Abstract
Background
Preventing acute complication of renal angiomyolipoma (AML), preserving renal parenchyma, and improving long-term renal function are the treatment targets of renal angiomyolipoma. Treatment should be considered for symptomatic lesions or those who are at risk of complications, especially bleeding symptoms, which are linked to tumor size, angiogenic component grade, and presence of tuberous sclerosis complex (TSC). Selective arterial embolization (SAE) has become the new norm for preventive or emergency treatment of renal AMLs with minimally invasive selective targeting of small arterial feeders, we aimed to assess the efficacy and safety of selective renal arterial embolization (SAE) in the management of complicated renal angiomyolipoma and to detect the predictors of prophylactic SAE in cases of non-complicated AML.
Results
Bleeding symptoms were significantly more frequent in patients with TSC-associated renal AMLs (C = 0.333 and p = 0.036) and patients with intra-lesional aneurysm > 3 mm (C = 0.387 and p = 0.013). Overall success rate: thirty-three (91.7%) renal AMLs were successfully embolized with no recurrence. While three (8.3%) renal AMLs were not; one (2.8%) renal AML was not embolized due to technical failure and two (5.5%) renal AMLs showed recurrence. Primary (technical) success rate: thirty-three (86.9%) successful embolization, five (13.1%) arteriographies were done with failed embolization. The maximum diameter and volume of the lesions after SAE showed statistically significant reduction (z = 4.25 and p < 0.001).
Conclusions
SAE is an effective and safe technique to manage renal AMLs preoperatively or in an emergency. TSC-associated lesions, and intra-lesional aneurysms (aneurysms > 3 mm in diameter) were significantly more associated with bleeding symptoms, considering them significant predictors for prophylactic SAE in non-complicated AML.
Similar content being viewed by others
Background
Renal angiomyolipoma (AML) is the most common benign renal tumor with a prevalence varying between 0.2 and 0.6% and a high female predilection [1]. In 80% of cases, renal AMLs occur as sporadic, isolated individuals. Tuberous sclerosis complex (TSC) or pulmonary lymphangio-leiomyomatosis (LAM) was responsible for the remaining 20% of renal AMLs [2].
AMLs are considered as a heterogeneous group of neoplasms. Despite the fact that many forms have different pathology, radiological characteristics, and clinical behavior, they all have varying proportions of the same three elements: adipose skin, smooth muscle, and blood vessels [3].
They are also classically detected by the characteristic presence of fat on computed tomography (CT), magnetic resonance imaging (MRI) or ultrasonography (US) of the kidneys [4]. So, Imaging is crucial in the diagnosis and management of renal AMLs [2].
Even though some renal AMLs are asymptomatic, they have a tendency to grow in size and may cause local complications. Flank pain, palpable mass, gross hematuria, anemia, and symptoms linked to a mass effect such as abdominal pain, abdominal fullness, abdominal visceral compression, and anorexia are all common symptoms of renal AMLs [4].
Renal AMLs have irregular blood vessels with no internal elastic lamina and fibrous tissue replacing smooth muscle, rendering the vessels stiff, tortuous, and vulnerable to aneurysm formation and rupture [5]. Renal AMLs have been shown to have a high risk of rupture during their clinical course, with symptoms such as hematuria, retroperitoneal bleeding, and hemorrhagic shock [6].
Management recommendations are based on tumor size and symptoms. Primary therapeutic targets focus on preventing acute events, preserving renal parenchyma and improving long-term renal function [7].
Potential interventions include selective arterial embolization (SAE), nephron-sparing surgery, total nephrectomy, cryo- and radiofrequency ablation and treatment with mammalian target of rapamycin (mTOR) inhibitors [3].
SAE can be used as a prophylactic treatment of high-risk renal AMLs, as an emergency management of bleeding renal AMLs or as a pre-operative adjunct treatment to avoid intra-operative blood loss during surgery [8].
Since it is less invasive than surgery and allows for targeted treatment of bleeding vessels with a low risk of serious complications, this minimally invasive interventional radiology procedure has been the preferred treatment for renal AMLs for many years [9].
There are no well-established criteria to justify SAE as prophylactic procedure for high-risk renal AMLs.
This research work aims to evaluate the efficacy and safety of selective renal arterial embolization in the management of complicated renal AML, and to assess the predictors of prophylactic SAE in non-complicated renal AML.
Methods
Our study included thirty-seven patients, thirty-three patients with renal AMLs managed by SAE, then follow-up was done by clinical assessment, laboratory investigations and medical imaging. While, four patients were excluded, three patients without follow-up, and one patient who underwent arteriography without an attempt for embolization then was managed with partial nephrectomy.
All patients were referred to the Interventional Radiology Unit of Urology and Nephrology Center, Mansoura University from the Urology Department of the same center, or from the emergency hospital and underwent follow-up in the period from August 2017 to September 2021.
We recorded demographic information (age and gender). Type of AML (sporadic or TSC-associated), location and number of lesions, type of intervention (prophylactic in case of high risk renal AMLs, emergency as an acute management of bleeding renal AMLs or preoperative adjunct treatment for surgery to prevent intra-operative blood loss), clinical symptoms and complications before and within 4 weeks after SAE, recurrence (defined as recurrent symptoms or increased tumor size > 2 cm on follow-up images requiring re-intervention), and the need for further treatment. Levels of serum creatinine prior to SAE and during follow-up, complete blood count including leucocytic count, hematocrit and hemoglobin levels, coagulation profile including prothrombin time, prothrombin concentration and International Normalized Ratio (INR) were measured.
