Abstract
Background
One of the drawbacks in contrast-enhanced T1-weighted imaging (CE-T1WI) is the enhancing cortical vessels which can be confused with meningeal enhancement. Previous studies reported that post-contrast FLAIR could be better for diagnosing the superficial brain abnormalities. So the purpose of this study was to evaluate the role of delayed post-contrast FLAIR, in comparison with post-contrast T1, in the detection and evaluation of brain metastases.
Results
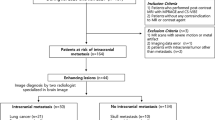
The study was conducted on 40 patients with suspected/known brain metastases scanned in order to detect and evaluate brain metastases. All patients were subjected to the following: full history taking, review of clinical examination reports, and other imaging modalities whenever available, followed by brain MRI examination using 1.5 T closed magnet including pre-contrast series, axial and sagittal T1-weighted spin echo (SE), axial and coronal T2-weighted turbo spin echo (TSE) and axial FLAIR, while post-contrast series included axial, coronal and sagittal T1-weighted spin echo (SE) and lastly DPC-FLAIR sequence 10 min after contrast administration. This study included 18 males and 22 females, ranging in age from 26 to 75 years. Six out of a total of 40 patients had brain metastases of unknown origin, while 34 of them were presented with different types of known primary tumors. The detected lesions were subdivided into five groups according to their detectability by DPC-FLAIR and contrast-enhanced T1WI: Group (I): lesions detected only by DPC-FLAIR: 16 lesions; Group (II): lesions detected only by CE-T1WI: 1 lesion; Group (III): lesions detected by both DPC-FLAIR and CE-T1WI with equal conspicuity by both: 28 lesions; Group (IV): lesions detected by both, showing more obvious enhancement with DPC-FLAIR: 43 lesions; and Group (V): lesions detected by both, showing more obvious enhancement with CE-T1WI: 11 lesions. DPC-FLAIR had a sensitivity of 98.98% and a specificity of 100% for the detection of metastatic brain lesions and for CE-T1WI; sensitivity of 83.83%; and a specificity of 50%.
Conclusions
Delayed post-contrast FLAIR is a reliable sequence for the detection of metastatic brain lesions as it can detect more metastatic brain lesions compared to contrast-enhanced T1WI.
Similar content being viewed by others
Background
The most common malignancy of the brain is the metastatic brain tumor which is considered an important cause of morbidity and mortality. In the autopsy of the metastatic cancer patients, the cerebral metastasis is represented by 20–40% [1]. Most of the brain metastatic patients have a known primary cancer (metachronous presentation) which is mostly located in the lung, breast or gastrointestinal tract. All are considered the most common sites of the primary tumor of the brain metastasis. However, in 5–10% of brain metastatic patients, no primary tumor is detected [2].
Contrast-enhanced T1-weighted imaging (CE-T1WI) is the preferred magnetic resonance sequence in contrast imaging at most organizations [3]. One of the CE-T1WI sequences’ drawbacks is the enhancing cortical vessels which can be confused with meningeal enhancement. Hence, and based on the preliminary experience of the preceding studies, it is proposed that post-contrast FLAIR could be better for diagnosing the superficial brain abnormalities, especially meningeal disease [4].
In addition, various clinical studies showed that DPC-FLAIR usually offers more information than CE-T1WI alone [3]. FLAIR image sequence is an unusual sequence acquired by applying long TR (time to repetition), long TE (time to echo) and inversion time (TI) that suppresses successfully the CSF signals at all sites including sulcal spaces leading to better recognition and demarcation of the lesions, especially which is lying adjacent to or abutting the borders of meninges, particularly sulcal spaces as variable hyperintensities. Now, the mild T1-weighting of T2 FLAIR is also useful to the post-contrast FLAIR images in lesions demarcation, making it a highly sensitive sequence in diagnosing meningeal inflammation, infections, and carcinomatosis [5].
