Abstract
Background
A retrospective study of 44 patients with autologous arteriovenous fistula (AVF) presenting with cephalic arch stenosis was carried out. The aim is to assess the effectiveness of angioplasty and stenting in cephalic arch stenosis in autologous AVF in hemodialysis patients and also to assess the outcome of metal stents at this distinctive anatomical site. All patients were subjected to Doppler examination, where the stenotic lesion and the AVF flow volume were assessed prior to intervention. The follow-up period reached up to 57 months in some patients. All patients were under surveillance and were assessed for patency and flow volume. The primary and secondary stenosis-free rates were calculated. Re-intervention during the follow-up period was recorded.
Results
The technical success rate was 100%. Twenty-six patients had balloon angioplasty. Eighteen patients had primary and/or secondary stents inserted. The primary patency rate at 6 and 12 months for the balloon angioplasty group was 80% and 60% and for the stent group was 86% and 71%, respectively. The mean primary patency rate in balloon angioplasty patients was 12.9 months, while in the primary stented patients was 19.9 months. Twenty-six patients had secondary intervention. The average secondary patency rate for patients with balloon angioplasty was 25.5 months, while it was 33.6 months in the stented patients.
Conclusion
Cephalic arch angioplasty and stenting is an effective intervention increasing the longevity of the AVF that is crucial for hemodialysis patients. The use of metal stents whether bare metal stents or covered stents is safe and adds significant increase in patency rates.
Similar content being viewed by others
Background
A well-functioning vascular access is the pre-requisite for chronic hemodialysis management [1]. An autogenous arteriovenous fistula is the access of choice for hemodialysis [2].
The cephalic vein is the superior conduit for hemodialysis. It is the outflow vein for radiocephalic and brachiocephalic AVF [3]. The cephalic arch is the final part of the cephalic vein which passes beneath the clavicle and bends sharply piercing the clavipectoral fascia to join the axillary vein forming the subclavian vein [4, 5].
The cephalic arch region is particularly vulnerable to stenosis. This vulnerability is believed to be caused by its anatomic location and by hemodynamic factors [6].
One of the factors is that the cephalic vein of patients with renal failure shows intimal hyperplasia compared with the cephalic vein in normal subjects [7]. The cephalic arch has limited ability to dilate to support AVF blood flow as it passes through the very dense clavipectoral fascia, and the failure to dilate in the face of intimal hyperplasia will result in luminal narrowing and obstruction to flow [6].
Other factors include the turbulence and shear stress related to the curve which may lead to intimal injury that induces endothelial proliferation, vasoconstriction, and platelet aggregation. Also, there are at least twice as many valves in the cephalic arch compared to any portion of the cephalic vein, and these valves hypertrophy in the presence of high blood flow reduce the lumen diameter significantly [4,5,6].
Endovascular and surgical therapy can be used to treat cephalic arch stenosis [8]. Percutaneous venoplasty is considered the standard treatment for dialysis arteriovenous fistula stenosis associated with access dysfunction [9]. Although venoplasty of the cephalic arch stenosis (CAS) is associated with good technical results, primary patency rates are poorer than in other parts of the dialysis access circuit. Restenosis in this region is common, and repeated angioplasty is necessary to maintain patency. However, even after deployment of bare stents, patency after stenting of AVF is unsatisfactory due to the rapid development of in-stent stenosis [10]. The use of stent grafts in angioplasty for recurrent cephalic arch stenosis significantly improved short-term restenosis rates and long-term patency [11].
Methods
A retrospective study of 44 consecutive hemodialysis patients with autologous cephalic AVF presenting with cephalic (terminal) arch stenosis was carried out over 3 years of period.
Patients were diagnosed with AVF stenosis during surveillance Doppler ultrasound or referred by nephrologists suspecting malfunctioning fistula. All patients had a Doppler ultrasound examination to assess patency and flow volume of the AVF. Visible lumen narrowing on grayscale ultrasound (30% luminal stenosis or more) and aliasing on color Doppler at the site of stenosis and/or a flow volume < 600 ml/min warranted angioplasty.
All procedures were performed under local anesthesia. Antegrade puncture of the outflow cephalic vein was performed in a standard Seldinger technique, and an introducer sheath was inserted.
The cephalic arch stenosis was confirmed with venography. Then, the stenosis was negotiated with a 0.035-inch hydrophilic-coated guide wire (Terumo, Tokyo, Japan) and a diagnostic catheter, followed by the balloon catheter passed over the guide wire.