Diagnosis, embolization technique and post-procedure care
On admission, Un-enhanced CT scan was done for all patients to detect the actual size of intra-renal or peri-nephric hematoma.
Interventional procedure including diagnostic and therapeutic renal angiography was done by using (Toshiba, Infinix CC CAS-8000V/Cx, Japan) machine under fluoroscopic guidance.
Interventional radiologists, urologists, and/or nephrologists proposed a multidisciplinary treatment strategy.
Using the modified Seldinger's technique via the normal femoral artery under local anesthesia, SAE was performed using five Fr cobra catheter (Cordis, USA) and Renegade HI-FLO 18 microcatheter (Boston Scientific, USA). Non-ionic contrast media (Omnipaque 350 mg/ml, Schering, Germany) was used in all arteriographies.
Several embolic materials, either alone or in combination, were used including microcoils (Pushable fibered platinum™, Boston Scientific, USA) ranges from 3 to 5 mm in diameter and 4 to 9 mm in length, microspheres (Embosphere™, Guerbet, France) or absolute alcohol (Concentrated ethanol 95–99%)]. Coils were used to occlude massive aneurysmal formations that would have been unsuitable for particle embolization alone.
All patients were followed up until they become hemodynamically stable, hematuria stopped, hemoglobin loss ceased, general condition improved and hemoglobin level started to build up.
We defined overall success as no intra-operative blood loss in pre-operative SAE and no recurrence on follow-up visits in prophylactic and urgent SAE regardless the number of arteriographies done for complete embolization of the lesion, while we defined primary (technical) success as immediate and complete devascularization of the lesion on control angiogram in the first instance with no need for re-embolization.
Follow-up
Medical history, outpatient charts, follow-up appointments, and radiological data were checked. Medical examination, laboratory investigations, and diagnostic imaging were performed on all patients by the urology and nephrology teams; 1, 3, and 6 months after SAE, and once a year thereafter. If there was no improvement or decrease in size, a CT or MR follow-up was performed 3 months after the SAE and annually if there was no change or decrease in size.
Outcome measures
The primary outcome entailed primary endpoint that was technical success as well as clinical success of the procedure as regard short- term outcomes in the form of management of renal AMLs as a prophylaxis of high-risk renal AMLs, as an acute management of bleeding renal AMLs or as a pre-operative adjunct treatment for surgery to avoid intra-operative blood loss.
Secondary outcomes were substantial reduction in tumor size, low recurrence rates and acceptable complications.
Statistical analysis
Analysis was performed using IBM SPSS® software package version 25.0 for Windows.
Qualitative data were described using number (n) and percentage (%) and analyzed using Chi-Square, Fischer Exact and Monte Carlo tests. Quantitative data were described using median (range) [minimum and maximum] for nonparametric data and mean ± standard deviation (SD) for parametric data and analyzed using Mann–Whitney U, Kruskal–Wallis H and Wilcoxon signed-rank tests for nonparametric data, while Student t, One Way ANOVA and Paired t tests for parametric data.
We used the contingency coefficient (C) to identify the predictors of prophylactic SAE associated with increased risk of bleeding in the included patients. Correlation between tumor size-reduction and initial tumor size was determined using Spearman's rank correlation coefficient (Rs). p values < 0.05 were considered as statistically significant.
Results
Our study included 33 patients with 36 AMLs. Patients’ ages ranged from 12 to 64 years with mean age ± SD of 38.27 ± 13.9 years. The demographic and clinical data are presented in Table 1. During a 4-year study period, 33 patients underwent 38 arteriographies for 36 renal AMLs; one large TSC-associated AML was not embolized due to difficulty to reach the arterial feeder and total nephrectomy was decided without attempt for re-embolization.
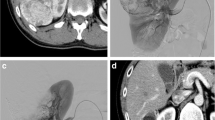
Therefore, thirty-seven arteriographies with SAE for 35 renal AMLs were carried out. Three SAE were done as a prophylaxis of high-risk asymptomatic renal AMLs (tumor size > 4 cm and/or abnormal vasculature on imaging) (Fig. 1), Twenty-nine SAE were done as an acute management of bleeding renal AML (Fig. 2). Three SAE were done as a pre-operative adjunct treatment for surgery to prevent intra-operative blood loss (Fig. 3).