Previous studies suggested that in order to improve the diagnosis of leptomeningeal tumoral diseases, DPC-FLAIR images and delayed post-contrast T1 images are preferably used, especially when compared to unenhanced FLAIR images, or early enhanced T1 images [6].
Other studies proved that delayed post-contrast MR images increase sensitivity for detection of a larger number of lesions than immediate post-contrast images, especially lesions smaller than 5 mm [7].
So the purpose of this study will be to evaluate the role of delayed post-contrast FLAIR (DPC-FLAIR), in comparison with post-contrast T1 (CE-T1WI), in the detection and evaluation of brain metastases.
Methods
The study was prospective and carried out on 40 patients with suspected or known metastatic brain disease referred to the Radiodiagnosis Department in our university hospitals as well as private radiobiology centers in our city for MRI evaluation, in the period between June 2018 and April 2020.
Exclusion criteria included patients contraindicated for MRI study (claustrophobia, MRI non-compatible devices) and patients contraindicated for intravenous contrast injection (those with renal impairment or history of allergy to gadolinium).
All patients included in this study were subjected to the following: Thorough history taking, revision of full clinical examination reports, review of previous imaging studies as well as laboratory data if available, principles of medical ethics were checked and followed and informed consents were obtained from each patient or his relatives.
All the patients were examined using Philips Ingenia 1.5 T (Philips Medical System, Amsterdam, the Netherlands) and Signa Explorer 1.5 T (GE medical system, USA) by applying a quadrangular head coil. The contrast material used in the study was Gadolinium diethylenetriamine pentaacetic acid (Gad-DTPA) (Magnevist). The dose was 0.1 mmol/kg body weight injected manually utilizing a butterfly needle or cannula.
Each MRI examination included: pre-contrast series, axial and sagittal T1-weighted spin echo (SE), axial and coronal T2-weighted turbo spin echo (TSE) and axial FLAIR, while post-contrast series included axial, coronal and sagittal T1-weighted spin echo (SE) and lastly DPC-FLAIR sequence 10 min after contrast administration. This 10-min delay timing of image acquisition following contrast acquisition was unified to ensure standardization of the technique in different centers where the study was conducted.
The following parameters were utilized: slice thickness 4 mm, interslice gap 0.4 mm, field of view (FOV) 230–240 mm2, rectangular field of view (RFOV) 80%, and number of signals averaged (NSA): 2 to 3. For T1W sequences: repetition time (TR) = 565 ms; echo time (TE) = 12 ms. For T2W sequences: TR = 6629 ms; TE = 110 ms; turbo spin echo (TSE) factor = 12.5. For FLAIR: TR = 10,000 ms; TE = 94 ms, TI = 2500 ms.
Image analysis was conducted by and an expert neuroradiologist with 15 years of experience who was asked to assess both detectability of enhancing lesions in both CET1WI and DPC-FLAIR and compare the degree of conspicuity of enhancement for each lesion in both sequences.
Data were collected, coded, revised and entered to the Statistical Package for R Studio software version 3.6.2. (Dark and stormy night copyright ©). The data were presented as number and percentages for the qualitative data, mean, standard deviations and ranges for the quantitative data with parametric distribution, and median with interquartile range (IQR) for the quantitative data with the nonparametric distribution. Chi-square test was used in the comparison between two groups with qualitative data, and Fisher’s exact test was used instead of the chi-square test when the expected count in any cell is found to be less than 5. ROC curve analysis was used to obtain the sensitivity and specificity.
AUC statistics were tabled on the basis of a control group of the non-contrast sequences.
The confidence interval was set to 95%, and the margin of error accepted was set to 5%. So, the p value was considered significant as the following: P > 0.05: nonsignificant (NS), P < 0.05: significant (S) and P < 0.01: highly significant (HS).
Results
The current work is a prospective study conducted on 40 patients with suspected or known metastatic brain disease. Our study included 18 males (45%) and 22 females (55%). The 40 patients’ ages ranged between 26 and 72 years (mean 56.23 years with standard deviation 11.04 years and median 57).