The balloon sizes used ranged from 7 to 10 mm depending on the diameter of the normal vessel adjacent to the stenosis. Under pressure control, the balloon was inflated slowly until the waist disappeared. Gentle manual compression was done at the puncture site after removal of the sheath using one finger, followed by applying a small dressing which is tapped gently to the skin. No anticoagulant or antiplatelet medication was given post-procedure.
When post-balloon angioplasty result is not satisfactory (i.e., residual stenosis > 30%), a metallic stent was inserted. In 10 patients, self-expandable bare metal stent Misago (Terumo, Tokyo, Japan) was inserted. In four patients, a Fluency stent-graft (Bard, USA) was inserted; two 10 mm × 4 cm stents were inserted due to AVF rupture during balloon angioplasty and two 8 mm × 4 cm stents inserted for fear of rupture due to the small size of the vein.
All patients had a Doppler examination one month post-procedure, then every three to six months. No patients were lost for follow-up. The flow volume in the AVF was recorded on the first follow-up and at 1-year follow-up.
When re-stenosis was diagnosed during follow-up Doppler examination, re-intervention was performed. Twenty-six patients had secondary intervention within the follow-up period. Eighteen patients had balloon angioplasty, and 8 patients were stented in the secondary intervention session; 4 of them had stents in the primary intervention. Six bare-metal stents and 2 stent-grafts were used. The stent size ranged from 8 to 12 mm.
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). The Kolmogorov–Smirnov test was used to verify the normality of distribution of variables, and Mann–Whitney test was used to compare between two groups for not normally distributed quantitative variables. Spearman coefficient was used to correlate between quantitative variables. Significance of the obtained results was judged at the 5% level.
Results
30 female and 14 male patients with mean age of 57.32 years presenting with cephalic arch stenosis referred for endovascular management were included.
Thirty-eight patients had brachiocephalic (BC) AVF, and 6 had radiocephalic (RC) AVF. The mean fistula age was 26.75 months; in two patients, the AVF was 6 weeks old, and the patients had not started dialysis through the fistula. The mean pre-intervention flow volume was 1243 ml/min and ranged from 600 to 2700 ml/min.
The range of luminal stenosis on Doppler examination was 30–90% with an average stenosis of 60%.
Table 1 shows the distribution of the studied cases according to age of the patient, age, and site of the AVF and pre-plasty flow volume.
The technical success rate of the procedures was 100%. Our indication for technical success was the achievement of < 30% residual stenosis on completion angiography.
Eighteen (40.9%) patients did not need re-intervention during the follow-up period. Fourteen patients of the 18 patients had balloon angioplasty, and 4 had primary stents.
Twenty-six (59.1%) patients had secondary intervention within the follow-up period ranging from 18 to 57 months. Sixteen patients of the 26 patients had primary balloon angioplasty, and 10 had primary stents. Eighteen patients had secondary balloon angioplasty, and 8 patients were stented in the secondary intervention session; 4 of them had stents in the primary intervention.
Six of the 26 patients had multiple re-interventions, while 20 patients had a single re-intervention. There were 38 secondary interventions with an average of 1.46 intervention per patient.
Those interventions were 16 bare-metal stents, 10 primary stents, 4 secondary stent after balloon angioplasty, and 2 after primary bare metal stents. Six covered stents were used: 4 primary inserted and 2 inserted after primary bare metal stent. The stent was inserted if there is more than 30% residual stenosis visualized on post-angioplasty venogram.
In 2 cases, AVF rupture was encountered during primary angioplasty and a covered stent was used in each case with preservation of the fistula. In both cases, there was a resultant subcutaneous hematoma which was managed conservatively.
Table 2 shows the distribution of cases according to the performed primary and secondary procedure.
The primary stenosis-free (patency) rate at 6 and 12 months for the angioplasty group was 80% and 60% and for the stent group was 86% and 71%, respectively. The primary stenosis-free (patency) rate is defined as the uninterrupted patency without any additional procedure of the previously treated lesion.
The mean primary stenosis-free rate in the 44 patients during the follow-up period was 15.3 months. The mean primary stenosis-free rate in the balloon angioplasty only (30 patients) was 12.9 months; while in the primary stented 14 patients was 19.9 months.
The average secondary stenosis-free (patency) rate for patients with balloon angioplasty only was 25.5 months ranging from 18 to 43 months, while was 33.6 months in the stented 14 patients either primary or secondary stenting ranging from 19 to 57 months. The secondary stenosis-free (patency) rate is that patency is never lost but is maintained by prophylactic secondary intervention.
Table 3 shows a comparison between the primary and secondary stenosis-free rates between balloon angioplasty and stent patients.
There is a significant increase in primary and secondary stenosis-free rate in the stent group of patients compared to balloon angioplasty only, P 0.032 and 0.027, respectively.