A 63-year-old female patient presented with mild left loin pain. She was diagnosed with huge left renal AML and underwent prophylactic SAE using 3 microcoils. She suffered from post-embolization syndrome (PES); pain and fever few days after SAE that were treated with conservative treatment. On follow-up visits, there was a tumor size reduction of about (16%) 6 months after SAE. Digital subtraction angiography (a, b); a Pre-embolization angiography of the left renal artery using cobra catheter showing single renal artery with early pre-hilar division and its lower division supplying large lesion with multiple abnormal tortuous vascularities. b Post-embolization control angiogram showing no further opacification of the lesion after SAE using 3 microcoils. c T2-W (coronal image) d T2-W (axial image) and e Gadolinium-enhanced T1-W fat-saturated (axial image) MRI sequences before SAE. Huge left renal parenchymal fat-containing lesion (AML). f T2-W (axial image) MRI sequence 6 months after SAE. Tumor size reduction of about (16%)
A 20-year-old male patient with TSC presented with left loin pain for 3 months and recurrent attacks of gross hematuria for 1 month. He was hemodynamically stable and was admitted with hemoglobin level of 11.5 g/dL with no need of blood transfusion. He underwent urgent SAE using microspheres and 2 microcoils; hematuria stopped, his hemoglobin level reached 12.2 mg/dl and there was a significant tumor size reduction of about (48%) on follow-up. a UECT scan of the brain (axial image): Hyperdense calcified subependymal hamartomas (tubers) (yellow arrow). b Arterial phase of CECT scan (axial image) and c Arterial phase of CECT scan coronal MIP images: Single left renal artery with midzonal intra-lesional aneurysm (yellow arrow) supplied by the middle segmental branch and multiple left renal AMLs with multiple abnormal vascularities. Left renal digital subtraction angiography (d, e, f); d Pre-embolization angiography of the left renal artery using cobra catheter showing multiple lesions with multiple abnormal vascularities, then e Superselective catheterization of the middle segmental branch supplying midzonal lesion with multiple intra-lesional aneurysms. f Post-embolization control angiogram showing no further opacification of the lesion after SAE using microspheres. g CECT scan (axial image) before SAE: Exophytic left midzonal renal AML with multiple abnormal vascularities. h UECT scan (axial image) 9 months after SAE: Significant tumor size reduction of about (48%) Note the 2 microcoils (yellow arrow)
A 37-year-old female patient presented with right loin pain for 4 months. She was diagnosed with a large upper zonal renal AML with direct tumor extension into renal vein and infra-hepatic part of IVC on CECT. She underwent preoperative SAE using absolute alcohol and 2 microcoils followed by total nephrectomy with no intra-operative blood loss. Resected lesion was sent to pathologic analysis which revealed epithelioid renal AML. CECT scan (axial images): a large upper zonal right renal AML with multiple abnormal vascularities and direct tumor extension into renal vein (a) and infra-hepatic part of IVC (yellow arrow), b Arterial phase of CECT scan (coronal MIP images): c Double right renal arteries; main artery and higher accessory one supplying lower renal pole. d Upper segmental branch of the main renal artery supplying large upper zonal right renal AML with multiple abnormal vascularity. Right renal digital subtraction angiography (e, f, g): e Pre-embolization angiography of the right renal artery using cobra catheter showing large upper zonal lesion with multiple abnormal feeders. f Superselective catheterization of the arterial feeder of the lesion. g Post-embolization control angiogram showing no further opacification of the lesion after SAE using absolute alcohol and 2 microcoils. h Histopathologic image: Epithelioid renal AML that is formed of a mixture of polygonal and spindle cells of variable size where inflammatory cells are mingled with neoplastic cells. The resected specimens of renal vein and IVC show the same microscopic findings with no mural infiltration
Thirty-one lesions underwent twenty-nine arteriography sessions and were embolized at the first session (two patients, each has two AMLs). Four lesions were successfully embolized after the second session due to technical failure, so they underwent eight arteriographies.
Bleeding symptoms were significantly more frequent in patients with TSC-associated renal AMLs (C = 0.333 and p = 0.036) and patients with intra-lesional aneurysm more than 3 mm in diameter (C = 0.387 and p = 0.013); there were nine lesions with micro-aneurysms, 24 lesions with aneurysms > 3 mm in diameter and one lesion with a giant aneurysm.
Twenty (57.1%) lesions were embolized using microcoils only, while four (11.4%) lesions were embolized using microspheres. Embolic agent combinations were used as [microcoils and microspheres] in seven (20%) lesions and [microcoils and absolute alcohol] in four (11.4%) lesions.
Overall success rate
Out of 36 renal AMLs; thirty-three (91.7%) renal AMLs were successfully embolized and showed no recurrence on follow-up.
Meanwhile, three (8.3%) renal AMLs were not successfully embolized as one (2.8%) renal AML was not embolized due to technical failure as it TSC-associated AML and there was difficulty reaching the arterial feeders as they were multiple and tortuous and underwent total nephrectomy,
While 2 (5.5%) renal AMLs showed recurrence on follow up (the first case was a sporadic AML, that showed recurrent bleeding symptoms after 3 months and was embolized with microcoils. However, the other case was TSC-associated AML showed an increase in its size at 6 months follow-up visit and was embolized with microspheres) (Table 2).
Primary (technical) success rate
Thirty-three (86.9%) arteriographies were done with successful embolization in the first instance with immediate and complete devascularization on control angiogram, while five (13.1%) arteriographies were done with incomplete or failed embolizations. [Re-embolization during early follow-up was necessary in four (10.5%) times and further renal surgery was required]. Total nephrectomy was decided without re-embolization once (2.6%).
There were three (60%) technical failures due to difficulty reaching the arterial feeders as they were multiple and tortuous even with use of microcatheters, while there were two (40%) failed embolizations due to spasm of the arterial feeders during manipulation even after performing the standard management protocol of vascular spasm (Table 3).