Out of the 40 studied patients, 30 patients (75%) had extracranial systemic metastasis. Ten percent of the patients (4 out of 40) had nodal metastasis. Two patients had bone metastasis (5%), and also another two patients had liver, lung and bone metastasis. One patient (2.5%) had calvarial, spine and nodal metastasis, and one patient (2.5%) had pulmonary and bone metastasis.
The most common primary lesion was breast cancer in 15 patients (37.5%). The second most common primary lesion was lung cancer in six patients (15%). The remaining sites were non-Hodgkin lymphoma (12.5%), prostate (7.5%), bladder (5%), colon (5%) and orbit (2.5%). The site of primary lesion was unknown in six patients (15%) at the time of brain examination.
All the studied patients (except two patients (5%) who had no significant neurological symptoms) presented with a range of clinical presentation occurring separately or in conjugation with other symptoms. The most common clinical presentation was headache in 13 patients out of 40 patients (32.5%), followed by vomiting in nine patients (22.5%), followed by seizures, vertigo and cognitive dysfunction in eight, five and three patients (20.5%, 12.5% and 7.5%), respectively.
The 40 patients had a total of 99 brain metastatic lesions distributed as follows: Twenty patients had solitary lesions (n = 20), eight patients had two lesions (n = 16) and 12 patients had more than two lesions (the remaining 63 lesions). The distribution of the lesions regarding locations, size and shapes is detailed in Table 1.
The enhancement patterns of the included 99 lesions were either solid homogeneous (57.5%), peripheral ring (20.2%), leptomeningeal (15%) or pachymeningeal (7.1%).
The DPC-FLAIR demonstrated 16 enhancing lesions that were not detected by post-contrast T1 (Figs. 1 and 2). On the other hand, CE-T1WI detected one enhancing subependymal lesion that was not detected by DPC-FLAIR (Fig. 3).
Male patient aged 49 years, with known prostatic cancer, presented with dizziness and vertigo. The right cerebellar hemisphere shows leptomeningeal enhancement, which is noted only at delayed post-contrast FLAIR(D red circle), with no enhancement seen in post-contrast T1 (C). A: Axial pre-contrast T1WI. B Axial FLAIR., C Axial CE-T1WI. And D Axial DPC-FLAIR
Female patient aged 28 years with known breast cancer. MRI study of the brain revealed subependymal tiny nodule noted at the right lateral ventricle, that shows enhancement on the CE-T1WI (C) but no enhancement detected on DPC-FLAIR (D). A Axial pre-contrast T1WI. B Axial FLAIR. C Axial CE-T1WI. D Axial DPC-FLAIR
Among the remaining 82 enhancing lesions that were detected by both CE-T1WI and DPC-FLAIR, 28 lesions showed equal conspicuity by both sequences (Fig. 4), 43 lesions showed more obvious enhancement in the DPC-FLAIR than in post-contrast T1 (Fig. 5), while the remaining 11 lesions showed more obvious enhancement in CE-T1WI than in DPC-FLAIR. (Fig. 6).
A 52-year-old female patient with unknown primary. MRI study of the brain revealed a T1 hypo-intense lesion is noted at the left cerebellar hemisphere that shows minimal surrounding vasogenic edema on FLAIR. It shows better enhancement on DPC-FLAIR (D red circle) compared to the post-contrast T1WI (C). A Axial pre-contrast T1WI. B Axial FLAIR. C Axial CE-T1WI. D Axial DPC-FLAIR
A 51-year-old female patient with breast cancer. MRI of the brain shows an intra-axial focal lesion, epicentered upon the right side of tectum of the midbrain, with homogeneous intralesional post-contrast enhancement on CE-T1WI (C red circle). DPC-FLAIR demonstrates less intense lesion enhancement (D red circle). A Axial pre-contrast T1WI. B Axial FLAIR. C Axial CE-T1WI. D Axial DPC-FLAIR
DPC-FLAIR was superior to CE-T1WI in detecting leptomeningeal and tiny cortical lesions while the reverse is true for subependymal lesions, while both DPC-FLAIR and CE-T1WI had more or less similar performance in subcortical and infratentorial metastatic lesions.