Table 4 shows a comparison between the primary and secondary stenosis-free rates between BMS and covered stent patients.
There is no significant difference between BMS and covered stent in terms of primary and secondary stenosis-free rates; however, the number of cases in each group is small.
Table 5 shows a comparison between the primary and secondary stenosis-free rates between patients with brachiocephalic and radiocephalic AVF.
There is significant increase in secondary stenosis-free rate between BC and RC patients; however, the number of patients in the RC group is small.
The mean flow volume at 1-month follow-up scan was 1603 ml/min ± 532 SD, and the range was 900–2800 ml/min with a median of 1550 ml/min, while the mean flow volume at 1-year follow-up was 1479 ml/min ± 667 SD ranging from 600 to 3500 ml/min with a median of 1500 ml/min.
We had two cases of fistula rupture managed successfully by covered stents. No stent fracture noted during the follow-up period.
Table 6 shows a comparison between the p-value of the primary and secondary stenosis-free rates between patients in relation to the age of the patient, the age of the fistula, and the pre-plasty flow volume.
There is significant increase in the primary stenosis-free rate with the increase in the pre-angioplasty flow volume of the AVF. Also, there is significant increase in the secondary stenosis-free rate related to the increase in the age of the AVF.
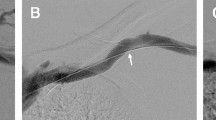
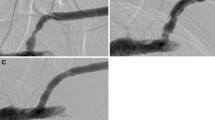
Figures 1, 2 and 3 show selected cases of the study and their management.
a Antegrade diagnostic venogram in left AVF showing tight stenosis at the terminal arch. b Angioplasty at the terminal arch using 8 mm × 4 cm balloon. c Post-angioplasty venogram showing no significant residual stenosis at the terminal arch. d Antegrade diagnostic venogram in Left AVF after 5 months showing recurrent tight stenosis at the terminal arch. e Angioplasty at the terminal arch using 8 mm × 4 cm balloon. f Post-angioplasty venogram showing no significant residual stenosis at the terminal arch
a Antegrade diagnostic venogram in right AVF showing tight stenosis at the terminal arch. b Angioplasty at the terminal arch using 8 mm × 4 cm balloon. c Post-angioplasty venogram showing no significant improvement of the stenosis at the terminal arch. d Deployment of 10 mm × 4 cm self-expandable stent at the terminal arch with completion venogram showing no significant residual stenosis
Discussion
Venous stenosis affects up to 50% of AVF and is the most common cause for vascular access dysfunction. Cephalic arch stenosis (CAS) is recognized as a distinct clinical entity that is implicated in up to 75% of dysfunctional brachiocephalic fistulae [12].
The treatment of CAS remains a matter of debate, and the data are limited. Angioplasty, stent insertion, and surgical intervention have all been proposed in small series [13, 14].
The mean age of the AVF from creation to initial radiological intervention was 26.8 months; this corresponded to 25.2 months in the study of Rajan et al. [10].
Our technical success rate was 100%; this was in agreement with Jones et al. [15] who reported the same technical success rate and was higher than Rajan et al. [10] who reported 76% success rate.
We had two ruptured fistula during angioplasty (4.5%) managed successfully by covered stents; Rajan et al. [10] reported 6% rupture rate (3 of 50), one of which led to fistula loss and the other two were salvaged with prolonged balloon inflation or stenting.
In the current study, the primary patency rate at 6 and 12 months for balloon angioplasty was 80% and 60%, respectively. This is compared to Jackson et al.’s [14] study who reported 68.8% and 31% at 3 and 12 months, respectively.
We reported patency rate at 6 and 12 months for primary stent of 86% and 71%, respectively. Shemesh et al. [11] reported primary patency at 6 months of 82% in the stent graft group and 39% in the bare stent group. Jones et al. [15] reported primary patency 67% and 42% at 6 and 12 months for covered stents.
We reported a significant increase in primary and secondary stenosis-free rate in the stent group of patients compared to balloon angioplasty only, P 0.032 and 0.027, respectively. However, there was no significant difference between BMS and covered stent in terms of primary and secondary stenosis-free rates.
59% of our patients had a secondary intervention with an average of 1.5 interventions per patient. Jackson et al. [14] reported a re-intervention rate of 2.3 per patient.
We studied the relation between age of the patient, age of the AVF, and its flow volume at the time of primary intervention and patency rates. There was no significant effect of the patient’s age on patency rates, and this matched Jones et al. [15], who found no significant difference in patency with regard to sex or age.