Need for renal surgery
Surgery was performed seven times; total nephrectomy was performed immediately after failure of primary embolization once. While urologists had decided on other renal surgeries after technically successful primary embolization six times due to abnormal lesion composition, recurrence, or poor residual functional renal parenchyma; partial nephrectomy was performed once, and total nephrectomy was performed five times.
Complications related to arteriography and embolization
Minor complications occurred fourteen times that were treated with conservative measures; puncture site hematoma occurred once, post-embolization syndrome occurred twelve times and persistent hematuria occurred once. While major complications affected one patient with renal abscess formation 1 month after SAE and treated with percutaneous drainage was done.
Patient outcomes, follow-up times and changes in tumor sizes are presented in Table 4.
The efficacy of embolization was determined over a mean follow-up of 6.91 ± 3.41 months (range 1–12 months). No patients were lost to follow- up.
In calculation of changes in tumor sizes, we used both maximum diameter & volume. The median maximum diameter of the lesions was 13.9 (6.60–25.5) cm before SAE & became 10.7 (4.7–30.0) cm after SAE with median maximum diameter reduction of 1.8 (− 7.2–7.0) cm and reduction rate about 16%, while the median volume of the lesions before SAE was 715.91 (134.18–3533.81) cc and became 283.89 (37.33–6322.45) cc after SAE with median volume reduction of 213.1 (− 3266.67–1711.69) cc and reduction rate about 48.44%. Both maximum diameter and volume of the lesions after SAE showed statistically significant reduction (using Wilcoxon signed-rank test; z = 4.25 and p < 0.001).
Kruskal–Wallis H test showed that there was no statistically significant difference in tumor size-reduction with using different embolic agents (X2 = 1.360 and p = 0.715).
Spearman's rank correlation coefficient (Rs) was used to determine the strength of the relationship between tumor size-reduction and initial tumor size; a positive correlation was noted (Rs = 0.381 and p = 0.035).
Laboratory investigations before and after SAE
Four patients had renal impairment before SAE and had baseline creatinine levels above 1.8 mg/dL. Only one patient, after SAE serum creatinine level rose in a mild and self-limiting way at early follow-up visits and recovered his initial value in a few days. There was no statistically significant change in median serum creatinine levels, mean hemoglobin levels and mean leucocytic count after SAE (Table 4), (Figs. 4, 5).
Minor complications affected 14 patients who were treated with conservative measures (n = 14): puncture site hematoma (n = 1), post-embolization syndrome (PES): (n = 12) and persistent hematuria (n = 1). While major complications affected one patient with renal abscess formation 1 month after SAE and treated with percutaneous drainage.
Regarding the hospital stay after SAE, 33 admissions were done. The median hospital stay duration was 6.0 (1.0–20.0) days; 11 (33.3%) admissions were described as very short hospital stay (1–3 days), eight (24.2%) admissions were categorized as short hospital stay (4–7 days) and nine (27.3%) were categorized as reasonable stay (8–11 days), while only five (15.2%) admissions were categorized as relatively long stay (> 14 days).
In this study, thirty-one renal AMLs were successfully embolized in the first instance, with immediate and complete devascularization on control angiogram in twenty-nine arteriographies (two patients, each with two AMLs), During the early follow-up, four re-embolizations were needed due to incomplete embolization due to difficulty reaching the arterial feeders because they were numerous and tortuous (n = 2) or due to arterial feeder spasm during manipulation (n = 2).
Recurrence affected two patients during follow-up; one showed recurrent bleeding symptoms and the other showed an increase in tumor size on follow-up and both were treated with total nephrectomy.
Urologists had decided on other renal surgeries after technically successful primary embolization 6 times due to abnormal lesion composition, recurrence, or poor residual functional renal parenchyma; partial nephrectomy was performed once, and total nephrectomy was performed five times.
Discussion
Renal AML is the most common benign renal tumor, with a prevalence of 0.2 percent to 0.6 percent and a high female proclivity [1].
Potential interventions include SAE, nephron-sparing surgery, total nephrectomy, cryo- and radiofrequency ablation and treatment with mTOR inhibitors [3].
Preventing acute events, preserving renal parenchyma, and improving long-term renal function are the treatment targets [7]. Treatment should be considered for symptomatic lesions or those who are at risk of complications, especially bleeding symptoms, which are linked to tumor size, angiogenic component grade, and presence of TSC [8].
Since neoplasia could not be ruled out, more than 90% of sporadic renal AMLs were treated with complete nephrectomy prior to 1976 [10]. Thanks to the advancements in cross-sectional imaging, and even in cases of low-fat containing tumors, renal AMLs can now be accurately diagnosed with an MRI specificity of up to 99% [11].
SAE has become the new norm for preventive or emergency treatment of renal AMLs with minimally invasive selective targeting of small arterial feeders after the first study by Adler et al. [12]. Soulen et al. confirmed in 1991 that embolization of renal angiomyolipomas is safe and well tolerated, and that it can help avoid life-threatening hemorrhage [13, 14].
There is limited literature compared complete nephrectomy and nephron-sparing surgery with SAE in the treatment of renal AMLs [15, 16] with medical and economic analysis supporting SAE in symptomatic renal AMLs or renal AMLs at high-risk of complications.