So we have classified the 99 metastatic lesions into five groups according to the enhancement conspicuity which are detailed in Tables 1 and 2.
In order to identify the significance of DPC-FLAIR as compared to post-contrast T1, ROC analysis was performed and revealed that DPC-FLAIR had a sensitivity of 98.98% and a specificity of 100%. The estimated p value was < 2.2e−16 for the detectability of metastatic brain lesions for DPC-FLAIR (Table 3), while CE-T1WI had a sensitivity of 83.83% and a specificity of 50%. The estimated P value was 1.65e−11 for the detectability of metastatic brain lesions (Table4).
Our results showed that DPC-FLAIR was significantly more valuable in detection of metastatic brain lesions compared to CE-T1WI (p value < 0.001).
Discussion
Intracranial metastasis is considered a common neoplasm representing up to 40% of all adult brain neoplasms and 25% of all types of metastases [8]. For cancer patients, both the survival time and the quality of life are determined by the good management of brain metastases. The successful brain metastases management is dependent on different factors such as the extent of the primary tumor, the extent of systemic disease, and the number as well as the location of metastases [9].
In case of cancer patients with single brain metastasis, surgical resection followed by postoperative whole-brain radiation therapy (WBRT) is the treatment of choice providing a suitable site for resection and limited progress of cancer. On the other hand, in case of patients with multiple brain metastases (> 3 lesions), the preferred treatment is the WBRT alone. However, the treatment of choice in oligometastases (2–3 lesions) remains controversial whether WBRT alone, radiosurgery alone, or both [9].
In about 20% of patients with malignancy, brain metastases are diagnosed concurrently or even before the diagnosis of the primary lesion. Although the metastases are growing rapidly, they may remain small for several years. This proposes that at the time of diagnosis of the primary tumor, the intracranial metastasis may have been already existed; however, due to the lesion small size, it could not be detected easily by the traditional radiological scans [8].
In approximately 50% of all patients with intracranial metastases, the routine radiological imaging may demonstrate only a solitary lesion. So it is necessary for optimal management of brain metastases; all the other occult lesions must be detected [8].
Recently, the era of using post-contrast FLAIR sequences in intracranial lesions has attracted the attention of neuroradiologists [10,11,12,13].
We studied 40 cases, having 99 metastatic lesions, that were subdivided into five groups according to the number and conspicuity of metastatic brain lesions with DPC-FLAIR, compared with CE-T1WI, among them 16 lesions were only detected by DPC-FLAIR, one lesion was detected only by CE-T1WI, and the remaining 82 lesions were detected by both CE-T1WI and DPC-FALIR and were further subdivided into three groups; a group with better detection with CE-T1WI (11 lesions), a group better detected by DPC-FLAIR (43 lesions) and lastly a group with equal conspicuity with both CE-T1WI and DPC-FLAIR (28 lesions).
Our results indicate that the addition of DPC-FLAIR imaging to pre- and CE-T1WI increases diagnostic confidence in the evaluation of brain metastases. DPC-FLAIR had a high AUC value of 74.5%, a sensitivity of 98.98%, and a specificity of 100% for the detection of metastatic brain lesions.
In the current study DPC-FLAIR was superior to CE-T1WI in detecting leptomeningeal and tiny cortical lesions, while the reverse is true for subependymal lesions. On the other hand, both DPC-FLAIR and CE-T1WI had more or less similar performance in subcortical and infratentorial metastatic lesions.
Regarding cortical and subcortical lesions, in our study, eight tiny cortical lesions were detected only by DPC-FLAIR, four showed more evident enhancement with DPC-FLAIR compared to CE-T1WI, and only two tiny cortical lesions were of equal enhancement intensity in both T1 and DPC-FLAIR.