We found significant increase in patency rate related to age of the AVF and its flow rate; however, we could not find a study in the available literature in this regards.
Conclusion
Cephalic arch angioplasty and stent is effective in increasing the longevity of the hemodialysis AVF that is crucial for hemodialysis patients. The use of metal stents is safe and adds significant increase in patency rates.
It is recommended that patients on dialysis through an AVF should be on a close surveillance scheme including clinical and Doppler evaluation from the time of creation of the fistula and throughout their life to detect signs of dysfunction and allow early intervention.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AVF:
-
Arteriovenous fistula
- BC:
-
Brachiocephalic
- CAS:
-
Cephalic arch stenosis
- RC:
-
Radiocephalic
References
Janicki K, Pietura R, Radzikowska E, Zaluska W, Bicki J (2001) The obtention of vascular access on the arm for hemodialysis. Ann Univ Mariae Curie Sklodowska Med 56:206–211
Kingdon EJ, Holt SG, Davar J, Pennell D, Baillod RA, Burns A, Sweny P, Davenport A (2001) Atrial thrombus and central venous dialysis catheters. Am J Kidney Dis 38:631–639
Neumann I, Meisl FT, Kopriva G, Manker W, Sinzinger H (2001) Positive imaging of an inflammatory process at an arteriovenous access site with 111 Indium-labeled platelets. Rev Esp Med Nucl 20:120–122
Au FC (1989) The anatomy of cephalic vein. Am Surg 55(10):638–639
Hallock GG (1993) The cephalic vein in microsurgery. Microsurgery 14(8):482–486
Daoui R, Asif A (2012) Cephalic arch stenosis: mechanisms and management strategies. Semin Nephrol 32:538–544
Wali MA, Eid RA, Dewan M, Al-Homrany MA (2003) Intimal changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. J Smooth Muscle Res 39(4):95–105
Davies MG, Hicks TD, Haidar GM, El-Sayed HF (2017) Outcomes of intervention for cephalic arch stenosis in brachiocephalic arteriovenous fistulas. J Vasc Surg 66:1504–1510
Clinical practice guidelines for vascular access (2006) Am J Kidney Dis 48(Suppl 1):S176–S247
Rajan DK, Clark TW, Patel NK et al (2003) Prevalence and treatment of cephalic arch stenosis in dysfunctional autogenous hemodialysis fistulas. J Vasc Interv Radiol 14:567–573
Shemesh D, Goldin I, Zaghal I, Berlowitz D, Raveh D, Olsha O (2008) Angioplasty with stent graft versus bare stent for recurrent cephalic arch stenosis in autogenous arteriovenous access for hemodialysis: a prospective randomized clinical trial. J Vasc Surg 48:1524–1531
Aitken EL, Jackson AJ, Hameed H, Chandramohan M, Kasthuri R, Kingsmore DB (2014) Cephalic arch stenosis: angioplasty to preserve a brachiocephalic fistula or new brachiobasilic fistula? A cost-effectiveness study. Ren Fail 36(10):1550–1558
Heerwagen ST, Lonn L, Schroeder TV, Hansen MA (2010) Cephalic arch stenosis in autogenous brachiocephalic hemodialysis fistulas: Results of cutting balloon angioplasty. J Vasc Access 11:41–45
Jackson AJ, Aitken EL, Kasthuri R (2014) Kingsmore DB venous outflow stenosis of the brachiocephalic fistula: a single entity, or is the cephalic arch different? J Vasc Med Surg 2:154
Jones RG, Willis AP, Tullett K, Riley PL (2017) Results of stent graft placement to treat cephalic arch stenosis in hemodialysis patients with dysfunctional brachiocephalic arteriovenous fistulas. JVIR 28(10):1417–1421
Acknowledgements
Not applicable.
Funding
There was no funding for this study.
Author information
Authors and Affiliations
Contributions
Corresponding author has contributed to conception and design of the work, acquisition, analysis, and interpretation of data and drafted the work and substantively revised it. The author has approved the submitted version (and any substantially modified version that involves the author's contribution to the study); the author has agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated and resolved and the resolution documented in the literature. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee (Ethics committee, Faculty of Medicine, Alexandria University). Informed consent was obtained from all individual participants included in the study. This article does not contain patient data.
Consent for publication
Not applicable.
Competing interests
The author declares that he has no conflict of interest and certifies that he has no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visithttp://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelsalam, H. Cephalic arch stenosis in autologous hemodialysis fistula; to stent or not to stent? Long-term follow up. Egypt J Radiol Nucl Med 53, 96 (2022). https://doi.org/10.1186/s43055-022-00772-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00772-6