When compared to partial nephrectomy (12%), SAE for renal AMLs has less post-operative morbidity (6.9%), is minimally invasive, and needs shorter hospitalization [12, 17]. It also allows for rapid stabilization in cases of acute hemorrhage while preserving maximum renal function by sparing the normal renal parenchyma, which is particularly critical in TSC patients. Surgery allows for complete tumor resection and pathologic examination to confirm diagnosis, but in some cases with complicated vascular anatomy, hilar position, or lesions, surgery remains difficult [18]. Nephron-sparing surgery can be considered in the event of primary or repeated SAE failure [19].
Our study was a prospective descriptive mono-centric study that included 33 patients with renal AMLs diagnosed by various radiological modalities (CT, MRI, and US of the kidneys) and underwent 38 arteriographies for 36 renal AMLs that were managed by SAE. While Bardin et al. [8] included 23 patients who underwent SAE of 34 lesions.
Patients’ ages in our study ranged from 12 to 64 years with mean age ± SD of 38.27 ± 13.9 years with a female predominance (n = 24; 72.7%), similar results were mentioned by Bardin et al. [8], Duan et al. [9] and Hocquelet et al. [20] in their carried out studies. These results demonstrate that renal AMLs have a strong female predilection.
Our study included (n = 12; 36.4%) patients with TSC, a similar percentage were reported by Bardin et al. [8] that included (n = 6; 26.1%).
Bleeding symptoms in our study as intra-lesional, retro-peritoneal bleeding and/or gross hematuria (77.1%, 57.1% and 34.3%, respectively) were the most serious complication of renal AMLs and the main indication for urgent SAE, while loin pain and lesion size were common indications in all embolizations in our study.
The majority of SAE in our study were done as an acute management of bleeding renal AMLs (n = 29; 82.9%), while the remaining embolizations were done as a prophylaxis of high-risk renal AMLs (n = 3; 8.6%) or as a pre-operative adjunct treatment for surgery to prevent intra-operative blood loss (n = 3; 8.6%).
Hocquelet et al. [20] focused on prophylactic SAE of high-risk renal AMLs in 19 patients and Duan et al. [9] focused on urgent SAE of bleeding renal AMLs in 25 patients. Bardin et al. [8] discussed both prophylactic and urgent SAE of renal AMLs in 23 patients (73.9% versus 26.1%). Hongyo et al. reported that prophylactic selective SAE for AMLs has good tumor-reduction effects, especially for AMLs with tumor diameter < 70 mm [21], while very limited studies discussed preoperative SAE of renal AMLs. Wang et al. reported that Nephron sparing surgery with preoperative SAE can be considered a viable and effective treatment option for giant renal AMLs, for it avoids excess blood loss and shortens warm ischemia time during operative management [22].
Since Oesterling et al. [23] reported that 82–94% of lesions larger than 4 cm were symptomatic and 50–60 percent bleed spontaneously, the threshold diameter for prophylactic therapy has been 4 cm [10] for several years. However, this historical threshold has since been discussed by authors recommending treatment for asymptomatic tumors greater than 6 [24] or 8 cm [25] as the rate of symptomatic renal AMLs > 4 cm seems to have been over-evaluated in old records [26].
Recent research suggests that although tumor size is significant, type of the lesion and size of associated intra-lesional aneurysms may be more significant regarding the risk of progression and bleeding of renal AMLs [8].
Yamakado et al. [27] discovered that the estimated cut-off of 4 cm had significantly lower specificity (38%) than aneurysm of 5 mm or greater (86%) in their multiple regression analysis, and that aneurysm size was the only factor significantly related to rupture (p = 0.001). CT scans may reveal intra-lesional aneurysms but small ones are easier to be detected with conventional angiography [28].
In our study, bleeding symptoms were significantly more frequent with TSC-associated lesions (p = 036) and with intra-lesional aneurysms (p = 0.013) confirming the importance of tumor type.
We reported that prophylactic SAE to decrease the risk of bleeding can be considered in TSC-associated renal AMLs as well as patients with intra-lesional aneurysm more than 3 mm in diameter.
The type of embolization agent used is primarily determined by the degree of vascularization, artery size, existence of aneurysms, and arterial distribution of the treated AML. Aneurysms are treated with microcoils on a regular basis. The downstream vascular bed can be excluded using microspheres and absolute alcohol. Microspheres were used systemically to reduce tumor flow, allowing minimal levels of absolute alcohol to be infused without causing complications. To prevent reperfusion and recurrence, proximal occlusion of feeding arteries with microcoils was desired wherever possible [20].
To our knowledge, microcoils are the most commonly used embolic agent [29, 30], and the most available in our hospital. However, glue, thrombin, and particles are less commonly used [31].
In our study, we used various embolic agents; microcoils only were used in embolization of (n = 20; 57.1%) lesions, while microspheres only were used in embolization of (n = 4; 11.4%) lesions. Embolic agent combinations were used as [microcoils and microspheres] in embolization of (n = 7; 20%) lesions and [microcoils and absolute alcohol] in embolization of (n = 4; 11.4%) lesions. Microcoils were the most embolic agents used in our study.
There was no difference in tumor size reduction or the need for re-embolization in studies comparing smaller and larger embolic agents for SAE of renal AMLs [32] and this coincided with our study results (p = 0.715).