Essig et al. [10] in a study including 28 patients, (57% with enhancing gliomas, and 43% with cerebral metastases), found that the small subcortical lesions were not easily detected by water-sensitive techniques such as T2-weighted fast SE or fast DPC-FLAIR imaging. Significantly more metastases were detected with contrast-enhanced fast DPC-FLAIR images than with non-enhanced fast FLAIR and T2- or proton density-weighted fast SE images; however, significantly more metastases were detected with contrast-enhanced T1-weighted SE images than with all other modalities (p < 0.01). They stated the small lesions mostly did not cause vasogenic edema or mass effect, and only showed mild enhancement with contrast. However, DPC-FLAIR was as highly sensitive as T1-weighted SE images to detect the very small cortical lesions because these enhanced lesions are easily detected on CSF suppressed background. However, they reported that lesions > 10 mm could be detected easily on all imaging sequences.
Concerning the meningeal metastases, in our study, DPC-FLAIR images were superior to CE-T1WI in 13 meningeal lesions, among them eight were detected only by DPC-FLAIR, and five lesions were better detected by DPC-FLAIR. DPC-FLAIR was inferior in two meningeal lesions which were pachymeningeal.
These results are in agreement with a study conducted by Park and Ahn [14], who compared between DPC-FLAIR and contrast-enhanced 3D T1 black-blood fast spin echo (FSE) imaging regarding the diagnosis of leptomeningeal metastases. The visual conspicuity of DPC-FLAIR was significantly greater than that of 3D T1-BB FSE (p = 0.014 for reviewer 1 and p = 0.023 for reviewer 2). They suggested the higher sensitivity of DPC-FLAIR over the conventional contrast-enhanced T1 sequence in terms of visualization of leptomeningeal metastases due to the greater ability of DPC-FLAIR to demonstrate lower contrast concentrations than T1W1 images. This is due to the fact that, through the damaged vessels, gadolinium leakage into the adjacent CSF occurred resulting in contrast dilution and accordingly its low concentration. Hence, they concluded the predilection of post-contrast DPC-FLAIR over the standard contrast-enhanced T1.
Yet we disagree with the study conducted by Singh et al. [15] who stated that for diagnosis of intracranial neoplastic leptomeningeal disease, DPC-FLAIR sequences are of lower sensitivity than the conventional contrast-enhanced T1W1 images. The sensitivity and specificity for DPC-FLAIR images for detecting leptomeningeal metastases were 41% and 88%, respectively, and those of contrast-enhanced T1-weighted MR images were 59% and 93%. They supposed that this is because of using standard contrast-enhanced T1W1 MRI instead of magnetization transfer saturation with the T1W1 MRI in the previous studies [11].
Additionally, DPC-FLAIR is highly effective in the diagnosis of parenchymal metastasis. In our study, DPC-FLAIR, regarding parenchymal lesions, was superior in 34 lesions, inferior in nine lesions, and equal in 19 as compared to contrast T1WI.
Similarly, the significance of DPC-FLAIR imaging in the detection of superficial parenchymal lesions was reported by Lee et al. [3] who studied the DPC-FLAIR in several pathological diseases, including intracranial metastases. CSF signal intensity suppression, minimal blood vessels enhancement, diminished phase shift artifacts caused by blood vessels or dural sinuses enhancement, as well as easy detection of peritumoral edema are the causes that appreciate the use of the DPC-FLAIR images in the diagnosis of the superficial and deep metastatic tumors over CE-T1WI [3].
This was in agreement with Ercan et al. [16] who compared between DPC-FLAIR and T1WI in patients with identified or suspected brain metastases. Concerning the number, conspicuity, and parenchymal metastases enhancement, the DPC-FLAIR images were more sensitive than the CE-T1WIimages in five patients. However, these results were allocated to the delayed enhancement as in all patients, the CE-T1WI was firstly performed followed by the DPC-FLAIR.
