging characteristics of dementia in multiple system atrophy. Parkinsonism Relat Disord 19(6):617–621
Catafau AM, Bullich S (2015) Amyloid PET imaging: applications beyond Alzheimer’s disease. Clin Transl Imaging 3(1):39–55
Matias-Guiu JA, Cabrera-Martin MN, Matias-Guiu J, Oreja-Guevara C, Riola-Parada C, Moreno-Ramos T et al (2015) Amyloid PET imaging in multiple sclerosis: an (18)F-florbetaben study. BMC Neurol 25(15):243
Bodini B, Veronese M, Garcia-Lorenzo D, Battaglini M, Poirion E, Chardain A et al (2016) Dynamic imaging of individual remyelination profiles in multiple sclerosis. Ann Neurol 79(5):726–738
Zeydan B, Lowe VJ, Schwarz CG, Przybelski SA, Tosakulwong N, Zuk SM et al (2018) Pittsburgh compound-B PET white matter imaging and cognitive function in late multiple sclerosis. Mult Scler 24(6):739–749
Schubert JJ, Veronese M, Marchitelli L, Bodini B, Tonietto M, Stankoff B et al (2019) Dynamic (11)C-PiB PET shows cerebrospinal fluid flow alterations in alzheimer disease and multiple sclerosis. J Nucl Med 60(10):1452–1460
Kolanko M, Win Z, Patel N, Malik O, Carswell C, Gontsarova A et al (2020) Using amyloid PET imaging to diagnose Alzheimer’s disease in patients with multiple sclerosis. J Neurol 267(11):3268–3273
Pytel V, Matias-Guiu JA, Matias-Guiu J, Cortes-Martinez A, Montero P, Moreno-Ramos T et al (2020) Amyloid PET findings in multiple sclerosis are associated with cognitive decline at 18 months. Mult Scler Relat Disord 2(39):101926
Zeydan B, Lowe VJ, Reichard RR, Przybelski SA, Lesnick TG, Schwarz CG et al (2020) Imaging biomarkers of Alzheimer disease in multiple sclerosis. Ann Neurol 87(4):556–567
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ et al (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83(3):278–286
Howard J, Trevick S, Younger DS (2016) Epidemiology of multiple sclerosis. Neurol Clin 34(4):919–939
Weiner HL (2004) Multiple sclerosis is an inflammatory T-cell-mediated autoimmune disease. Arch Neurol 61(10):1613–1615
Hu D, Notarbartolo S, Croonenborghs T, Patel B, Cialic R, Yang TH et al (2017) Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat Commun 8(1):1600
Kolarova M, Garcia-Sierra F, Bartos A, Ricny J, Ripova D (2012) Structure and pathology of tau protein in Alzheimer disease. Int J Alzheimer’s Dis 2012:731526
Luo J, Wärmländer SKTS, Gräslund A, Abrahams JP (2016) Cross-interactions between the Alzheimer disease amyloid-β peptide and other amyloid proteins: a further aspect of the amyloid cascade hypothesis. J Biol Chem 291(32):16485–16493
Roth KA (2001) Caspases, apoptosis, and Alzheimer disease: causation, correlation, and confusion. J Neuropathol Exp Neurol 60(9):829–838
Campanholo K, Pitombeira M, Rimkus C, Mendes M, Apóstolos-Pereira S, BusattoFilho G et al (2022) Myelin imaging measures as predictors of cognitive impairment in MS patients: a hybrid PET-MRI study. Mult Scler Relat Disord 57:103331
Popescu V, Agosta F, Hulst HE, Sluimer IC, Knol DL, Sormani MP et al (2013) Brain atrophy and lesion load predict long term disability in multiple sclerosis. J Neurol Neurosurg Psychiatry 84(10):1082–1091
Pietroboni AM, Colombi A, Carandini T, Contarino VE, Ghezzi L, Fumagalli GG et al (2019) Low CSF beta-amyloid levels predict early regional grey matter atrophy in multiple sclerosis. Mult Scler Relat Disord 19(39):101899
Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR (2000) Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev 33(1):95–130
Stankoff B, Wang Y, Bottlaender M, Aigrot MS, Dolle F, Wu C et al (2006) Imaging of CNS myelin by positron-emission tomography. Proc Natl Acad Sci USA 103(24):9304–9309
Mitew S, Kirkcaldie MT, Halliday GM, Shepherd CE, Vickers JC, Dickson TC (2010) Focal demyelination in Alzheimer’s disease and transgenic mouse models. Acta Neuropathol 119(5):567–577
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5(2):146–156
Quintanilla RA, Orellana DI, Gonzalez-Billault C, Maccioni RB (2004) Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp Cell Res 295(1):245–257
Ojala JO, Sutinen EM, Salminen A, Pirttila T (2008) Interleukin-18 increases expression of kinases involved in tau phosphorylation in SH-SY5Y neuroblastoma cells. J Neuroimmunol 205(1–2):86–93
Ahmad MH, Fatima M, Mondal AC (2019) Influence of microglia and astrocyte activation in the neuroinflammatory pathogenesis of Alzheimer’s disease: rational insights for the therapeutic approaches. J Clin Neurosci 59:6–11
Lu TT, Wan C, Yang W, Cai Z (2019) Role of Cdk5 in amyloid-beta pathology of Alzheimer’s disease. Curr Alzheimer Res 16(13):1206–1215
Wang Y, Yin H, Li J, Zhang Y, Han B, Zeng Z et al (2013) Amelioration of beta-amyloid-induced cognitive dysfunction and hippocampal axon degeneration by curcumin is associated with suppression of CRMP-2 hyperphosphorylation. Neurosci Lett 557 Pt B:112–117
Ikezu S, Ingraham Dixie KL, Koro L, Watanabe T, Kaibuchi K, Ikezu T (2020) Tau-tubulin kinase 1 and amyloid-beta peptide induce phosphorylation of collapsin response mediator protein-2 and enhance neurite degeneration in Alzheimer disease mouse models. Acta Neuropathol Commun 8(1):12
Gordon-Lipkin E, Banwell B (2017) An update on multiple sclerosis in children: diagnosis, therapies, and prospects for the future. Expert Rev Clin Immunol 13(10):975–989
Matías-Guiu JA, Cabrera-Martín MN, Pytel V, Montero P, Carreras JL, Matías-Guiu J (2020) Amyloid positron emission tomography in multiple sclerosis: between amyloid deposition and myelin damage. Ann Neurol 87(6):988
Stankoff B, Freeman L, Aigrot MS, Chardain A, Dollé F, Williams A et al (2011) Imaging central nervous system myelin by positron emission tomography in multiple sclerosis using [methyl-11C]-2-(4’-methylaminophenyl)- 6-hydroxybenzothiazole. Ann Neurol 69(4):673–680
Mintun MA, LaRossa GN, Sheline YI, Dence CS, Lee SY, Mach RH et al (2006) [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67(3):446–452
Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P et al (2013) Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement 9(1):e1–e16
Villemagne VL, Ong K, Mulligan RS, Holl G, Pejoska S, Jones G et al (2011) Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J Nucl Med 52(8):1210–1217
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The paper is exempt from ethical committee approval because this is a scoping review study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Electronic search strategies, using medical subject headings (MeSH) terms.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalafi, M., Rezaei Rashnoudi, A., Rahmani, F. et al. Amyloid PET scan diagnosis of Alzheimer’s disease in patients with multiple sclerosis: a scoping review study. Egypt J Radiol Nucl Med 54, 12 (2023). https://doi.org/10.1186/s43055-023-00964-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-00964-8