We encountered low complication rates; major complications (n = 1; 2.6%) and minor complications (n = 14; 36.8%). Our results were comparable to those described by Chick et al. [13] that reported low major complication rates (n = 1; 2.90%), while our results were favorable compared with those described by Bardin et al. [8] that reported higher major complication rates (n = 3; 13.00%) and this may be due to heightened attention of post-procedure care during our study.
Just one patient developed a major complication, a renal abscess formation because of necrosis and liquefaction of tumor after SAE, which was managed with IV antibiotics and percutaneous drainage.
PES, an inflammatory reaction results in pain and fever that can last for many days after SAE, was the most common minor complication (n = 12; 31.6%). Analgesics were used to treat any patient pain and/or fever. Bissler et al. [33] used a short-term tapering dose of prednisone over a two week period after SAE of renal AMLs instead of acetaminophen and it seemed to decrease PES and improved patient comfort. Other minor complications, as puncture site hematoma (n = 1; 2.6%) and persistent hematuria (n = 1; 2.6%) were treated with conservative measures.
We defined overall success as no intra-operative blood loss in pre-operative SAE and no recurrence on follow-up visits in prophylactic and urgent SAE regardless the number of arteriographies done for complete embolization of the lesion, while recurrence was defined as recurrent bleeding symptoms no increase in tumor size.
In addition, we defined primary (technical) success as immediate and complete devascularization of the lesion on control angiogram in the first instance with no need for re-embolization.
We were able to achieve an overall success rate of 91.7% in our study as 33/36 renal AMLs were successfully embolized with no recurrence on follow-up visits. Meanwhile 2 lesions showed recurrence on follow-up visits; one showed recurrent bleeding symptoms and the other showed an increase in its size. Both recurrent lesions were treated with total nephrectomy and were sent to pathologic analysis; the first lesion was diagnosed as AML with xantho-granulomatous changes and the other was diagnosed as epithelioid AML variant. Only one renal AML was not embolized as it was difficult reaching the arterial feeder and underwent total nephrectomy.
Our study demonstrated a primary (technical) success rate of 86.9% as 33/38 arteriographies were done with successful embolization in the first instance, while four re-embolizations were necessary during early follow-up due to incomplete or failed embolizations and one failed embolization underwent total nephrectomy without re-embolization.
With 91.7% overall success rate, 86.9% technical success rate, 10.5% re-embolization and 5.5% recurrence, our results were to some extent favorable compared with those previously reported by Bardin et al. [8] with 17.4% re-embolization and 13% recurrence and Hocquelet et al. [8, 20] with 10.5% re-embolization and 10.5% recurrence.
Several studies found that no significant changes in serum creatinine levels before and after SAE [8, 9, 20]. Our results were similar confirming the safety and efficacy of SAE in renal AMLs as regard preservation of renal functions on follow-up visits. Median serum creatinine levels before SAE were 0.8 (0.5–2.8) and after SAE was 0.70 (0.40–3.1) mg/dL. Just four patients had renal impairment before SAE and had baseline creatinine levels above 1.8 mg/dL. Only one patient, after SAE serum creatinine level rose in a mildly and self-limiting way at early follow-up visits and recovered his initial value in a few days.
To our knowledge, no studies put an eye on hemoglobin levels and leucocytic count before and after SAE in renal AMLs. However, in our study, there were no significant changes in hemoglobin levels and leucocytic count before and after SAE on follow-up visits. These results demonstrated the safety and efficacy of SAE in renal AMLs as regard controlling the hemodynamic status of the patients on follow-up visits with considerable minimally invasive and aseptic procedure.
Whereas we used tumor size-reduction as a predictor of successful embolization, our study showed a statistically significant tumor size-reduction after SAE; maximum diameter reduction rate of 16% and volume reduction rate of 48.44% (p < 0.001). We also found a positive correlation between tumor size-reduction and initial tumor size (p = 0.035). Although our results were satisfactory but they were to some extent less than those reported by other authors in their carried out studies [8, 9, 20] as our study was a prospective study with short and variable follow-up [mean follow-up of 6.91 ± 3.41 (1–12) months] unlike their retrospective studies with relatively intermediate to long follow-up.
In their study, Hocquelet et al. [20] found that the percentage of fat content before SAE was a predictive factor of volume reduction with a volume reduction significantly more important for renal AMLs with less than 50% of fat than for those with more than 50% of fat (p < 0.00001). Only the percentage fat content (p0.0001) was found to be the best predictor of volume decrease in their multivariate study. Unfortunately, due to the limited number of unenhanced CT scans required for density histogram calculations, we were unable to test this theory. However, only two “fat-poor” renal AMLs in our study showed marvelous tumor size reduction after SAE on follow-up imaging, with 75.38% and 44.32%, respectively.
Limitations
This study has some limitations, first, our population was heterogeneous with sporadic cases (n = 21) and TSC patients (n = 12) as well as different indications of SAE; urgent (n = 29) versus prophylactic (n = 3) and preoperative (n = 3) embolizations.
Second, as in other studies, we encountered difficulty to measure with accuracy large renal AMLs associated with intra-lesional and/or retro-peritoneal bleeding, particularly as several modalities were used (US, CT and MRI). Data were particularly difficult to collect in cases of ruptured renal AML which was often the mode of discovery of emergency cases.