It was hypothesized that in some patients, with a delay in the imaging time, the intracranial metastatic lesions are filled up with the gadolinium contrast agent, allowing better visualization of more small lesions. The more the imaging time the more leakage of the contrast material through the BBB leading to intralesional gadolinium accumulation with subsequent higher signal intensity [7].
Kushnirsky et al. [17] proposed the delay in imaging could lead to increase signal intensity as the imaging delay permits a longer time for the aberrant and leaky neovasculature inside the metastasis to be perfused with the contrast agent. The minute metastatic foci have a small vascular surface making the contrast diffusion very limited in the first-pass kinetics. However, by the time the contrast material recirculates through the cerebral vasculature leading to additional contrast extravasation [17].
Notwithstanding, the accuracy of CE-T1WI for the diagnosis of subependymal enhancing lesions remains higher than that of the DPC-FLAIR images [6]. In our study, the only lesion which was detected by post-contrast T1 and not by DPC-FLAIR was a tiny subependymal nodule.
So, due to the probability of the presence of leptomeningeal abnormalities with intra-parenchymal or subependymal lesions, it is clear that both CE-T1W1 and DPC-FLAIR sequences should be acquired within the same acquisition protocols.
Furthermore, concerning the CSF flow artifacts, it is well established that T1W1 images are less sensitive to this type of artifact predominantly in the posterior fossa rather than DPC-FLAIR images. Subsequently, to avoid the false-positive diagnosis of leptomeningeal abnormalities, these artifacts must be taken into consideration.
Limitation of our study included relatively small sample size and lack of comparison between delayed CE-T1WI and DPC-FLAIR to obviate the effect of the delay on the contrast concentration within the lesions; however, we still believe that the effect of delay is not the major factor of the higher accuracy of DPG-FALIR because it is more sensitive for detecting enhancing tiny cortical and leptomeningeal lesions by avoiding the confusion caused by enhancing cortical vessels that can be seen on CE-T1WI as well as omitting the bright signal of CSF.
Conclusions
Delayed post-contrast FLAIR is a reliable sequence for the detection of metastatic brain lesions as it can detect more metastatic brain lesions compared to contrast-enhanced T1WI.
We recommend it to be a routine sequence in cases with suspected brain metastasis.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CE-T1WI:
-
Contrast-enhanced T1-weighted imaging
- DPC-FLAIR:
-
Delayed post-contrast FLAIR
- WBRT:
-
Whole-brain radiation therapy
References
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC et al (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. The Lancet 363(9422):1665–1672
Sawaya R, Bindal RK, Lang FF. Metastatic brain tumors. In: Kaye AH, Laws ER (eds). Brain tumors: An encyclopedic approach. 2nd ed: Edinburgh: Churchill Livingstone; 2002. p. 999–1026 p.