Furthermore, the pre-arteriography images of some patients were taken from other hospitals with different equipment than those available in our hospital.
Finally, our research was a prospective study with short and variable follow-up period, but this should have little bearing on our results because the bulk of tumor size-reduction happens during the first few years after SAE [34]. This was confirmed in two out of the last three patients of our study with short-term follow-up; the first one presented a 16.41% tumor size-reduction two months after SAE and the second presented an impressive 38.4% tumor size-reduction one month after SAE, while the third underwent SAE as a pre-operative adjunct before partial nephrectomy. Nevertheless, long-term follow-up should be performed to define durable efficacy after successful SAE of renal AMLs.
Conclusions
SAE is an effective and safe technique for managing renal AMLs preventively or in emergency to treat bleeding with significant decrease in tumor size, low recurrence rates and acceptable complications as well as preservation of renal functions.
Based on our results, the type of renal AMLs, especially TSC-related lesions and intra-lesional aneurysms > 3 mm in diameter, were found to be significantly more associated with bleeding symptoms in our research. As a result, we conclude that, in addition to size, the type of lesion and the presence of aneurysms larger than 3 mm should be included in treatment plans, and that they can be used as important predictors of prophylactic SAE in non-complicated AML situations.
In every case, discussion between interventional radiologists and urologists is essential to determine the optimal management.
Long-term follow-up is required to determine long-term effectiveness after successful SAE of renal AMLs and further research is needed to determine the precise role of all the currently available therapeutic modalities with a particular emphasis on TSC-associated lesions, which are typically larger, numerous, and therefore more difficult to be managed.
Availability of data and materials
Due to privacy regulations, the clinical data collected in this study are not deposited in a public registry, but the data can be made available via a request to the corresponding author.
Abbreviations
- AML:
-
Angiomyolipoma
- TSC:
-
Tuberous sclerosis complex
- SAE:
-
Selective arterial embolization
- LAM:
-
Lymphangio-leiomyomatosis
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- US:
-
Ultrasonography
- mTOR:
-
Mammalian target of rapamycin
- INR:
-
International normalized ratio
- PES:
-
Post-embolization syndrome
References
Flum AS, Hamoui N, Said MA, Yang XJ, Casalino DD, McGuire BB et al (2016) Update on the diagnosis and management of renal angiomyolipoma. J Urol 195(4 Part 1):834–846
Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M (2014) Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging 39(3):588–604
Vos N, Oyen R (2018) Renal angiomyolipoma: the good, the bad, and the ugly. J Belgian Soc Radiol 102(1)
Wang C, Yang M, Tong X, Wang J, Guan H, Niu G et al (2017) Transarterial embolization for renal angiomyolipomas: a single centre experience in 79 patients. J Int Med Res 45(2):706–713
Schieda N, Kielar A, Al Dandan O, McInnes M, Flood T (2015) Ten uncommon and unusual variants of renal angiomyolipoma (AML): radiologic–pathologic correlation. Clin Radiol 70(2):206–220
Katada Y, Umehara I, Ohki T, Kishino M, Shibuya H (2009) Bilateral renal angiomyolipoma in a patient with tuberous sclerosis treated with resection of one kidney and transarterial embolization of other kidney using CT during selective arteriography: a case report. Cases J 2(1):6351
Sivalingam S, Nakada SY (2013) Contemporary minimally invasive treatment options for renal angiomyolipomas. Curr Urol Rep 14(2):147–153
Bardin F, Chevallier O, Bertaut A, Delorme E, Moulin M, Pottecher P et al (2017) Selective arterial embolization of symptomatic and asymptomatic renal angiomyolipomas: a retrospective study of safety, outcomes and tumor size reduction. Quant Imaging Med Surg 7(1):8–23
Duan X-H, Zhang M-F, Ren J-Z, Han X-W, Chen P-F, Zhang K et al (2016) Urgent transcatheter arterial embolization for the treatment of ruptured renal angiomyolipoma with spontaneous hemorrhage. Acta Radiol 57(11):1360–1365
Castle SM, Gorbatiy V, Ekwenna O, Young E, Leveillee RJ (2012) Radiofrequency ablation (RFA) therapy for renal angiomyolipoma (AML): an alternative to angio-embolization and nephron-sparing surgery. BJU Int 109(3):384–387
Sasiwimonphan K, Takahashi N, Leibovich BC, Carter RE, Atwell TD, Kawashima A (2012) Small (< 4 cm) renal mass: differentiation of angiomyolipoma without visible fat from renal cell carcinoma utilizing MR imaging. Radiology 263(1):160–168
Adler J, Greweldinger J, Litzky G (1984) “Macro” aneurysm in renal angiomyolipoma: two cases, with therapeutic embolization in one patient. Urol Radiol 6(3–4):201–203
Chick CM, Tan BS, Cheng C, Taneja M, Lo R, Tan YH et al (2010) Long-term follow-up of the treatment of renal angiomyolipomas after selective arterial embolization with alcohol. BJU Int 105(3):390–394
Soulen MC, Faykus MH Jr, Shlansky-Goldberg RD, Wein AJ (1994) Cope CJJoV, radiology I. Elective embolization for prevention of hemorrhage from renal angiomyolipomas. J Vasc Intev Radiol 5(4):587–591
Huyghe E, Dechier MC, Mottier ML, Rischmann P, Otal P, Soulie M et al (2010) 1365 Management of renal angiomyolipoma: medical and cost effectiveness comparison of selective embolization and surgery. J Urol 4(183):e527
Faddegon S (2011) Treatment of angiomyolipoma at a tertiary care centre: the decision between surgery and angioembolization. Can Urol Assoc J 5(6):138–141
Boorjian SA, Frank I, Inman B, Lohse CM, Cheville JC, Leibovich BC et al (2007) The role of partial nephrectomy for the management of sporadic renal angiomyolipoma. Urology 70(6):1064–1068
Seyam RM, Bissada NK, Kattan SA, Mokhtar AA, Aslam M, Fahmy WE et al (2008) Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology 72(5):1077–1082
Luca D, Rossetti R (1999) Management of renal angiomyolipoma: a report of 53 cases. BJU Int 83(3):215–218
Hocquelet A, Cornelis F, Le Bras Y, Meyer M, Tricaud E, Lasserre A et al (2014) Long-term results of preventive embolization of renal angiomyolipomas: evaluation of predictive factors of volume decrease. Eur Radiol 24(8):1785–1793
Hongyo H, Higashihara H, Osuga K, Kashiwagi E, Kosai S, Nagai K et al (2020) Efficacy of prophylactic selective arterial embolization for renal angiomyolipomas: identifying predictors of 50% volume reduction. CVIR Endovasc 3(1):1–7
Wang D, Li H, Ji Z (2017) Nephron-sparing surgery with preoperative selective arterial embolization in management of giant renal angiomyolipomas. Int J Clin Exp Med 10(3):4905–4912
Oesterling JE, Fishman EK, Goldman SM, Marshall FF (1986) The management of renal angiomyolipoma. J Urol 135(6):1121–1124
Kuusk T, Biancari F, Lane B, Tobert C, Campbell S, Rimon U et al (2015) Treatment of renal angiomyolipoma: pooled analysis of individual patient data. BMC Urol 15(1):123
Dickinson M, Ruckle H, Beaghler M, Hadley H (1998) Renal angiomyolipoma: optimal treatment based on size and symptoms. Clin Nephrol 49(5):281–286
Bhatt JR, Richard PO, Kim NS, Finelli A, Manickavachagam K, Legere L et al (2016) Natural history of renal angiomyolipoma (AML): most patients with large AMLs > 4 cm can be offered active surveillance as an initial management strategy. Eur Urol 70(1):85–90
Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K (2002) Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology 225(1):78–82
Yip K, Peh W, Tam P (1998) Spontaneous rupture of renal tumours: the role of imaging in diagnosis and management. Br J Radiol 71(842):146–154
Etezadi V, Gandhi RT, Benenati JF, Rochon P, Gordon M, Benenati MJ et al (2011) Endovascular treatment of visceral and renal artery aneurysms. J Vasc Interv Radiol 22(9):1246–1253
Minocha J, Parvinian A, Bui JT, Knuttinen MG, Ray Jr CE, Gaba RC (2015) Transcatheter renal interventions: a review of established and emerging procedures. J Clin Imaging Sci 5
Hislop SJ, Patel SA, Abt PL, Singh MJ, Illig KA (2009) Therapy of renal artery aneurysms in New York State: outcomes of patients undergoing open and endovascular repair. Ann Vasc Surg 23(2):194–200
Villalta JD, Sorensen MD, Durack JC, Kerlan RK, Stoller ML (2011) Selective arterial embolization of angiomyolipomas: a comparison of smaller and larger embolic agents. J Urol 186(3):921–927
Bissler JJ, Racadio J, Donnelly LF, Johnson ND (2002) Reduction of postembolization syndrome after ablation of renal angiomyolipoma. Am J Kidney Dis 39(5):966–971
Patatas K, Robinson G, Ettles D, Lakshminarayan R (2013) Patterns of renal angiomyolipoma regression post embolisation on medium-to long-term follow-up. Br J Radiol 86(1024):20120633
Acknowledgements
The authors thank all colleagues and technicians in the Radiology Department, Urology and Nephrology center, Mansoura University, for their helpful cooperation and all the study participants for their patience and support.
Funding
This research received no funding or specific grant from any funding agency.
Author information
Authors and Affiliations
Contributions
RA, ME, TA and TE were the guarantors of integrity of the entire study. RA, ME and SA were involved in manuscript preparation, study concept and design. RA and ME were involved in clinical studies. ME, RT and TA were involved in experimental studies/data analysis. ME, SA and TA were involved in statistical analysis. RA, ME and TE were involved in manuscript editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional research board of faculty of medicine, University of Mansoura, approved the study (proposal code: MS.18.10.323).
Consent for publication
A written consent to publish this information was obtained from study participants.
Competing interests
The authors declare that they have no relevant conflicts of interest, and no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abouelkheir, R.T., El-Ksas, M., Abdel Fattah, S. et al. Efficacy and safety of selective renal arterial embolization in renal angiomyolipoma: a prospective single-center study. Egypt J Radiol Nucl Med 53, 165 (2022). https://doi.org/10.1186/s43055-022-00848-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00848-3