Lee EK, Lee EJ, Kim S, Lee YS (2016) Importance of contrast-enhanced fluid-attenuated inversion recovery magnetic resonance imaging in various intracranial pathologic conditions. Korean J Radiol 17(1):127–141
Galassi W, Phuttharak W, Hesselink JR, Healy JF, Dietrich RB, Imbesi SG (2005) Intracranial meningeal disease: comparison of contrast-enhanced MR imaging with fluid-attenuated inversion recovery and fat-suppressed T1-weighted sequences. Am J Neuroradiol 26(3):553–559
Rastogi R, Jain S, Gupta Y, Joon P, Wani A, Pratap V (2016) Can postcontrast-T2FLAIR be a boon over postcontrast-T1GRE images in MR brain imaging. J Neuroinfect Dis 7(2):219
Kremer S, AbuEid M, Bierry G, Bogorin A, Koob M, Dietemann JL et al (2006) Accuracy of DPC-FLAIR MR imaging for the diagnosis of leptomeningeal infectious or tumoral diseases. J Neuroradiol. 33(5):285–91
Fan B, Li M, Wang X, Xu Y, Li F, Zhang L et al (2018) Time-dependent changes in the magnetic resonance detection of brain metastases using single-dose gadobutrol. Iran J Radiol 15(1):e66054
Yuh W, Tali ET, Nguyen HD, Simonson TM, Mayr NA, Fisher DJ (1995) The effect of contrast dose, imaging time, and lesion size in the MR detection of intracerebral metastasis. AJNR 16(2):373–380
Terae S, Yoshida D, Kudo K, Tha KK, Fujino M, Miyasaka K (2007) Contrast-enhanced FLAIR imaging in combination with pre- and postcontrast magnetization transfer T1-weighted imaging: usefulness in the evaluation of brain metastases. J Magn Resonance Imaging JMRI 25(3):479–487
Essig M, Knopp MV, Schoenberg SO, Hawighorst H, Wenz F, Debus J et al (1999) Cerebral gliomas and metastases: assessment with contrast-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 210(2):551–557
Mathews VP, Caldemeyer KS, Lowe MJ, Greenspan SL, Weber DM, Ulmer JL (1999) Brain: gadolinium-enhanced fast fluid-attenuated inversion-recovery MR imaging. Radiology 211(1):257–263
Melhem E, Bert R, Walker R (1998) Usefulness of optimized gadolinium-enhanced fast fluid-attenuated inversion recovery MR imaging in revealing lesions of the brain. AJR 171(3):803–807
Jackson EF, Hayman LA, Mathews V, Lowe M, Ulmer J (2000) Meningeal enhancement on fast FLAIR images [5](multiple letters). Radiology 215(3):922–924
Park YW, Ahn SJ (2018) Comparison of contrast-enhanced T2 FLAIR and 3D T1 black-blood fast spin-echo for detection of leptomeningeal metastases. Magn Reson Imaging 22(2):86–93
Singh SK, Leeds NE, Ginsberg LE (2002) MR imaging of leptomeningeal metastases: comparison of three sequences. AJNR 23(5):817–821
Ercan N, Gultekin S, Celik H, Tali TE, Oner YA, Erbas G (2004) Diagnostic value of contrast-enhanced fluid-attenuated inversion recovery MR imaging of intracranial metastases. AJNR 25(5):761–765
Kushnirsky M, Nguyen V, Katz JS, Steinklein J, Rosen L, Warshall C et al (2016) Time-delayed contrast-enhanced MRI improves detection of brain metastases and apparent treatment volumes. Neurosurgery 124(2):489–495
Acknowledgements
The authors would like to acknowledge Mr. Mohamed Abdelaziz, MRI technician in the Department of Radio-diagnosis, Faculty of Medicine, University of Alexandria, Egypt, for his efforts in MRI examinations of our patients.
Funding
None.
Author information
Authors and Affiliations
Contributions
AE (the corresponding author) is responsible for ensuring that the descriptions are accurate and agreed by all authors. All authors (RI, SA and AE) had made substantial contributions to all of the following: (1) the conception and design of the radiological work, (2) the acquisition, analysis and interpretation of radiological data; (3) drafting the work and revising it; (4) conduction of revision and corrections as per reviewers’ comments. All authors have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors approved the revised version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The medical ethics were considered and respected. The study was approved by Institutional Ethics Committee in Faculty of Medicine, Alexandria University IRB No: (00007555), FWA No: (00015712). Patient consents for participation in this research were signed by the patients or their relatives after explaining the procedures and risks with assuring respect of both patient and medical records confidentiality.
Consent for publication
Not applicable.
Competing interests
None of the authors has competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ibrahim, R.M., Rahman, S.A.A. & Elnekeidy, A.E.A.M. Added value of delayed post-contrast FLAIR in diagnosis of metastatic brain lesions. Egypt J Radiol Nucl Med 53, 205 (2022). https://doi.org/10.1186/s43055-022-00844-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00844-7